Introduction
Radiotherapy is one of the most common and effective
treatments for cancer (1). Over 40%
of patients with cancer require radiotherapy during the management
of the disease (2). Although clinical
radiotherapy treatment planning and delivery technologies have
improved, the toxicity of radiotherapy to non-cancerous tissues and
organs remains a problem (2,3). Thus, radioprotective compounds are
crucial in clinical radiotherapy (3),
and the administration of radioprotective compounds has been
suggested as an approach for preventing radiation-damage in normal
tissues (4,5).
Selenium is a trace element with a fundamental role
in human biology (6). It detoxifies
reactive oxygen species (ROS) produced by radiation treatment
(4,7).
In human antioxidant systems, selenium acts in the form of
selenocysteine, which is incorporated into various selenoproteins
(8,9).
At least 25 selenoproteins have been identified in humans,
including glutathione peroxidase (GPx), thioredoxin reductases,
iodothyronine deiodinase and the selenoproteins P, W and R
(10). Selenium exists in numerous
chemical forms, of which the most studied are selenomethionine,
sodium selenite, methylselenocysteine, 1,4-phenylenebis (methylene)
selenocyanate and methylseleninic acid (9). Sodium selenite is the chemical form of
selenium previously used in clinical studies of radiotherapy
supplementation between 1987 and 2012 (7).
Despite having been previously used as a
complementary medicine during clinical radiotherapy (11,12), the
effectiveness of selenium use in radiotherapy and the mechanisms
underlying the effect of selenium in reducing the side effects of
radiotherapy require further study (7). Schleicher et al (13) and Rodemann et al (14) performed in vitro studies of
selenium and radiotherapy and identified that sodium selenite has
potential as a protective agent for non-cancerous tissues during
radiotherapy (15). However, the
mechanisms underlying this protection have yet to be revealed.
Diamond et al (16)
demonstrated that low-level supplementation of culture media with
sodium selenite significantly protected CHO-AA8 cells, a hamster
ovary-derived cell line, from radiation-induced mutagenesis. Eckers
et al (17) also reported that
the overexpression of selenoprotein P suppressed radiation-induced
ROS accumulation and protected normal human fibroblasts from
radiation-induced toxicity. Tak et al (18) identified that, when U937 human
leukemic monocyte lymphoma cells were exposed to 2 Gy of
γ-radiation, a marked difference with respect to apoptotic features
and mitochondrial function was observed between the cells that were
and were not pre-treated with ebselen.
Further studies are required in order to determine
the mechanisms underlying selenium-induced prevention of
radiotherapy side effects, before it may be recommended as an
adjuvant to cancer radiotherapy. Sodium selenite is the only
chemical form of selenium that has previously been used in clinical
studies for this purpose; therefore, the present study investigated
the protective effects of sodium selenite supplementation on
non-cancerous human esophageal cells, a cell type with high
radiosensitivity, prior to X-ray irradiation.
Materials and methods
Cell culture
The CHEK-1 immortalized non-cancerous human
esophageal cell line was provided by Dr H. Matsubara (Department of
Academic Surgery, Chiba University, Japan) (19) and maintained in RPMI-1640 medium (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) and 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Waltham, MA, USA), and incubated at 37°C in a
humidified chamber containing 5% CO2. The culture medium
was replaced every 3 days and the cells were passaged on a weekly
basis using a 1:5 splitting ratio.
Selenium supplementation
Sodium selenite (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was the chemical form of selenium that was used
for supplementation of the cell medium. The sodium selenite
supplementation doses ranged from 0–200 nM, and the duration of
incubation was 24–72 h, 18 h following initial cell seeding at a
density of 1×106 cells in a 10 ml/10 cm culture dish or
2×103 cells/50 µl well. Supplementation with a dose of
50 nM sodium selenite for 72 h prior to radiation treatment was
used for the cell viability assay, cell cycle analysis and western
blot analysis.
Irradiation
Irradiation was performed using an X-Ray irradiation
machine (Titan-225S; Shimadzu Corporation, Kyoto, Japan) at a rate
of 1.3 Gy/min. The dose of irradiation was 2 Gy based on the common
fractionation dose for radiotherapy.
Protein extraction for GPx-1 activity
assay and western blot analysis
CHEK-1 cells were supplemented with 50 nM sodium
selenite for 72 h, washed twice with PBS and harvested by adding a
solution of 1 mM EDTA in PBS, then removing the cells from the
culture dish using a cell scraper. Proteins were then extracted
using a radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck
Millipore) with a 10% protein inhibitor cocktail (Sigma-Aldrich;
Merck Millipore). The protein concentrations were determined using
a DC™ protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) following the method of Lowry et al (20). The extracted samples were stored at
−80°C until the time of analysis.
GPx-1 activity assay
The enzymatic activity of GPx-1 in CHEK-1 cell
homogenates was determined using the method described by Paglia and
Valentine (21), with certain
modifications. Briefly, GPx-1 activity was indirectly monitored
using spectrophotometric methods to observe the reduction of
oxidized glutathione, using nicotinamide adenine dinucleotide
phosphate (NADPH) as the reducing agent. GPx-1 activity was
quantified by measuring the change in NADPH absorbance at 340 nm
and was expressed as the change in NADPH absorbance (Δ mM NADPH)
over time (min) and with various levels of protein (mg) in the
presence of the substrate tert-butyl hydroperoxide. The absorbance
was recorded using a SpectraMax Plus 384 microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Cytotoxicity assay
The cytotoxicity of sodium selenite in CHEK-1 cells
was examined with various concentrations of sodium selenite (0–8
µM) using a colorimetric assay. Briefly, cells (2×103 in
50 µl/well) were seeded into 96-well plates. Sodium selenite
solutions were added 18 h following the initial cell seeding, and
the cells were then incubated for 72 h at 37°C in a humidified
chamber containing 5% CO2. The cell proliferation rate
and half-maximal inhibitory concentration (IC50) were
then determined using a Cell Counting kit-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's protocol. The absorbance was measured at a
wavelength of 450 nm using a SpectraMax Plus 384 microplate reader.
The data was analyzed using linear regression analysis.
Clonogenic assay
The survival of the cells post-irradiation was
investigated by conducting clonogenic and cell viability assays. To
conduct the clonogenic assay, the CHEK-1 cell media was
supplemented with various concentrations of sodium selenite (0–200
nM) for 72 h from 18 h following the initial seeding, and were then
irradiated with 2 Gy X-ray radiation. Immediately following
irradiation, the cells (500 cells/4 ml medium) were seeded in
Falcon 25 cm2 tissue culture flasks. Following 14 days
of culture at 37°C, the cells were washed once with PBS, fixed with
99.5% ethanol and stained with 0.5% crystal violet in
H2O:methanol (1:1) for 30 min at room temperature. The
cells were then washed with tap water and air-dried. The total
number of colonies containing >50 cells were counted using a
binocular light microscope (Olympus Corporation, Tokyo, Japan).
Following the counting of the colonies, the plating efficiency (PE)
and survival fraction (SF) were calculated using the following
equations (22,23).
PE=NumberofcoloniesformedNumberofcellsseeded×100%
SF=Numberofcoloniesformed Post
irradiationNumberofcellsseeded×PE×100%
Cell viability assay
To observe cell viability post-irradiation, CHEK-1
cells (2×103 in 50 µl/well) were seeded in 96-well
plates. Sodium selenite (50 nM) solution was added at 18 h
following initial cell seeding, and the cells were incubated for 72
h prior to irradiation. Post-irradiation cell viability was
examined every hour for 72 h using a Cell Counting kit-8, according
to the manufacturer's protocol. The absorbance was measured at a
wavelength of 450 nm using a SpectraMax Plus 384 microplate
reader.
Cell cycle analysis
Detached and attached cells were collected at the
end of each post-irradiation time point (24, 48 and 72 h). Detached
cells were collected from the medium. Attached cells were collected
by adding a TrypLE™ Express solution (Thermo Fisher Scientific,
Inc.) to attached cells and incubating at 37°C in a humidified
chamber containing 5% CO2 until the cells had detached.
Detached and attached suspension cells were centrifuged at 168 ×
g for 4 min at room temperature, the supernatant was removed
and cells were washed twice with ice-cold PBS. The cells were fixed
with 70% cold ethanol and stored at −20°C until the time of
analysis (1–4 days). On the day of analysis, the cells were washed
with PBS, stained with 0.05 mg/ml of propidium iodide solution
(Sigma-Aldrich, St. Louis, MO, USA) with 0.002 mg/ml RNAse (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) and incubated at room
temperature for 30 min. The DNA content was analyzed using
fluorescence-activated cell sorting (FACSCalibur™; BD Biosciences,
San Jose, CA, USA).
Western blot analysis
Total proteins were extracted from the cells and
measured following sodium selenite supplementation at 72 h
post-irradiation. Protein (30 µg) samples were subjected to
electrophoresis on a 5–20% SuperSep™ Ace Ready Gel (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) and electrotransferred to
a nitrocellulose membrane (GE Healthcare Life Sciences, Chalfont,
UK). Prior to antibody treatment, the membrane was blocked with 5%
w/v non-fat dry milk in a solution of 1X Tris-buffered saline and
0.1% polysorbate 20 by agitating for 45 min at room temperature.
The protein levels were analyzed by incubating with (ADP ribose)
polymerase (PARP) polyclonal antibody (Cell Signaling Technology,
Inc., Danvers, MA, USA; cat. no. 9542; dilution 1:1,000) at 4°C,
overnight. A horseradish peroxidase-conjugated donkey anti-rabbit
IgG secondary antibody (GE Healthcare Life Sciences; cat. no.
NA934; dilution 1:10,000) was used by incubating with gentle
agitation for 90 min at room temperature. Protein bands were
detected using an enhanced chemiluminescence detection system (GE
Healthcare Life Sciences). A mouse anti-GAPDH antibody (cat. no.
MAB374; Abcam, Cambridge, UK; dilution 1:500) served as the loading
control. Scanning densitometry was performed using Image Quant LAS
4000 (Amersham, Buckinghamshire, UK) and the autoradiographs were
quantified using ImageJ software version 1.50a (National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
The data were presented as the mean ± standard error
from three independent experiments. The differences between
multiple variables were analyzed by one-way analysis of variance
and the Bonferroni pairwise comparison was used for post-hoc
analysis. All statistical analyses were performed using the EZR
(Easy R) statistical software program, an open-source statistical
software program which is based on R and R commander version 2.5.5
(24). P<0.05 was considered to
indicate a statistically significant difference.
Results
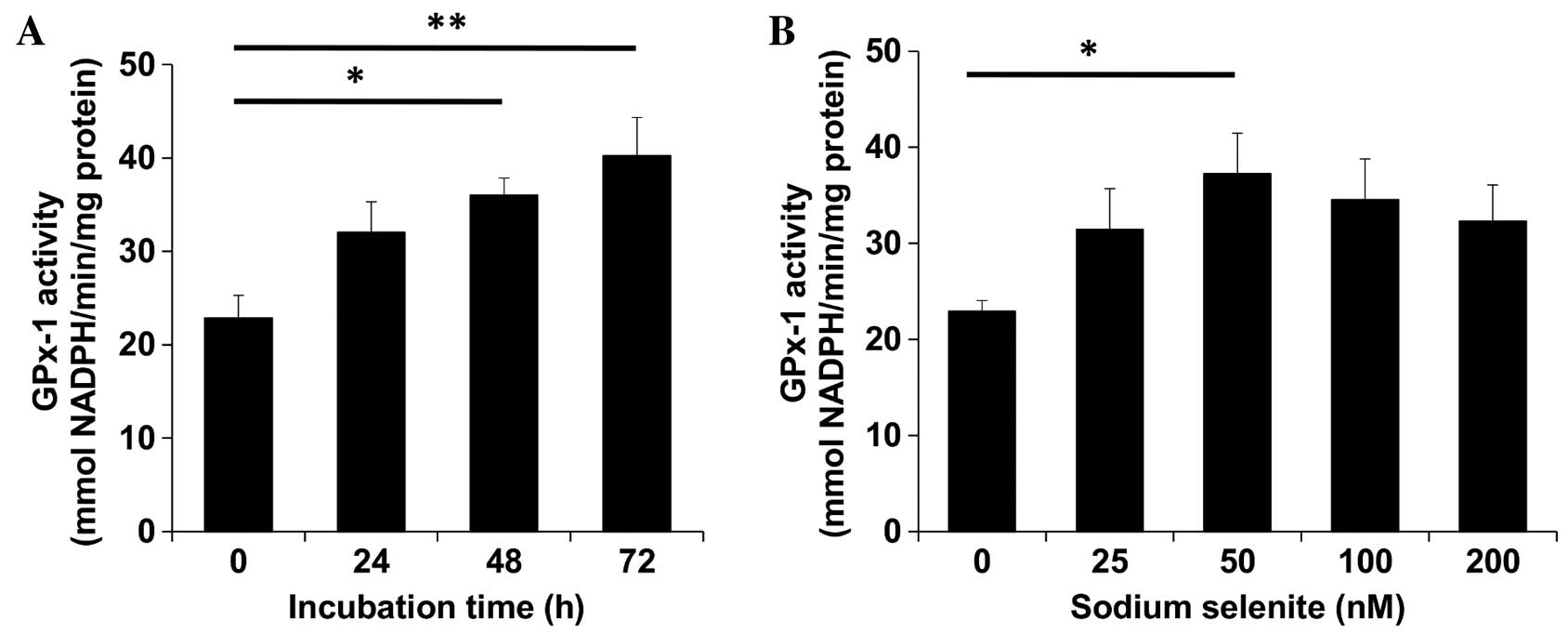
Sodium selenite increases GPx-1
activity
In CHEK-1 cells, sodium selenite supplementation was
observed to increase GPx-1 activity in a dose- and time-dependent
manner (Fig. 1). A previous study
reported that 50 nM sodium selenite was used as a supplementation
dose in primary keratinocytes prior to UV radiation treatment
(25). In the present study, when 50
nM sodium selenite solution was administered to CHEK-1 cells with a
72 h incubation time, maximal GPx-1 activity was achieved. By
examining various concentrations of sodium selenite with the same
72 h incubation time, GPx-1 activity was observed to become
saturated at a concentration of 50 nM. Therefore, the sodium
selenite dose that induced the highest GPx-1 activity was 50 nM for
72 h; these conditions were subsequently used for further
experiments.
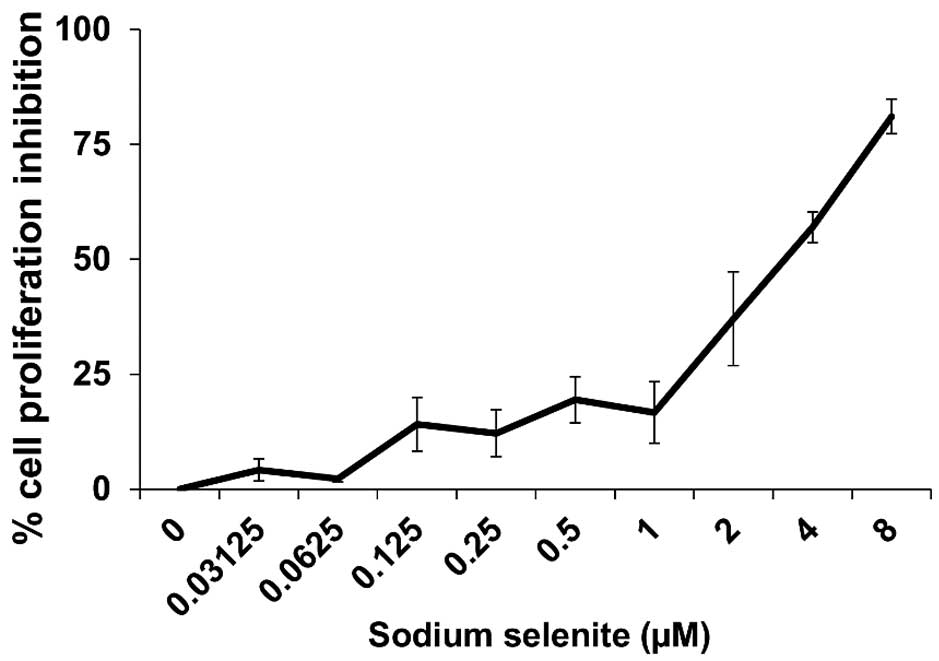
Cytotoxicity of sodium selenite in
CHEK-1 cells
Fig. 2 depicts the
cytotoxicity of sodium selenite in CHEK-1 cells based on the
percentage of cell proliferation inhibition. The IC50
was determined to be 3.6 µM. The dose of 50 nM sodium selenite was
assumed to be a low and safe dose for supplementation for the
cells, compared with the IC50 dose (3.6 µM). In
addition, using linear regression analysis, 50 nM sodium selenite
supplementation was estimated to inhibit ≤3% of cell
proliferation.
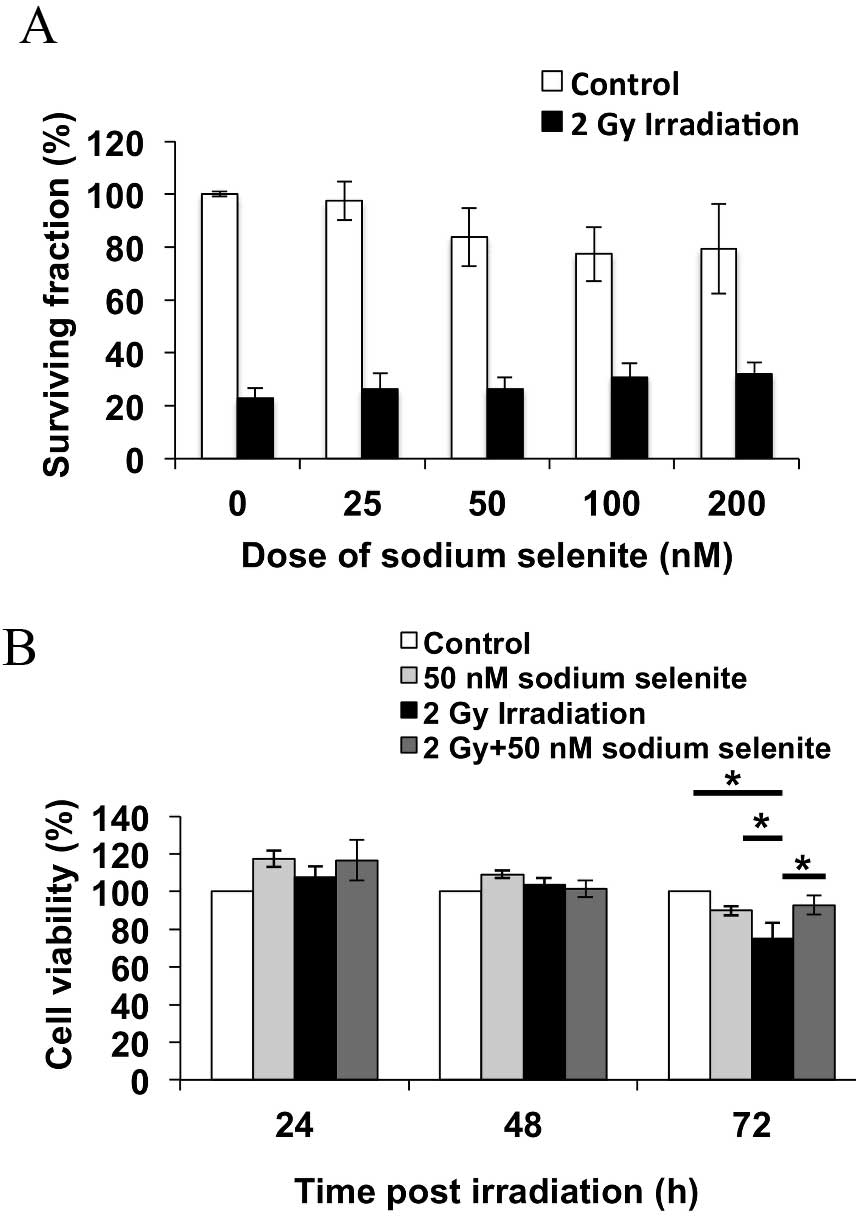
Sodium selenite supplementation
increases the post-irradiation cell survival rate
Post-irradiation survival of CHEK-1 cells was
observed using a clonogenic assay and cell viability assay.
Fig. 3A presents the colony formation
of the cells at 14 days post-irradiation. Sodium selenite
supplementation increased post-irradiation cell survival by
increasing the percentage of surviving cells in a dose-dependent
manner, though this trend was not statistically significant.
Fig. 3B depicts cell viability at
24–72 h post-irradiation; at 72 h post-irradiation, the viability
of the cells treated with 50 nM sodium selenite and 2 Gy
irradiation was increased, compared with 2 Gy irradiation alone
(P=0.031). These results suggested that sodium selenite
supplementation prior to irradiation protects the cells from
irradiation-induced damage.
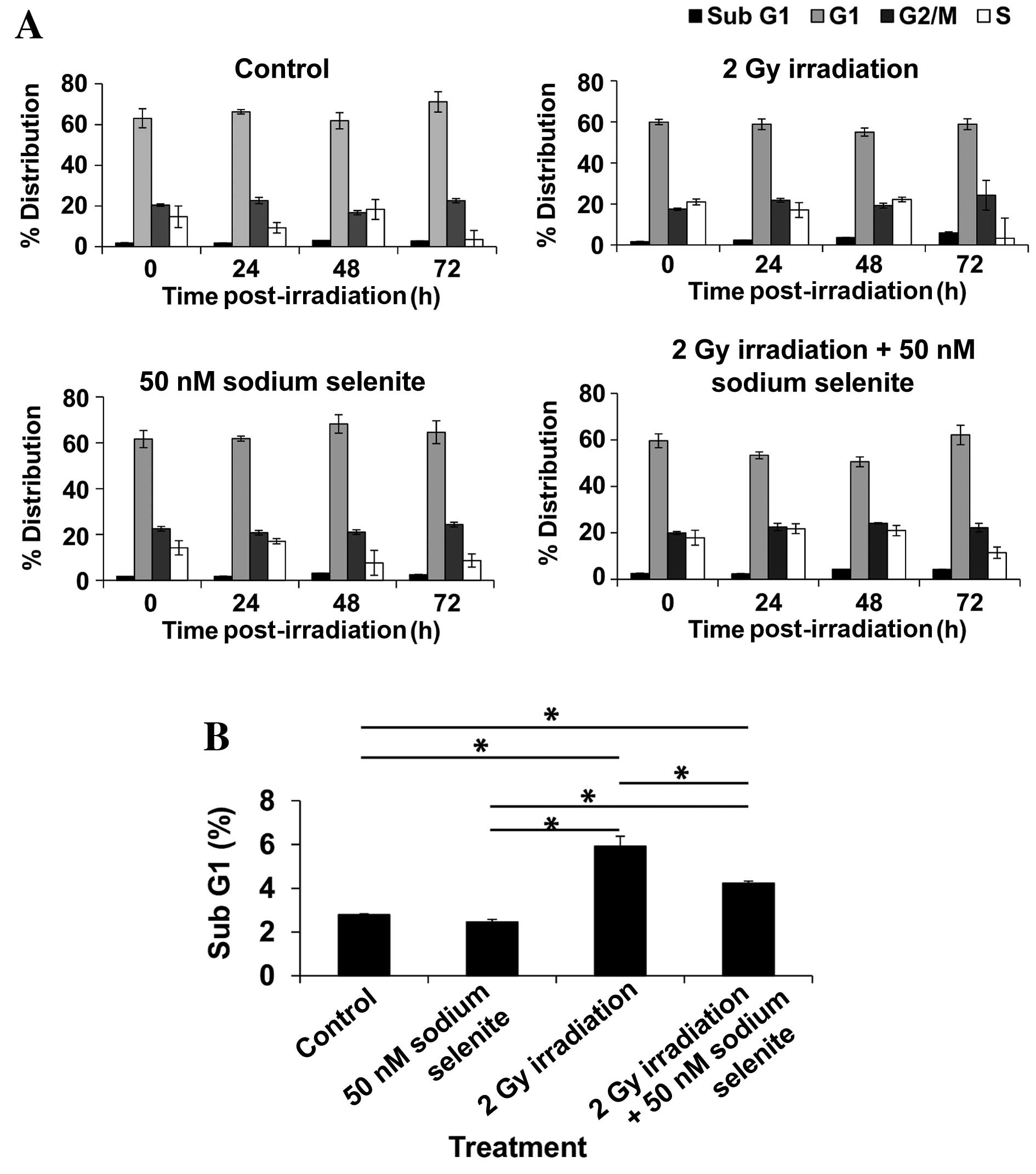
Sodium selenite supplementation
reduces the proportion of sub-G1 phase cells post-irradiation
The cell cycle distribution of CHEK-1 cells
post-irradiation is presented in Fig.
4. The percentages of cells observed to be in
sub-G1, G1, G2/M and S phases at
72 h in the untreated control groups were 2.7±0.03, 71.2±5.01,
22.5±1.03 and 3.5±4.44%, respectively, whereas these percentages
for cells in the 50 nM sodium selenite groups were 2.5±0.11,
64.6±3.21, 24.3±0.94 and 8.6±2.85, respectively. These results
indicate that the cell cycle profile was not affected by treatment
with sodium selenite, with the exception of a non-significant
decrease in the number of G1 cells from 71.2 to 64.6%
(P=1.00) between the control and sodium selenite groups. Treatment
with 2 Gy X-ray radiation increased the percentage of
sub-G1 phase cells from 2.8 to 5.9% (P=0.00087) and
non-significantly decreased the percentage of G1 phase
cells from 71.2 to 58.8% at 72 h post-irradiation (P=0.33).
Combined treatment with 50 nM sodium selenite and 2 Gy X-ray
irradiation resulted in a reduced percentage of sub-G1
cells (4.2 vs. 5.9%; P=0.0061) and a non-significant increase in
the percentage of G1 cells (62.1 vs. 58.8%; P=1.00) at
72 h post-irradiation, compared with 2 Gy X-ray irradiation alone.
These results indicate that sodium selenite supplementation prior
to irradiation may reduce the percentage of apoptotic and damaged
cells, and promote entry into the G1 phase following
irradiation.
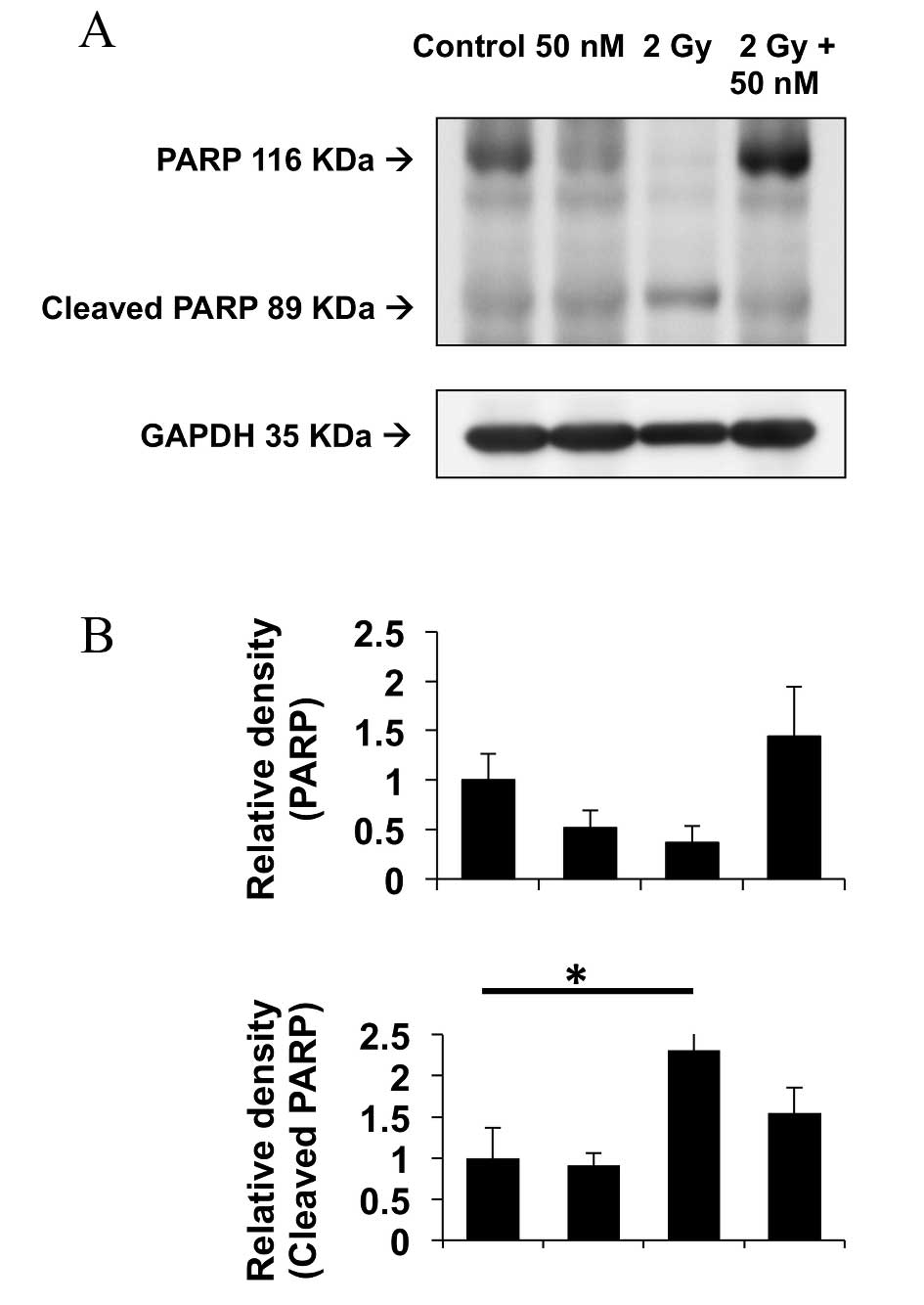
Protein expression levels of PARP, an
apoptosis biomarker
The expression levels of PARP protein, a principal
biomarker for apoptosis, were analyzed by western blotting 72 h
post-irradiation. The expression levels of PARP and cleaved PARP
proteins are depicted in Fig. 5.
GAPDH protein was used as a loading control. Treatment of the
CHEK-1 cells with 2 Gy X-ray radiation increased the expression
levels of cleaved PARP post-irradiation (P=0.0394). Additionally,
combination treatment with 50 nM sodium selenite and 2 Gy X-ray
irradiation reduced the expression levels of cleaved PARP, as
compared with irradiation alone; however, this difference was not
statistically significant (P=0.423). These results indicate that
sodium selenite may potentially inhibit radiation-induced apoptosis
in non-cancerous cells.
Discussion
The present study demonstrated that the
supplementation of non-cancerous human esophageal cells with 50 nM
sodium selenite prior to radiotherapy may protect the cells from
radiation-induced damage and that this protection may be due to the
inhibition of radiation-induced apoptosis. These results are
concordant with those of a previous study that used the
organoselenium compound ebselen for the supplementation of U937
cells prior to irradiation treatment (18).
Irradiation kills not only tumor cells but also
proliferating normal cells (2).
Additionally, irradiation stimulates the production of ROS, which
induce apoptotic cell death (26).
One method of radioprotection involves the inhibition of caspase
activation and PARP cleavage (2).
PARP cleavage is often associated with apoptosis and caspase
activation (27). By reducing the
expression levels of cleaved PARP, which is potentially a molecular
target for novel radioprotective compounds, sodium selenite
supplementation may serve as a radioprotective compound for normal
cells when administered prior to clinical radiotherapy.
GPx is an important enzyme of the cellular
antioxidant defense systems that detoxify peroxides and
hydroperoxides. Selenocysteine is present at the catalytic site of
GPx and the availability of selenium regulates GPx enzyme activity
(28). The stimulation of GPx
activity following selenium supplementation indicates that the
antioxidant function of this enzyme directly reduces the oxidative
DNA damage associated with radiation exposure (16). The current study demonstrated that
sodium selenite supplementation increases GPx-1 activity, which is
concordant with a previous study by Diamond et al (16), indicating that the low-level
supplementation of culture media with selenium, in the form of
sodium selenite, markedly protected CHO-AA8 Chinese hamster
ovary-derived cells from radiation-induced mutagenesis and that
this protection was associated with a significant elevation in
GPx-1 activity.
In conclusion, the present study investigated the
protective effects of sodium selenite supplementation against
irradiation-induced damage in non-cancerous esophageal cells. The
results suggest that treatment with 50 nM sodium selenite
supplementation for 72 h prior to irradiation protects normal cells
from irradiation-induced damage by inhibiting irradiation-induced
apoptosis; therefore, sodium selenite may be a potential
radioprotective compound for use in clinical radiotherapy. Husbeck
et al (29) previously
reported that alteration of the redox environment of prostate
cancer cells by sodium selenite supplementation increased their
apoptotic potential and sensitized them to radiation-induced cell
death. However, further studies are required to fully elucidate the
effects of sodium selenite supplementation on esophageal cancer
cells.
Acknowledgements
This study was supported by an annual Dean Award
grant in 2013 from Gunma University, Japan.
References
|
1
|
Shirazi A, Ghobadi G and Ghazi-Khansari M:
A radiobiological review on melatonin: A novel radioprotector. J
Radiat Res. 48:263–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greenberger JS: Radioprotection. Vivo.
23:323–336. 2009.
|
|
3
|
Nair CK, Parida DK and Nomura T:
Radioprotectors in radiotherapy. J Radiat Res. 42:21–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dörr W: Effects of selenium on radiation
responses of tumor cells and tissue. Strahlenther Onkol.
182:693–695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weiss JF and Landauer MR: Radioprotection
by antioxidants. Ann N Y Acad Sci. 899:44–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rayman MP: The importance of selenium to
human health. Lancet. 356:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puspitasari IM, Abdulah R, Yamazaki C,
Kameo S, Nakano T and Koyama H: Updates on clinical studies of
selenium supplementation in radiotherapy. Radiat Oncol. 9:1252014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rayman MP: Selenium in cancer prevention:
A review of the evidence and mechanism of action. Proc Nutr Soc.
64:527–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fritz H, Kennedy D, Fergusson D, Fernandes
R, Cooley K, Seely A, Sagar S, Wong R and Seely D: Selenium and
lung cancer: A systematic review and meta analysis. PLoS One.
6:e262592011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdulah R, Miyazaki K, Nakazawa M and
Koyama H: Chemical forms of selenium for cancer prevention. J Trace
Elem Med Biol. 19:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ponholzer A, Struhal G and Madersbacher S:
Frequent use of complementary medicine by prostate cancer patients.
Eur Urol. 43:604–608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Micke O, Buntzel J, Kisters K, Schafer U,
Micke P and Mucke R: Complementary and alternative medicine in lung
cancer patients: A neglected phenomenon? Front Radiat Ther Oncol.
42:198–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schleicher UM, Cotarelo C Lopez,
Andreopoulos D, Handt S and Ammon J: Radioprotection of human
endothelial cells by sodium selenite. Med Klin (Munich). 94:(Suppl
3). 35–38. 1999.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodemann HP, Hehr T and Bamberg M:
Relevance of the radioprotective effect of sodium selenite. Med
Klin (Munich). 94:(Suppl 3). 39–41. 1999.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Micke O, Schomburg L, Buentzel J, Kisters
K and Muecke R: Selenium in oncology: From chemistry to clinics.
Molecules. 14:3975–3988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diamond AM, Dale P, Murray JL and Grdina
DJ: The inhibition of radiation-induced mutagenesis by the combined
effects of selenium and the aminothiol WR-1065. Mutat Res.
356:147–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eckers JC, Kalen AL, Xiao W, Sarsour EH
and Goswami PC: Selenoprotein P inhibits radiation-induced late
reactive oxygen species accumulation and normal cell injury. Int J
Radiat Oncol Biol Phys. 87:619–625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tak JK and Park JW: The use of ebselen for
radioprotection in cultured cells and mice. Free Radic Biol Med.
46:1177–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdulah R, Faried A, Kobayashi K, Yamazaki
C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H and Koyama H:
Selenium enrichment of broccoli sprout extract increases
chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC
Cancer. 9:4142009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
21
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
22
|
Buch K, Peters T, Nawroth T, Sänger M,
Schmidberger H and Langguth P: Determination of cell survival after
irradiation via clonogenic assay versus multiple MTT Assay-a
comparative study. Radiat Oncol. 7:12012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rafferty TS, Green MH, Lowe JE, Arlett C,
Hunter JA, Beckett GJ and McKenzie RC: Effects of selenium
compounds on induction of DNA damage by broadband ultraviolet
radiation in human keratinocytes. Br J Dermatol. 148:1001–1019.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JH, Kim SY, Kil IS and Park JW:
Regulation of ionizing radiation-induced apoptosis by mitochondrial
NADP+-dependent isocitrate dehydrogenase. J Biol Chem.
282:13385–13341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Zhao S and Song J:
Caspase-dependent apoptosis and -independent poly(ADP-ribose)
polymerase cleavage induced by transforming growth factor beta1.
Int J Biochem Cell Biol. 36:223–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baker RD, Baker SS, LaRosa K, Whitney C
and Newburger PE: Selenium regulation of glutathione peroxidase in
human hepatoma cell line Hep3B. Arch Biochem Biophys. 304:53–57.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Husbeck B, Peehl DM and Knox SJ: Redox
modulation of human prostate carcinoma cells by selenite increases
radiation-induced cell killing. Free Radic Biol Med. 38:50–57.
2005. View Article : Google Scholar : PubMed/NCBI
|