Introduction
Esophageal carcinoma, which is the eighth leading
cause of cancer-related deaths worldwide (1), occurs with great variation along with
geography, ethnicity, and sociocultures. Esophageal squamous cell
carcinoma (ESCC) is the most common pathological type in some East
Asia countries, such as China and Japan, whereas adenocarcinoma
occurs more commonly in Europe and the USA (2). To date, Ivor-Lewis esophagectomy has
been chosen as the most valid means of resectable ESCC, as the
majority of ESCC tend to occur in the middle thoracic esophagus.
However, the benefits of radical resection through Ivor-Lewis
esophagectomy remain unsatisfactory. Although the local tumor is
completely removed via this method, >50% of patients experience
lymphatic metastatic recurrence in the 2 or 3 years after surgery
(3), which is the main recurrence
type.
As is well-known, the prognosis of patients with
ESCC is TNM staging-specific. However, TNM staging is not accurate
and sensitive enough to predict the prognosis of ESCC patients
(4,5).
Therefore, it is necessary to investigate biological markers to
predict the recurrence and prognosis of these patients. These
markers may help to detect candidates with a high recurrence risk
for postoperative adjuvant therapy. In our previous study, some
molecular indicators (including C-C chemokine receptor type 7 and
vascular endothelial growth factor-C) were identified that may be
useful to predict lymphatic metastatic recurrence in pN0 esophageal
squamous cell carcinoma (6). However,
there has been no report on the correlation between the
overexpression of centrosomal protein 55 (CEP55) and the prognosis
of patients with ESCC after Ivor-Lewis esophagectomy.
CEP55, which has as important role in maintaining
the proper function of the midbody structure, is the latest mitotic
phosphoprotein to be found. CEP55 has an important role in the
final stage of cell division, which involves the physical
separation of the two daughter cells (7–9).
Overexpression of CEP55 leads to cytokinesis defects and
multinucleated cells increase, which may cause tumorigenesis. CEP55
overexpression has been found in various human tumors (10–14) and
tumor cell lines (15).
Overexpression of CEP55 in mammalian cells correlates with
increased cell migration and invasion (16). Moreover, suppression of CEP55
expression predominantly impedes the growth of cancer cells, which
is associated with increased apoptosis (10). The findings of these previous studies
suggest that overexpression of CEP55 acts as a factor that
contributes to poor patient prognosis in malignancies. However, to
the best of our knowledge, there have been no reports that have
established a correlation between the overexpression of CEP55 and
the prognosis of ESCC. Therefore, the present study was designed to
investigate the correlation between CEP55 overexpression in cancer
tissues and prognosis in patients with ESCC after Ivor-Lewis
esophagectomy.
Materials and methods
Ethics statement
The study protocol was approved by the Research
Ethic Committee of Provincial Hospital Affiliated to Shandong
University (Jinan, China). All patients and their relatives
provided informed consent.
Patients and materials
Between March 2007 and May 2008, all of the patients
enrolled in the present study (n=110) suffered from mid-thoracic
ESCC and had undergone Ivor-Lewis esophagectomy with two-field
lymph node dissection in our department, and were retrospectively
studied. The inclusion criteria were as follows: i) Mid-thoracic
esophageal squamous cell carcinoma pathologically diagnosed as
postoperative pathological stage T2-4aN0-2M0; ii) no preoperative
radiotherapy or chemotherapy; iii) without surgical
contraindication; iv) metastasis of cervical or supra-clavicular
lymph node was excluded prior to surgery; v) All of the operations
were radical resections identified by the postoperative pathology;
vi) there was no serious operative complication; and vii) number of
lymph nodes dissected was >12. The study group consisted of 79
men and 31 women aged 43–72 years. Pathological staging complied
with the classification guidelines outlined by the International
Union Against Cancer in 2009 (17).
Patients were routinely followed-up during the first six years
following surgery. Data of recurrent disease were recorded in
completion. Clinicopathological characteristics of the 110 patients
are listed in Table I.
 | Table I.CEP55 expression and
clinicopathological characteristics in patients with esophageal
squamous cell carcinoma. |
Table I.
CEP55 expression and
clinicopathological characteristics in patients with esophageal
squamous cell carcinoma.
|
|
| CEP55 expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Patients | (−) | (+) | P-value |
|---|
| Total | 110 | 47 | 63 |
|
| Gender |
|
|
| 0.336 |
| Male | 79 | 36 | 43 |
|
|
Female | 31 | 11 | 20 |
|
| Age (years) |
|
|
| 0.392 |
|
<60 | 52 | 20 | 32 |
|
| ≥60 | 58 | 27 | 31 |
|
| Tumor length
(cm) |
|
|
| 0.06 |
|
<5 | 45 | 24 | 21 |
|
| ≥5 | 65 | 23 | 42 |
|
| Differentiation |
|
|
| 0.022 |
|
Well-moderate | 71 | 36 | 35 |
|
| Poor | 39 | 11 | 28 |
|
| pT |
|
|
| 0.019 |
| T2 | 38 | 22 | 16 |
|
| T3 | 72 | 25 | 47 |
|
| pN |
|
|
| 0.033 |
| N2 | 24 | 6 | 18 |
|
| N1 | 52 | 21 | 31 |
|
| N0 | 34 | 20 | 14 |
|
| TNM stage |
|
|
| 0.002 |
| II | 36 | 23 | 13 |
|
|
III | 74 | 24 | 50 |
|
| Recurrence |
|
|
| 0.021 |
|
Yes | 49 | 15 | 34 |
|
| No | 61 | 32 | 29 |
|
Each specimen from the 110 patients was cut into two
sections. An esophageal cancer specimen of at least 0.5×0.5×0.5 cm
in size was used for RNA extracting. Each esophageal cancer tissue
was labeled, wrapped quickly in foil, snap-frozen in liquid
nitrogen for 1 min, and stored at −80°C until subsequent RNA
extraction. The remaining esophageal cancer tissue specimen was
fixed in a 10% formaldehyde solution for histopathological
examination. Histological examination confirmed that all the cancer
tissues studied were squamous cell carcinomas.
CHEM samples were harvested for each patient from a
sample site located >5 cm from the margin of ESCC. All of the
CHEM samples from the 110 patients were analyzed for CEP55
expression via immunohistochemical and semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis.
Immunohistochemistry
Immunohistochemical analysis, using the
streptavidin-peroxidase (SP) method, was performed to detect the
levels of CEP55 expression in each tissue specimen. Slides were
stained according to the manufacturer's protocols for CEP55
(Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China).
Formalin-fixed paraffin-embedded sections (4 µm) were heated at
65°C for 30 min and were dewaxed in xylene, rehydrated through a
graded alcohol series and placed in an endogenous peroxide block
for 10 min. Antigen retrieval was performed using 10 mM of citrate
buffer in a microwave for 15 min. Tissue sections were subsequently
incubated at 4°C overnight with anti-CEP55 rabbit polyclonal
antibody (bs-7742R; Beijing Biosynthesis Biotechnology Co., Ltd.)
at a dilution of 1:150 in PBS. Secondary processing of the tissue
samples was performed with an SP kit and a universal secondary
antibody kit (SP-9001) according to the manufacturer's instructions
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China). Briefly, following incubation overnight with the primary
antibody, the secondary biotinylated antibody and subsequent
avidin-biotin complex reagent were incubated for 30 min at 37°C,
respectively. The tissues were then rinsed with PBS three times (5
min each time). Staining was visualized using diaminobenzidine and
tissues were counterstained with hematoxylin. As the negative
control, the primary antibody was replaced with PBS.
The immunohistochemical score was calculated by
combining the proportion score (percentage of positive stained
cells) with the staining intensity score. Specimens were examined
under a light microscope. In five randomly selected fields of
vision per-section, positively stained cells among 100 cells were
assessed and quantified (percentage). The mean percentage of the
five fields was used to identify the proportion score in a
six-category grading system (0, negative; 1, 1–10%; 2, 11–25%; 3,
26–50%; 4, 51–75%; and 5, >75%). Staining intensity was scored
as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. Both
of the scores were multiplied to form the immunohistochemical
score. All sections were examined by two independent pathologists
who were blinded to the clinical data.
Receiver operating characteristics
(ROC) curve
The cut-off score for CEP55 overexpression was
screened based on the ROC curve. Raw data of CEP55 expression
levels in the ESCC and CHEM groups were analyzed using a MedCalc
v13.0.2.0 statistical software package (MedCalc Software bvba,
Ostend, Belgium). The score closest to the point of both maximum
sensitivity and specificity was selected as the cut-off score.
Semi-quantitative RT-PCR analysis
Total RNA of each specimen was extracted using a
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc. Waltham, MA,
USA) one-step procedure according to the protocol provided by the
manufacturer. RNA purity and concentration were determined by a
standard ultraviolet spectrophotometric assay. RT-PCR was performed
using PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. To remove
genomic DNA, total RNA was mixed with 2.0 µl 5X gDNA Eraser Buffer
and 1.0 µl gDNA Eraser. RNase free water was then added to the
mixture to make a total reaction volume of 10 µl. The thermal
profile was 42°C for 2 min followed by 4°C, at which point the
fluid was ready for the next step. In the RT step, the reaction was
performed in a total volume of 25 µl, containing 4 µl of 5X
PrimeScript Buffer 2, 1 µl PrimeScript RT Enzyme Mix1, 1 µl RT
Primer Mix, 10 µl RNase Free dH2O and 10 µl of the
aforementioned fluid. The thermal profile was as follows: 37°C for
15 min, 85°C for 5 sec, followed by 4̊C. β-actin was used as an
internal control. Primers were designed according to previous
reports (16) as follows: CEP55,
5′-end primer 5′-TTGGAACAACAGATGCAGGC-3′ and 3′-end primer
5′-GAGTGCAGCAGTGGGACTTT-3′; and β-actin, 5′-end primer
5′-AGAGCCTCGCCTTTGCCGATCC-3′ and 3′-end primer
5′-CTGGGCCTCGTCGTCGCCCACATA-3′.
Following an initial denaturation at 94°C for 5 min,
samples were amplified by 35 cycles of 94°C for 30 sec, 58°C for 30
sec, 72°C for 30 sec and final extension at 72°C for 10 min. PCR
products were visualized by electrophoresis through 1% agarose gels
stained with ethidium bromide. Gel images were obtained using an
Alphalmager 2200 UV-image analyzer (Alpha Innotech, San Leandro,
CA, USA). Ratios of CEP55/β-actin were used to semi-quantify the
CEP55 expression levels. PCR was repeated in triplicate for all
samples and the data were analyzed using the comparative Cq method
(18).
Adjuvant therapies
To date in China, there have been no unanimous
guidelines on postoperative adjuvant therapy after radical
resection for the treatment of ESCC. Therefore, National
Comprehensive Cancer Network (NCCN) guidelines are often used,
which are not appropriate for the Chinese population with ESCC. In
our department the indications for postoperative adjuvant treatment
are on the basis of tumor stage, clinicians' preferences and
patients' willingness for treatment or economic status. Typically,
patients with pT3-4 are advised to receive radiotherapy and those
with pN1 should receive chemotherapy as a minimum. In the patients
enrolled in the present study, nine received postoperative
radiotherapy alone. Postoperative chemotherapy was administered to
12 patients for >4 cycles, predominantly paclitaxel and
cisplatin/carboplatin, and 64 patients received combined
chemoradiotherapy.
Follow-up after surgery and diagnosis
of recurrence
Patients were regularly reexamined every 3–6 months
in the three years following surgery. After three years, follow-up
took place every 6–12 months. These follow-up appointments included
a thorough physical examination and chest and upper abdomen
computed tomography scans. Positron emission tomography-computed
tomography scanning was administered to specific patients if
necessary. Examinations were compared with preoperative imaging
data. If there was progressive lymph node enlargement, biopsy was
the first choice to identify possible lymph node metastatic
recurrence. Patients with mediastinal lymph node enlargement
identified in CT scans were advised to undertake PET-CT examination
if biopsy was difficult to achieve. A total of 18 patients were
diagnosed with lymphatic metastatic recurrence by PET-CT. Some
patients' metastases were diagnosed using biopsies. If new lesions
were identified in other organs, the patient was clinically
diagnosed with metastatic cancer after excluding the primary tumor.
The study follow-up period ended in February 2014; the longest
follow-up period was six years.
Statistical methods
The Mann-Whitney U test was used to identify
differences in CEP55 expression after immunohistochemical analysis.
The χ2 test was employed to analyze the correlations
between CEP55 overexpression and clinicopathological factors.
Univariate analysis was performed by modeling Kaplan-Meier survival
curves. The log-rank test was used to calculate the survival rate.
Multivariate analysis was performed using the Cox proportional
hazard model. P<0.05 was considered to indicate a statistically
significant difference. Statistical data were obtained using an
SPSS software package (SPSS 17.0; SPSS, Inc., Chicago, IL,
USA).
Results
Expression of CEP55 in ESCC tissue and
CHEM
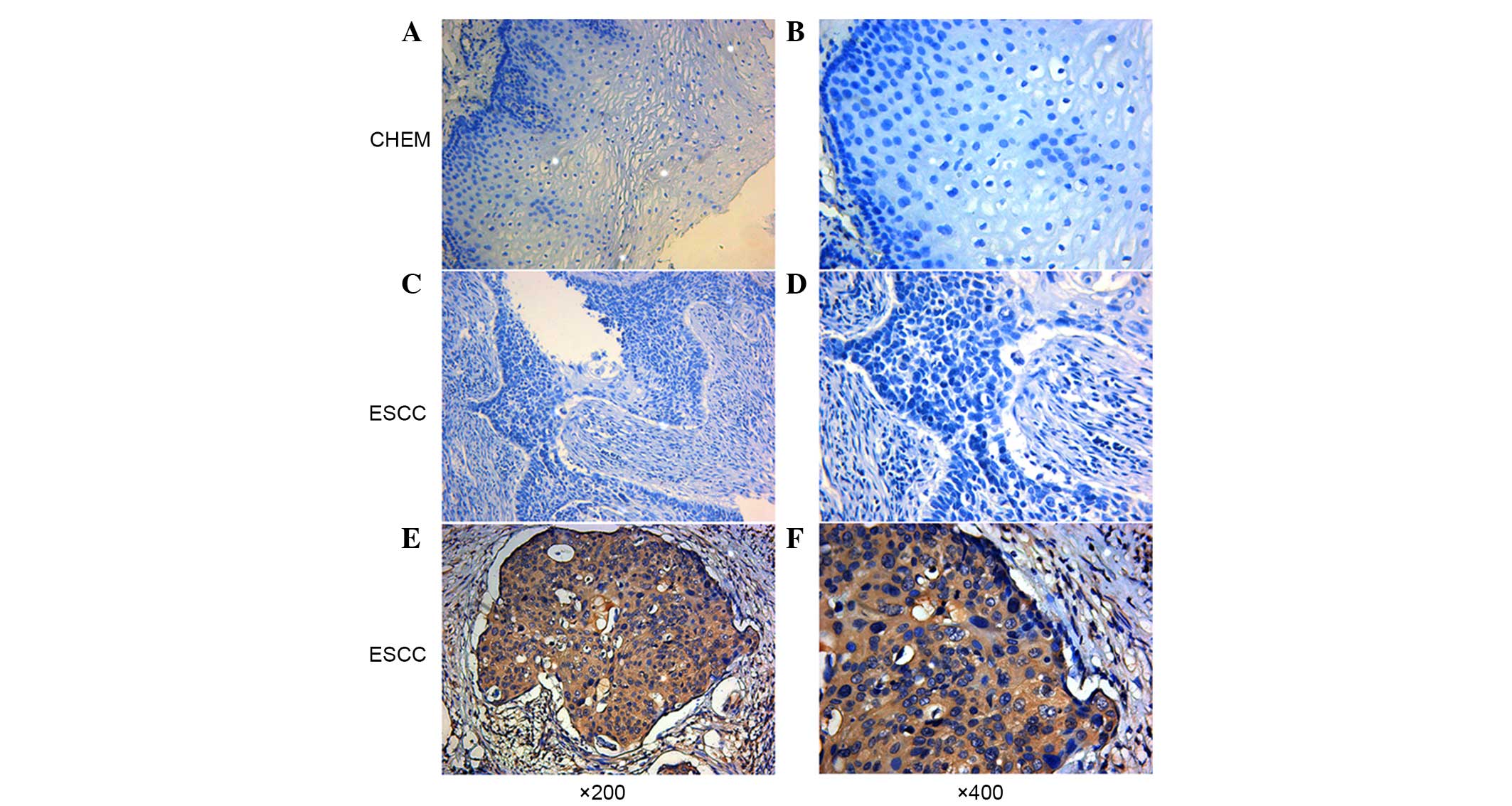
Using immunohistochemical analysis, positive
expression of CEP55 was detected as yellow or brownish yellow
staining in the cytoplasm. Positive CEP55 staining was readily
detected in ESCC, whereas negative or low staining was
predominantly observed in CHEM (Fig.
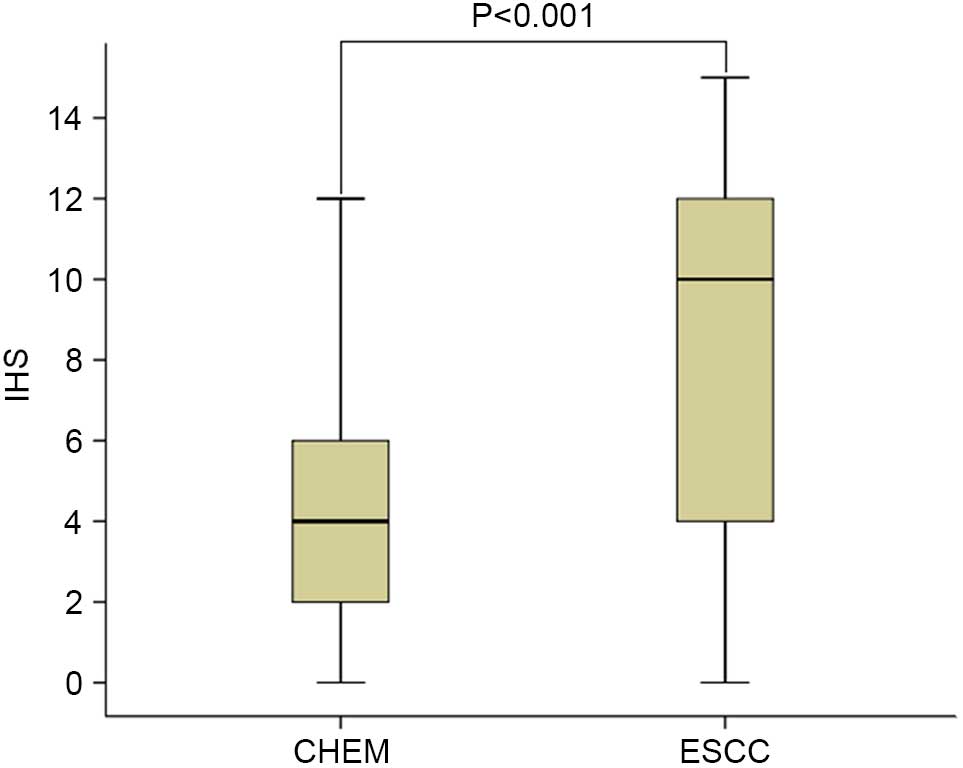
1). Expression levels of CEP55 in ESCC were markedly increased,
as compared with in CHEM (Fig. 2).
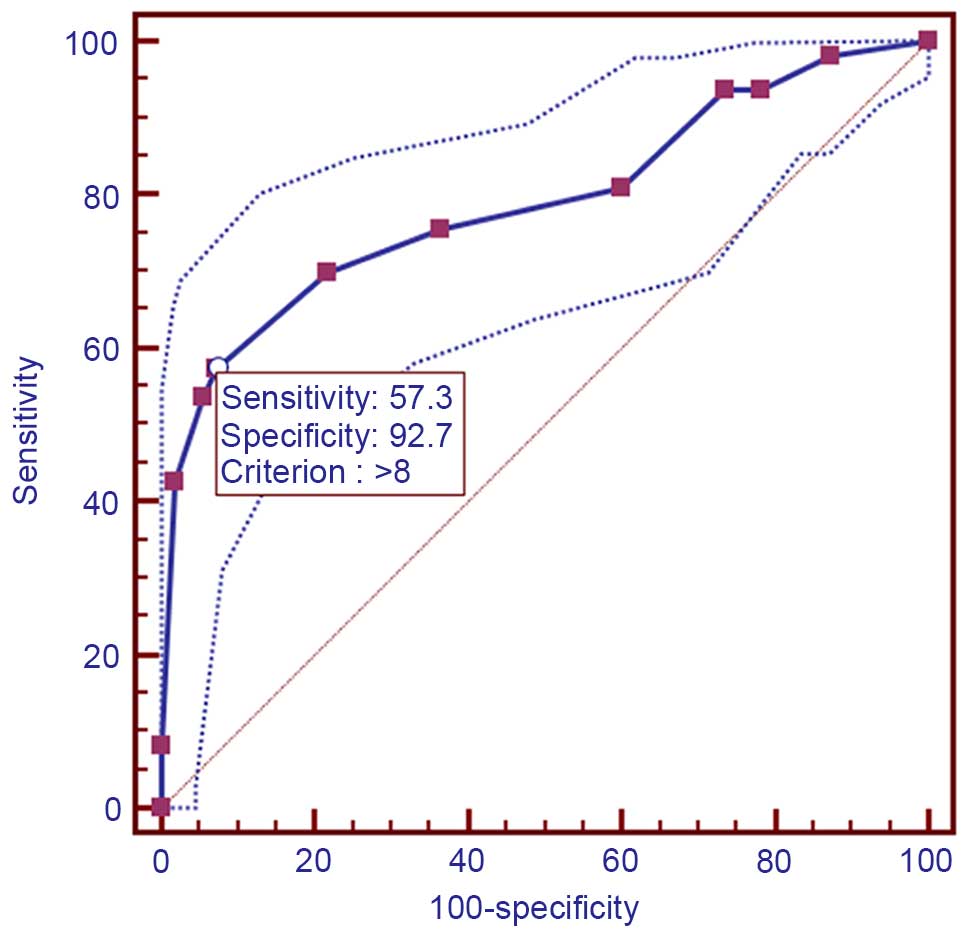
According to the ROC curve (Fig. 3),
the threshold value of 8 was the closest to the point with both
maximum sensitivity (57.3%) and specificity (92.7%); thus, a score
of 8 was selected as the cut-off value. The area under the curve
was 0.79 [95% confidence interval (CI): 0.730–0.842; P<0.0001].
Samples with a immunohistochemical score >8 were identified as
exhibiting high CEP55 expression, whereas samples with scores <8
were defined as having low or negative CEP55 expression. To
validate the results of immunohistochemical analysis, PCR was
employed to analyze CEP55 mRNA expression levels in different
tissues. The results were consistent with those determined through
the immunohistochemical method (Fig.
4).
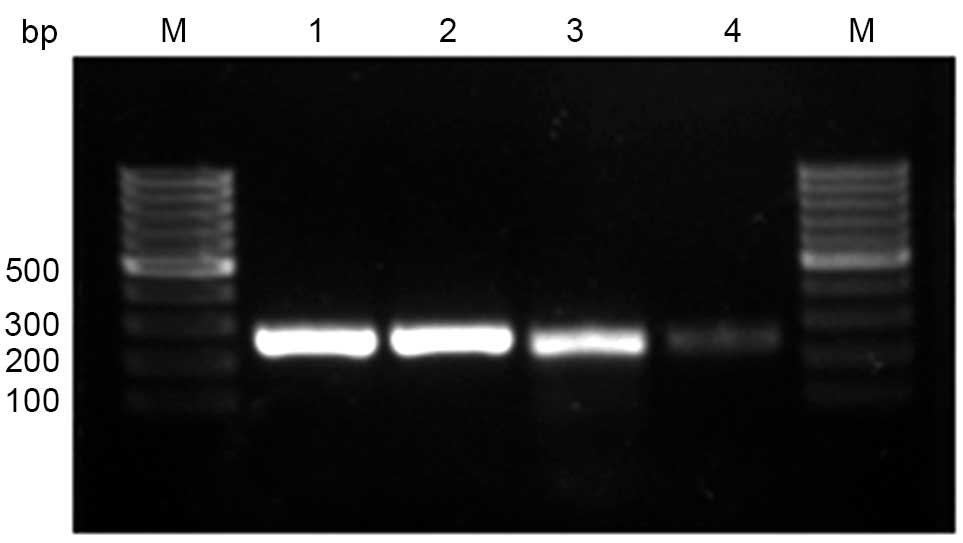 | Figure 4.Expression of CEP55 mRNA, as detected
by reverse transcription-quantitative polymerase chain reaction
analysis. Lane 1, representative β-actin mRNA expression in ESCC;
lane 2, representative β-actin mRNA expression in CHEM; lane 3,
representative CEP55 mRNA expression in ESCC; and lane 4,
representative CEP55 mRNA expression in CHEM. M, molecular marker;
bp, basepair; IHS, immunohistochemical scores; CEP55, centrosomal
protein 55; CHEM, corresponding healthy esophageal mucosa; ESCC,
esophageal squamous cell carcinoma. |
Correlation between CEP55
overexpression and clinical characteristics
CEP55 overexpression was identified in ESCC tissues
from 63 patients. Diagnostic sensitivity was demonstrated to be
57.3% (63/110; Table I). The
correlations between CEP55 expression and clinicopathological
features are shown in Table I.
χ2 analysis indicated that CEP55 overexpression was
significantly associated with tumor differentiation degree
(P=0.022), depth of invasion (P=0.019), lymph node metastasis
(P=0.033), TNM stage (P=0.002) and tumor recurrence (P=0.021;
Table I). No other
clinicopathological parameter was found to be associated with CEP55
overexpression (Table I). Notably,
all the positive correlations with CEP55 overexpression were
aggressive clinicopathological features of patients with ESCC.
Correlation between CEP55 expression
and prognosis
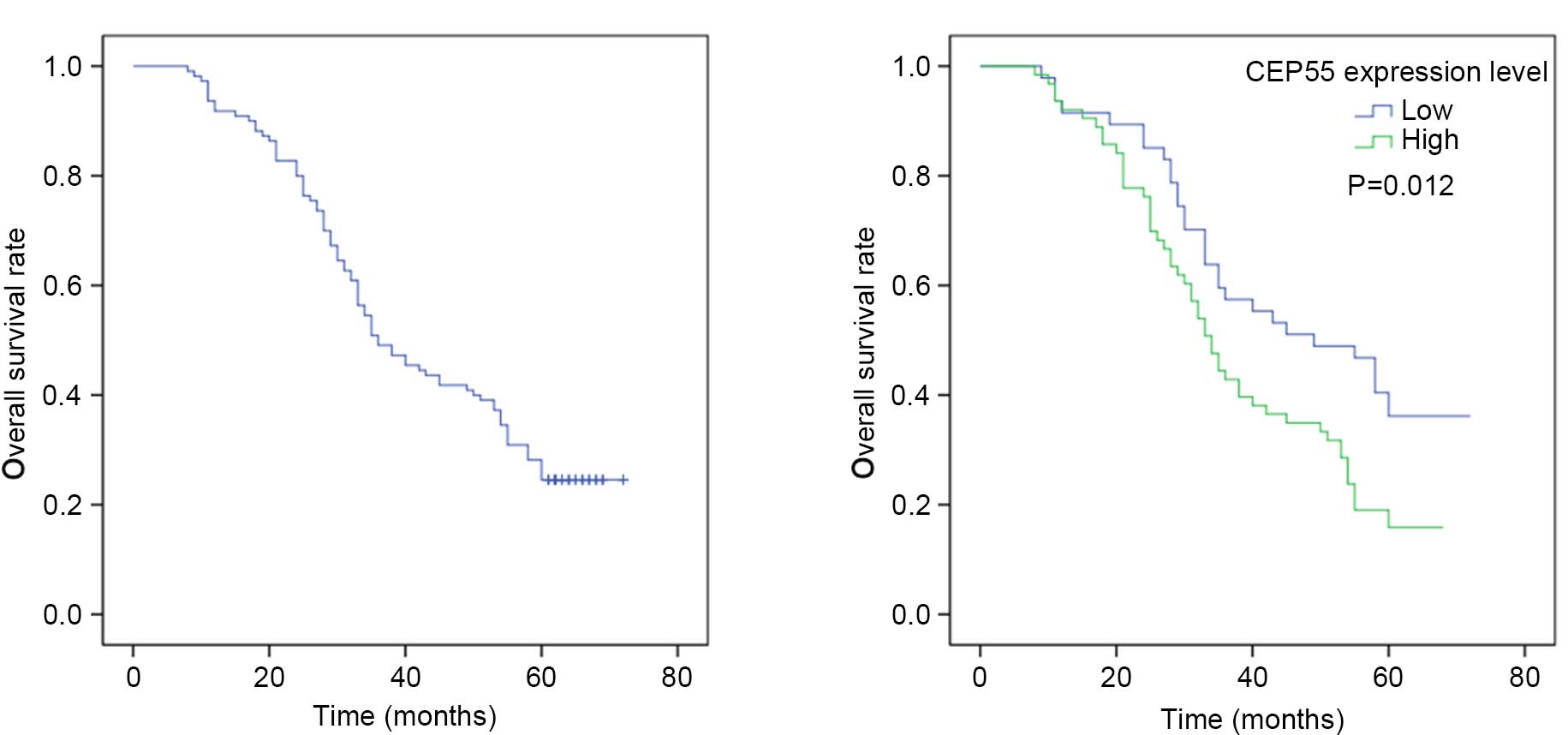
The 1-, 3-, and 5-year overall survival rates of 110
patients were 91.8, 49.8 and 30.4%, respectively. The median
survival time was 36.0 months (95% CI: 27.4–44.6 months).
Univariate analysis indicated that CEP55 expression levels
(P=0.012) were significant prognostic factors. The 5-year survival
rate of patients without CEP55 overexpression in ESCC tissues was
significantly higher than that of patients with CEP55
overexpression (Fig. 5). The results
of Cox regression multivariate analysis revealed that pT status,
lymph node metastasis, TNM stage and tumor differentiation degree
were independent relevant factors (Table
II).
 | Table II.Cox regression analysis for risk
factors of 5-year survival. |
Table II.
Cox regression analysis for risk
factors of 5-year survival.
| Characteristic | B | SE | Wald | P-value | OR | 95.0% CI |
|---|
| Gender | −0.265 | 0.268 | 0.981 | 0.322 | 0.767 | 0.454–1.297 |
| Age | 0.291 | 0.241 | 1.455 | 0.228 | 1.338 | 0.834–2.148 |
| Tumor size | 0.368 | 0.338 | 1.189 | 0.275 | 1.445 | 0.746–2.801 |
| Lymph node
metastasis | 2.429 | 0.356 | 46.484 | 0.000 | 11.348 | 5.645–22.813 |
| Invasion depth | 1.015 | 0.479 | 4.493 | 0.034 | 2.760 | 1.079–7.056 |
| TNM stage | 1.594 | 0.737 | 4.679 | 0.031 | 4.926 | 1.161–20.889 |
| CEP55
overexpression | 0.041 | 0.244 | 0.028 | 0.867 | 1.042 | 0.646–1.680 |
|
Differentiation | 0.531 | 0.267 | 3.941 | 0.047 | 1.700 | 1.007–2.870 |
Discussion
Esophageal cancer is a common lethal malignancy that
typically derives from esophageal mucosa. As a predominant
pathological type, ESCC has a higher incidence rate than
adenocarcinoma in China (2). As a
high-grade malignancy, the prognosis of ESCC is far from
satisfactory despite great advances in the compressive therapy of
ESCC. Even following surgery, patients suffer from an overall
5-year survival rate of 30–50% (19).
Despite radical resection, >50% of patients relapse in the 2 or
3 years following surgery. The most common recurrence type is
lymphatic metastatic recurrence (2).
The distinctive embryological structure of the esophagus is
characterized by the presence of lymph-vessels in muscularis
mucosae. The process of lymphatic drainage of the esophagus is
complex with a rich lymphatic network. Lymph node metastasis may
present as regional metastasis, skipping metastasis, or distant
metastasis (20–22). Lymph node metastasis of esophageal
cancer typically occurs when the primary tumor is particularly
small.
It is widely accepted that complete resection is the
preferred option to cure resectable mid-thoracic ESCC. On the basis
of oncology, subtotal oesophagectomy accompanied with three-field
lymphadenectomy (McKeown procedure) is regarded as the optimal
approach for mid-thoracic ESCC. However, this procedure is highly
invasive and was accompanied by a high incidence of complications,
such as recurrent nerve paralysis (23–26).
Through three-field lymph node dissection, only 20–30% of patients
with ESCC have been identified as having cervical or
supraclavicular lymphatic metastasis (27). For the majority of patients with ESCC,
three-field lymph node dissection is used for accurate staging
rather than an improvement in long-term survival. Therefore,
whether three-field lymph node dissection should be performed in
all patients with thoracic ESCC remains controversial, and this
procedure has not been widely adopted in clinics. Modified
Ivor-Lewis esophagectomy via a thoracoabdominal two-field lymph
node dissection is preferable to treat ESCC, as previously reported
(28), due to its predilection for
the middle thoracic esophagus. The disadvantage of this procedure
is that cervical lymph node dissection cannot be accomplished
simultaneously. However, some patients identified as pN0 stage via
postoperative pathology actually suffer from cervical lymph node
metastasis, as lymph node metastasis of ESCC is not detected.
Therefore, this creates a problem of how ESCC patients with a
probability of high lymphatic node metastasis can be identified.
For patients who have a high probability of lymphatic node
metastasis, cervical lymph node dissection can be performed
simultaneously or postoperative radiotherapy can be utilized, which
assesses the neck, supraclavicular region and superior mediastinum
for lymphatic node metastasis.
To date in China, there is no consensus on whether
an adjuvant therapy is required for ESCC patients with complete
resection. Thus, the NCCN esophageal cancer guidelines are often
referenced in the clinic. The NCCN treatment guidelines shows that
patients with ESCC do not have to accept an adjuvant therapy after
the complete removal of a tumor. However, considering the poor
prognosis of ESCC patients after radical surgery, we propose that
an adjuvant therapy is necessary for specific patients to improve
their prognosis, and patients with ESCC should be treated
individually. To achieve this aim, the invasiveness and the lymph
node metastatic potency of the ESCC must be identified. Therefore,
it is necessary to identify novel indicators to predict patient
survival more accurately in clinical practice.
A human gene, CEP55, which is located in 10q23.33
encodes a protein capable of homodimerization. It was reported that
CEP55 protein localizes to the centrosome in interphase cells and
transfers to the midbody during cytokinesis. CEP55 has an essential
role in membrane fission events (29,30). In
the final stage of the cell cycle, physical separation of the
cytoplasmic volumes occurs and the two prospective daughter cells
arise (31). During this stage, an
intercellular bridge containing the midbody ring is formed
(32). This ring regulates membrane
fission and fusion events (29,33).
Failure of cytokinesis results in tetraploid cells, which are
chromosomally unstable and hence more prone to tumorigenesis
(8). Overexpression of CEP55 causes
cytokinesis defects via an increase of chromosomally unstable
binucleated cells, suggesting CEP55 overexpression is associated
with tumorigenesis.
CEP55 overexpression is found in various cancer cell
lines, while its expression is barely detected in normal tissues by
expression-profile analyses using microarrays (34). As reported, CEP55 overexpression has
been detected in hepatocarcinoma (11), colon carcinoma (34), oral cavity squamous cell carcinoma
(13) and lung cancer (14). Notably, the present study is the first
to report CEP55 overexpression in the majority of ESCC specimens
examined.
The present study investigated the correlation
between CEP55 expression in cancer tissues and the prognosis of
patients with locally advanced ESCC after Ivor-Lewis esophagectomy.
The 5-year survival rate of patients with CEP55 overexpression in
tumor issue was significantly lower than that of patients without
CEP55 overexpression. Furthermore, overexpression of CEP55 was
demonstrated to be associated with the differentiation degree, T
stage, lymph node metastasis, TNM staging and tumor recurrence in
locally advanced ESCC patients.
In conclusion, CEP55 overexpression predicts poor
prognosis and is associated with aggressive clinicopathological
features in ESCC. This requires a decision on whether cervical
lymph node dissection should be performed and which patients with
ESCC should be selected to receive postoperative adjuvant therapy.
As a tumour accelerator, CEP55 may be a novel therapeutic target
for cancer therapy. Further research should be performed to
identify whether knockdown of the CEP55 gene can retard the
invasiveness of ESCC cells. The mechanism through which CEP55
regulates the growth of ESCC should be researched to confirm the
potential effectiveness of CEP55 as a therapeutic target of ESCC in
clinical practice.
References
|
1
|
Valverde CM, Macarulla T, Casado E, Ramos
FJ, Martinelli E and Tabernero J: Novel targets in gastric and
esophageal cancer. Crit Rev Oncol Hematol. 59:128–138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa S, Kanda T, Kosugi S, Ohashi M,
Suzuki T and Hatakeyama K: Recurrence pattern of squamous cell
carcinoma of the thoracic esophagus after extended radical
esophagectomy with three-field lymphadenectomy. J Am Coll Surg.
198:205–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D'Annoville T, D'Journo XB, Loundou A,
Trousse D, Dahan L, Doddoli C, Seitz JF and Thomas PA: Prognostic
impact of the extracapsular lymph node involvement on disease-free
survival according to the 7th edition of American joint committee
on cancer staging system. Eur J Cardiothorac Surg. 44:e207–e211;
discussion e211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou X, Wei JC, Fu JH, Wang X, Zhang LJ,
Lin P and Yang HX: Proposed modification of the seventh American
joint committee on cancer staging system for esophageal squamous
cell carcinoma in Chinese patients. Ann Surg Oncol. 21:337–342.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Y, Wang Z, Liu X, Jiang W and Shi M:
CCR7 and VEGF-C: Molecular indicator of lymphatic metastatic
recurrence in pN0 esophageal squamous cell carcinoma after
Ivor-Lewis esophagectomy? Ann Surg Oncol. 19:3606–3612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabbro M, Zhou BB, Takahashi M, Sarcevic
B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y and
Khanna KK: Cdk1/Erk2- and Plk1-dependent phosphorylation of a
centrosome protein, Cep55, is required for its recruitment to
midbody and cytokinesis. Dev Cell. 9:477–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morita E, Sandrin V, Chung HY, Morham SG,
Gygi SP, Rodesch CK and Sundquist WI: Human ESCRT and ALIX proteins
interact with proteins of the midbody and function in cytokinesis.
EMBO J. 26:4215–4227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao WM, Seki A and Fang G: Cep55, a
microtubule-bundling protein, associates with centralspindlin to
control the midbody integrity and cell abscission during
cytokinesis. Mol Biol Cell. 17:3881–3896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai M, Shimokawa T, Kobayashi T,
Matsushima S, Yamada Y, Nakamura Y and Furukawa Y: Elevated
expression of C10orf3 (chromosome 10 open reading frame 3) is
involved in the growth of human colon tumor. Oncogene. 25:480–486.
2006.PubMed/NCBI
|
|
11
|
Chen CH, Lu PJ, Chen YC, Fu SL, Wu KJ,
Tsou AP, Lee YC, Lin TC, Hsu SL, Lin WJ, et al: FLJ10540-elicited
cell transformation is through the activation of PI3-kinase/AKT
pathway. Oncogene. 26:4272–4283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoda S, Hirohashi Y, Torigoe T,
Nakatsugawa M, Kiriyama K, Nakazawa E, Harada K, Takasu H, Tamura
Y, Kamiguchi K, et al: Cep55/c10orf3, a tumor antigen derived from
a centrosome residing protein in breast carcinoma. J Immunother.
32:474–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CH, Chien CY, Huang CC, Hwang CF,
Chuang HC, Fang FM, Huang HY, Chen CM, Liu HL and Huang CY:
Expression of FLJ10540 is correlated with aggressiveness of oral
cavity squamous cell carcinoma by stimulating cell migration and
invasion through increased FOXM1 and MMP-2 activity. Oncogene.
28:2723–2737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CH, Lai JM, Chou TY, Chen CY, Su LJ,
Lee YC, Cheng TS, Hong YR, Chou CK, Whang-Peng J, et al: VEGFA
up-regulates FLJ10540 and modulates migration and invasion of lung
cancer via PI3K/AKT pathway. PLoS One. 4:e50522009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang YC, Chen YJ, Wu CH, Wu YC, Yen TC
and Ouyang P: Characterization of centrosomal proteins Cep55 and
pericentrin in intercellular bridges of mouse testes. J Cell
Biochem. 109:1274–1285. 2010.PubMed/NCBI
|
|
16
|
Tao J, Zhi X, Tian Y, Li Z, Zhu Y, Wang W,
Xie K, Tang J, Zhang X, Wang L and Xu Z: CEP55 contributes to human
gastric carcinoma by regulating cell proliferation. Tumour Biol.
35:4389–4399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talsma K, van Hagen P, Grotenhuis BA,
Steyerberg EW, Tilanus HW, van Lanschot JJ and Wijnhoven BPL:
Comparison of the 6th and 7th Editions of the UICC-AJCC TNM
Classification for Esophageal Cancer. Ann Surg Oncol. 19:2142–2148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dresner SM and Griffin SM: Pattern of
recurrence following radical oesophagectomy with two-field
lymphadenectomy. Br J Surg. 87:1426–1433. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanabe G, Baba M, Kuroshima K, Natugoe S,
Yoshinaka H, Aikou T and Kajisa T: Clinical evaluation of the
esophageal lymph flow system based on RI uptake of dissected
regional lymph nodes following lymphoscintigraphy. Nihon Geka
Gakkai Zasshi. 87:315–323. 1986.(In Japanese). PubMed/NCBI
|
|
21
|
Chen G, Wang Z, Liu XY, Zhang MY and Liu
FY: Abdominal lymph node metastasis in patients with mid thoracic
esophageal squamous cell carcinoma. World J Surg. 33:278–283. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Chen H, Xiang J and Zhang Y, Li C,
Hu H and Zhang Y: Pattern of lymphatic spread in thoracic
esophageal squamous cell carcinoma: A single-institution
experience. J Thorac Cardiovasc Surg. 144:778–785; discussion
785–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu Y, Swisher SG, Ajani JA, Correa AM,
Hofstetter WL, Liao Z, Komaki RR, Rashid A, Hamilton SR and Wu TT:
The number of lymph nodes with metastasis predicts survival in
patients with esophageal or esophagogastric junction adenocarcinoma
who receive preoperative chemoradiation. Cancer. 106:1017–1025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Natsugoe S, Yoshinaka H, Shimada M,
Sakamoto F, Morinaga T, Nakano S, Kusano C, Baba M, Takao S and
Aikou T: Number of lymph node metastases determined by presurgical
ultrasound and endoscopic ultrasound is related to prognosis in
patients with esophageal carcinoma. Ann Surg. 234:613–618. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baba M, Aikou T, Natsugoe S, Kusano C,
Shimada M, Nakano S, Fukumoto T and Yoshinaka H: Quality of life
following esophagectomy with three-field lymphadenectomy for
carcinoma, focusing on its relationship to vocal cord palsy. Dis
Esophagus. 11:28–34. 1998.PubMed/NCBI
|
|
26
|
Matsubara T, Ueda M, Takahashi T, Nakajima
T and Nishi M: Localization of recurrent disease after extended
lymph node dissection for carcinoma of the thoracic esophagus. J Am
Coll Surg. 182:340–346. 1996.PubMed/NCBI
|
|
27
|
Yu Y, Wang Z, Liu XY, Zhu XF and Chen QF:
Therapeutic efficacy comparison of two surgical procedures to treat
middle thoracic esophageal carcinoma. World J Surg. 34:272–276.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Wang Z, Yang Z, Shang B, Liu X and
Chen G: Prospective study of adjuvant radiotherapy on preventing
lymph node metastasis after Ivor-lewis esophagectomy in esophageal
cancer. Ann Surg Oncol. 20:2721–2726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gromley A, Yeaman C, Rosa J, Redick S,
Chen CT, Mirabelle S, Guha M, Sillibourne J and Doxsey SJ:
Centriolin anchoring of exocyst and SNARE complexes at the midbody
is required for secretory-vesicle-mediated abscission. Cell.
123:75–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujiwara T, Bandi M, Nitta M, Ivanova EV,
Bronson RT and Pellman D: Cytokinesis failure generating
tetraploids promotes tumorigenesis in p53-null cells. Nature.
437:1043–1047. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glotzer M: The molecular requirements for
cytokinesis. Science. 307:1735–1739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paweletz N: Walther flemming: Pioneer of
mitosis research. Nat Rev Mol Cell Biol. 2:72–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carlton JG and Martin-Serrano J: Parallels
between cytokinesis and retroviral budding: A role for the ESCRT
machinery. Science. 316:1908–1912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakai M, Shimokawa T, Kobayashi T,
Matsushima S, Yamada Y, Nakamura Y and Furukawa Y: Elevated
expression of C10orf3 (chromosome 10 open reading frame 3) is
involved in the growth of human colon tumor. Oncogene. 25:480–486.
2006.PubMed/NCBI
|