Introduction
Breast cancer is a leading cause of
cancer-associated mortalities among women worldwide. Risk factors,
in addition to familial history and chromosomal instability,
include diet and lifestyle, high body weight, oral contraception,
age at menarche/menopause/first pregnancy, and oestrogen treatment
(1). Previous data supports a
causative role of oxidative stress in breast cancer development and
a paradoxical effect elicited by physical activity, which increases
the production of reactive oxygen species (ROS), as well as
increasing antioxidant capabilities in order to counteract
subsequent oxidative insults (2). ROS
are dual-faceted molecules. Whereas modest levels are useful as
cell signalling molecules in various physiological cellular
processes, including proliferation, apoptosis, differentiation,
migration, invasion and angiogenesis (3), high levels cause severe oxidative damage
to cell components, such as lipids, proteins and DNA (4,5).
Antioxidants counteract increases in the production
of free radicals, protect the body from oxidative damage by
maintaining redox balance, and are critical for preserving optimum
health and well-being. Antioxidant defence mechanisms may be
enzymatic [superoxide dismutase (SOD), catalase, glutathione
peroxidase (GPx)] or non-enzymatic (vitamins and/or certain
polyphenol molecules introduced through diet); for example, reduced
glutathione (GSH) is particularly able to scavenge hydrogen
peroxide. When ROS production occurs in the absence of sufficient
defence mechanisms, a number of harmful genomic modifications may
occur, including chromosomal instability, permanent DNA damage and
acquisition of mutations, which may also be disposed by alterations
in DNA repair systems; this can contribute to the development of
various diseases, including carcinogenesis (6).
Numerous studies have indicated that upper body
exercise programs, and particularly dragon boat racing, in
association with cancer therapy (7)
may confer benefits to cancer patients, including improving
emotional and physical functioning, decreasing treatment-induced
symptoms (such as nausea, fatigue or pain) and problems associated
with lymphedema (8), and improving
survival and quality of life. However, little is known about the
biochemical/clinical factors underlying such health benefits, such
as the improvement in oxidative stress pathways induced by physical
activity (9,10). To date, to the best of our knowledge,
no studies of the association between dragon boat racing and
oxidative stress have been conducted.
Considering the contribution of regular exercise to
ROS production and the subsequent biological adaptation by enhanced
antioxidant enzymatic capacity (11),
the present study focused on the possible association between redox
status and physical activity by examining oxidant and antioxidant
biomarkers in 75 breast cancer survivors involved in one of two
training programs [dragon boat racing (n=25) or walking (n=25)] or
at rest (n=25). Walking as well as jogging, cycling and swimming
are examples of aerobic conditioning exercise that the doctors
advise to cancer patients as they may enhance physical well-being
and improve recovery, in addition to enhancing cardiovascular
fitness and effective weight management, all of which are
beneficial to individuals with lymphedema (12).
In addition, in order to evidence a possible link
between breast cancer susceptibility and DNA instability,
individual DNA fragmentation and nucleotide excision repair (NER)
DNA repair system capability were examined by comet assay.
Materials and methods
Experimental design and subjects
Breast cancer patients (n=75; aged 35–65 years) were
enrolled at the Department of Surgery of University of Catania
(Catania, Italy). Additionally, healthy women (n=30; aged 40–59
years) were evaluated as control group. The characteristics of the
population studied, including body mass index (BMI), age, height,
weight and maximal oxygen consumption (VO2 max) are reported in
Table I. Smokers or patients with
diabetes mellitus, liver disease, thyroid disease, nephrotic
syndrome, hypertension and rheumatoid arthritis were not included
in the study.
 | Table I.Characteristics of the enrolled
subjects. |
Table I.
Characteristics of the enrolled
subjects.
| Characteristic | Healthy control
subjects (n=30) | Breast cancer
patients (n=75) |
|---|
| Age (years) | 49±9 | 51±12 |
| Height (cm) | 160±5 | 164±7 |
| Weight (kg) | 70±5 | 68±4 |
| Body mass
index | 24±3 | 23±5 |
| Maximal oxygen
consumption (ml/kg/min) | 40±3 | 42±5 |
At ~1 month after commencing their individual
therapeutic protocols, the cancer patients were separated into
three groups depending on their freely chosen physical activity
program (twice per week for ≥7 months) or no activity, as follows:
Dragon boat racing (Group A, n=25), walking (Group B, n=25) and at
rest (Control BrC, n=25). In the present experimental design the
walking exercise consisted of walking briskly outdoors for 3–4 h a
week along freely chosen paths.
All patients followed a controlled
fruit/vegetable-rich diet. The patients were examined at different
time points: i) before surgical treatment (BST); ii) after surgical
treatment (AST); and iii) within 3 days after training.
The research protocol was granted ethical approval
by the Hospital Committee for Research on Human Subjects and the
written informed consent was obtained from each patient.
Blood samples
Blood samples obtained by venepuncture were
centrifuged to collect either plasma (10 min at 800 × g) or
lymphocytes by the Ficoll-Hypaque density gradient centrifugation
method, as described below.
Derivatives of reactive oxygen
metabolites (d-ROMs) test
Plasma samples (10 µl) were utilised to determine
ROS levels by colorimetric d-ROMs test at a wavelength (λ) of 505
nm, according to the manufacturer's protocol (Diacron International
srl, Grosseto, Italy). Colour intensity was expressed in Carratelli
Units (CARR U), with 1 CARR U corresponding to 0.8 mg/l of hydrogen
peroxide (13). Reference values of
healthy subjects are 250–300 CARR U, while high/very high levels
are in the range of 401–450 CARR U or >500 CARR U,
respectively.
Biological plasmatic antioxidant
potential (BAP) test
Individual antioxidant power was evaluated by
measuring BAP. The BAP test (Diacron International srl)
spectrophotometrically (λ=504 nm) measures the capacity of the
plasma to reduce iron from the ferric form (Fe3+) to the ferrous
form (Fe2+) (14). The results are
expressed in µmol/l; reference values of healthy subjects are
considered to be >2,200 µmol/l.
Determination of lipid hydroperoxides
(LPO)
Plasma LPO levels were evaluated by a modified
ferrous oxidation/xylenol orange assay, at λ=560 nm, as described
by Di Giacomo et al (15). The
absorbance, measured by a Hitachi U-2000 spectrophotometer, was
expressed as nmol/ml plasma using hydrogen peroxide (0.2–20 µM) for
calibration.
Total plasmatic thiol groups
Plasmatic thiol groups, containing predominantly
reduced GSH, were determined spectrophotometrically at λ=412 nm by
Ellman's reagent [acid, 5,5′-dithiobis-(2-nitrobenzoic acid)]
(15). Results are expressed as
µmol/ml of plasma.
Analysis of GPx activity
The analysis of plasmatic GPx was performed using a
Glutathione Peroxidase Assay Kit, according to manufacturer's
protocol (Cayman Chemical Company, Ann Arbor, MI, USA; item no.
703102), which refers to the Paglia and Valentine method (16). GPx activity was indirectly measured by
a decrease in absorbance at λ=340 nm (A340) due to the oxidation of
NADPH to NADP. Under conditions in which GPx activity is limiting,
the rate of decrease in A340 is directly proportional to GPx
activity in the sample, expressed as nmol/min/ml.
Analysis of SOD activity
The activity of all three types of SOD (Cu/Zn-, Mn-
and Fe-SOD) was measured in plasma samples using a Superoxide
Dismutase Assay Kit, according to the manufacturer's protocol
(Cayman Chemical Company; item no. 706002), at 440–460 nm and
expressed as U/ml; 1 unit of SOD is defined as the amount of enzyme
required to have 50% conversion of the superoxide radical into
molecular oxygen and hydrogen peroxide (17).
Alkaline and neutral comet assay
The alkaline and neutral Comet assay protocol was
performed as previously described by Tomasello et al
(18). Triplicate samples (each 40
µl) mixed with 0.5% low-melting point agarose were spread on FLARE™
Slides (Trevigen, Inc., Gaithersburg, MD, USA), immersed in cold
lysis solution for 1 h, and electrophoresed for 20 min in alkaline
(pH >13; 0.7 V/cm) or neutral buffer (pH 8; 0.5 V/cm). Following
electrophoresis, slides were neutralised, dehydrated by immersion
in 70% ethanol and stained with SYBR Green. Analysis was conducted
using an epifluorescence microscope (Leica Microsystems GmbH,
Wetzlar, Germany) with Casp Comet Assay Software (version 1.2.2;
CASPLab, University of Wroclaw, Poland), by measuring DNA damage as
the percent of DNA in the comet Tail (% TDNA). Each phase of the
procedure was performed according to European Standards Committee
on Oxidative DNA Damage guidelines (19). A CometAssay® Control Cell
population from Trevigen, Inc. was co-electrophoresed.
Human umbilical vein endothelial cell
(HUVEC) cultures, isolation of lymphocytes and DNA repair
assay
Ultraviolet C (UVC)-induced cell damage and the
efficacy of lymphocytes extracted from cancer survivors were tested
on agarose-embedded HUVECs (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), cultured according to Russo et al
(20). Confluent HUVECs were detached
with trypsin-ethylenediaminetetraacetic acid, and the number of
viable cells/ml was determine by trypan blue staining; only plates
with total viable cells ≥70% were used for the NER Comet test,
according to method proposed by Collins et al (21) and modified by Gaivão et al
(22). The individual capability for
NER of DNA from HUVECs damaged by UVC irradiation was measured to
evaluating the activity of repair enzymes present in individual
lymphocyte samples. Lymphocytes were isolated from heparinised
venous blood on Ficoll-Hypaque gradients by centrifugation;
lymphocytes extracted from each patient were prepared as described
by Gaivão et al (22).
Agarose-embedded HUVECs were irradiated with 1 Jm-2 UVC on ice;
this creates pyrimidine dimers and 6,4-photoproducts, which are
repaired by NER. Subsequently, the contents of the slides were
lysed (pH 10.0–10.5; 2.5 M NaCl, 100 mM Na2 EDTA, 10 mM Tris and 1%
lauroyl sarcosine, 1% Triton X-100 and 10% dimethyl sulfoxide were
added directly prior to use) at 4°C for 60 min to obtain naked DNA
(17) and washed for 3×5 min with
reaction buffer (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA, 1,6 mM MgCl2,
0.2 mg/ml bovine serum albumin, adjusted to pH 8.0 using 6 M KOH)
(21,22). Extracts (45 µl) were added to each gel
and incubated for 30 min in a humidified chamber at 37°C. Reaction
buffer alone was used as a negative control, and T4 endonuclease V
(Trevigen, Inc.) was used as a positive control. Following
incubation, the slides were processed according to the standard
protocol for the alkaline comet assay to measure the DNA breaks
introduced by the initial incision events of repair. The strand
breaks produced were detected by comet assay; the increase in %
tail DNA over time reflects the DNA repair activity of the cell
extract.
Statistical analysis
The data is presented as the mean ± standard
deviation in the tables and as median with interquartile range in
the figures. The differences among the control groups and groups A
and B were analysed by one-way analysis of variance. Statistical
analysis was conducted using GraphPad Prism 5 statistical software
(GraphPad Software, Inc., La Jolla, CA, USA). Boxplots were used to
represent data and the differences between the individual groups
were assessed with Bonferroni's post hoc test, with P≤0.05
considered to indicate a statistically significant difference.
Results
Determination of SOS
The levels of oxidative and antioxidative parameters
in the plasma of the subjects prior to training activity are shown
in Table II. d-ROMs levels, a
measure of oxidative status, differed significantly between the
groups, being higher in the BST group (500±50 CARR U) compared with
the control values of healthy women (265±45 CARR U). The AST
measures were significantly lower than those obtained BST (420±45
CARR U). In parallel, BAP levels, a measure of antioxidative
status, were significantly lower compared with the control values
in cancer patients (BST and AST patients).
 | Table II.Results of systemic oxidative status
tests in breast cancer patients and healthy controls, prior to
commencing physical activity. |
Table II.
Results of systemic oxidative status
tests in breast cancer patients and healthy controls, prior to
commencing physical activity.
| Test | Control group
(n=30) | BST group
(n=75) | AST group
(n=75) |
|---|
| d-ROMs (CARR
U) | 265±45 | 500±50a | 420±45a,b |
| BAP (µmol/l) | 2,380±200 | 2,060±150a | 2,000±100a |
In order to verify a possible link between oxidative
stress conditions and physical activity in cancer, women affected
by breast cancer were assessed in three groups: Group A (dragon
boat racing), Group B (walking) and Control BrC (breast cancer
survivors at rest). The following results regarding the SOS
assessed are represented by boxplots, where the range of normal
values derived from the healthy control group (n=30) are indicated
by the thick black line on the y-axis of each graph.
As shown in Fig. 1A,
the levels of radical species were above the normal range of
values; indeed, the majority of the examined subjects were in the
high or very high range (between 400 and 550 CARR U). In
particularly, ROS were induced by the two physical activities:
459±61 CARR U for Group A and 502±76 CARR U for Group B; however,
this difference was not statistically significant (P=0.332). The
increase in ROS was significant with respect to the Control BrC
group for each of the two activity groups (P=0.038 and P<0.001,
respectively).
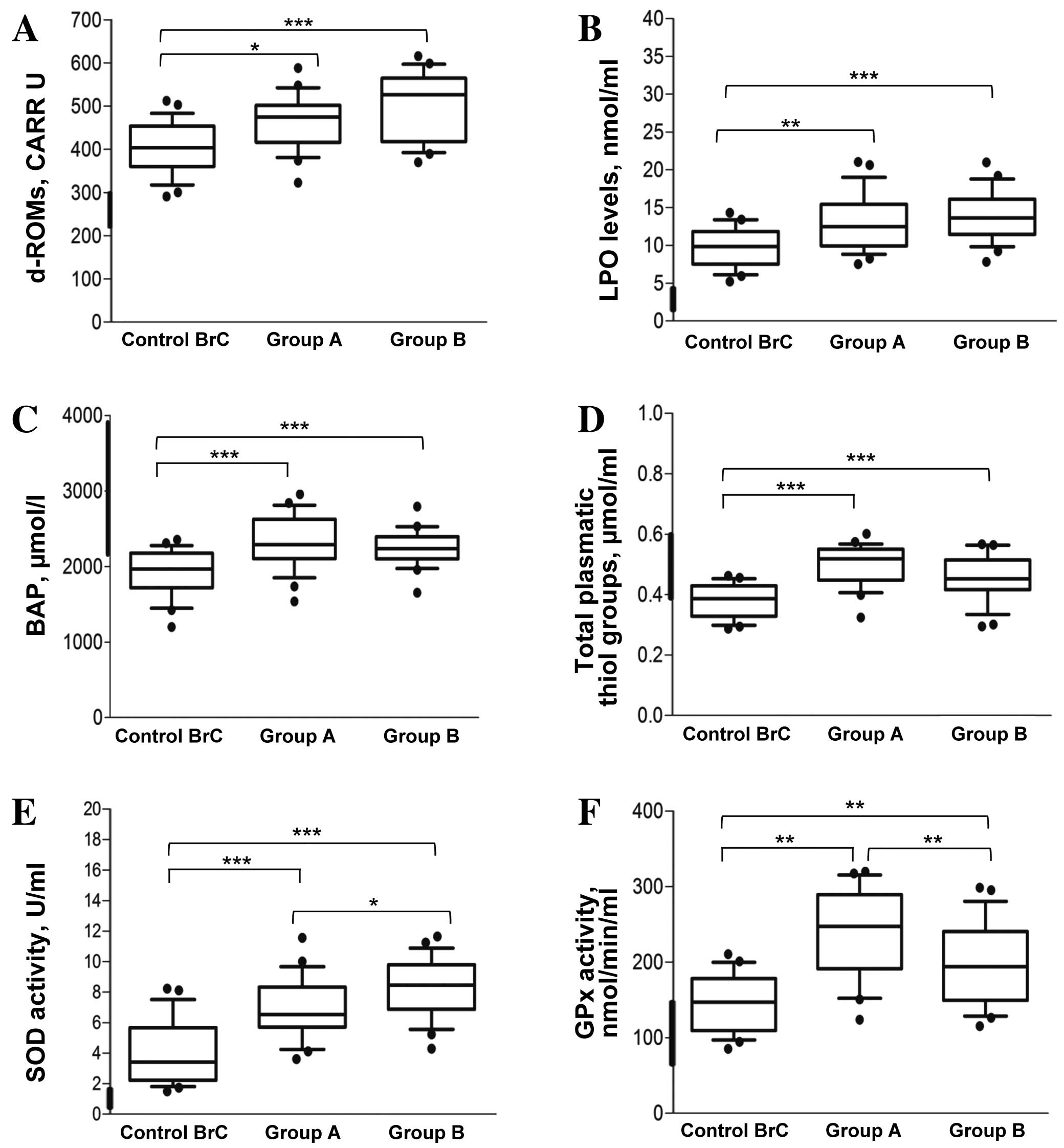 | Figure 1.Determination of systemic oxidative
status in breast cancer survivors. (A) d-ROMs test results; the
levels of oxidative stress are expressed in CARR U (1 CARR
U=H2O2 0.08 mg/dl; control range, 250–300
CARR U). (B) Plasmatic LPO levels are expressed as nmol/ml (control
range 8–10 nmol/ml). (C) BAP test represents the power of
antioxidant capability and is expressed as µmol/l (control range,
>2200 µmol/l). (D) Total plasmatic thiol groups are expressed as
µmol/ml (control range, 0.4–0.6 µmol/ml). (E) SOD activity is
expressed as U/ml (control values, 1.82±0.039 U/ml). (F) GPx
activity values are expressed in nmol/min/ml (control values,
113.41±20.77 nmol/min/ml). For each group, the line in the middle
of the box represents the median, the black dash represents the
mean value, and the lower and the upper edges of the box represent
the 1st and 3rd quartiles, respectively. Whiskers represent the
minimum and maximum values; observations denoted as black circles
are considered outliers. The thick black line on the y-axis of the
graph indicates the range of the healthy control group at rest
(n=30). ***P<0.001; **P<0.01; *P<0.05. Group A, dragon
boat racing group; Group B, walking group; Control BrC, breast
cancer survivors at rest; d-ROMs, derivatives of reactive oxygen
metabolites; LPO, lipid hydroperoxides; BAP, biological plasmatic
antioxidant potential; SOD, superoxide dismutase; GPx, glutathione
peroxidase. |
As presented in Fig.
1B, the evaluation of LPO revealed higher levels in the three
groups of cancer survivors compared with the healthy control values
(4.22±0.064 nmol/ml; n=30). The average values for Groups A and B
were 13.2±3.6 and 15.08±2.7 nmol/ml, respectively; the difference
between these two activity groups was not statistically significant
(P=0.224). Conversely, the differences between each of the physical
activity groups and the Control BrC group (9.7±2.5 nmol/ml) were
statistically significant (P=0.007 and P<0.001,
respectively).
BAP data revealed that the majority of the subjects
examined had a high plasmatic antioxidant potential (2,275±337 and
2,236±223 µmol/l for Groups A and B, respectively), without a
significant difference between the physical activity groups
(Fig. 1C). Additionally, following
physical training, BAP levels were significantly increased compared
with pre-exercise basal levels (2,000±100 µmol/l, refer to AST
values in Table II) and control BrC
levels.
In addition, as shown in Fig. 1D, the total plasmatic thiol levels all
three cancer survivor groups overlapped with the range of values
from control group (0.4–0.6 µmol/ml), and no statistically
significant differences were identified between the two physical
activity groups (P=0.173). However, the thiol levels in a
proportion of the women in Group B and Control BrC were below the
levels of the control. The Control BrC values were also
significantly lower than those of the two physical activity groups
(both P<0.001).
The estimated SOD activity in each of the cancer
survivor groups (Fig. 1E), was
markedly higher than control values (1.82±0.039 U/ml). In
particular, the activity of this enzyme was statistically
significantly higher in Group B compared with that in Group A
(8.4±1.9 vs. 6.8±2 U/ml; P=0.044), as well as compared with that in
the Control BrC group, which was the lowest (3.90±2.04, both
P<0.001).
GPx activity levels were significantly higher in the
two physical activity groups relative to the healthy control values
(113.41±20.77 nmol/min/ml, both P<0.001) and the Control BrC
group (147.10±37.6 nmol/min/ml, P=0.007 for Group A and P=0.007 for
Group B). In particular, the level in Group A was 246±57.7
nmol/min/ml, while in Group B a slightly lower mean of 197±53.3
nmol/min/ml was observed (Fig.
1F).
DNA damage and DNA repair
capability
The results regarding DNA fragmentation, measured
with alkaline and neutral versions of the comet assay, are
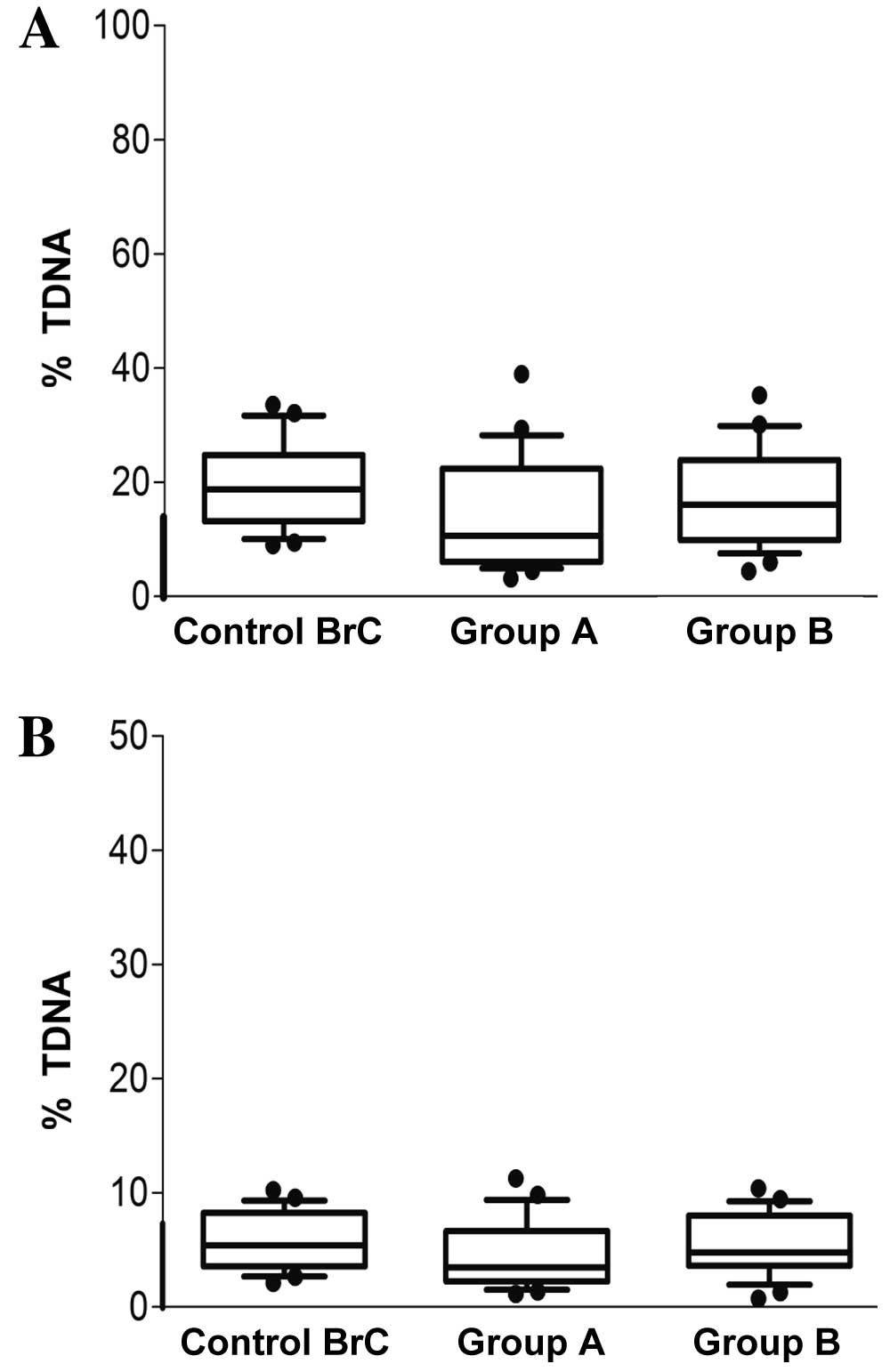
presented as % TDNA in Fig. 2A and B.
The alkaline comet assay data revealed that DNA damage was higher
in Group B (17.10%) compared with in Group A (14.05%), and the
damage in each of these two groups was lower than in the Control
BrC group (19.59%). However no statistical significance was
observed among the groups. Additionally, the majority of subjects
had % TDNA values within the range considered ‘normal’ for the
comet assay (22). Conversely, very
little double-strand break DNA damage was indicated by the neutral
comet assay analysis for all groups, as shown in Fig. 2B.
The NER analysis revealed no significant difference
in % TDNA between the two physical activity groups (Group A,
31.5±7.6 vs. Group B, 30.3±8.4%; P=0.80); however, the individual
repair capabilities were below that calculated with T4 endonuclease
V, which was used as the reference value in the present study (%
TDNA, 70%; dotted line in Fig. 3).
The two physical activity groups exhibited significantly greater
repair capabilities compared with the Control BrC group (24.5±6%)
following UVC-induced damage (Group A, P=0.008; Group B,
P=0.045).
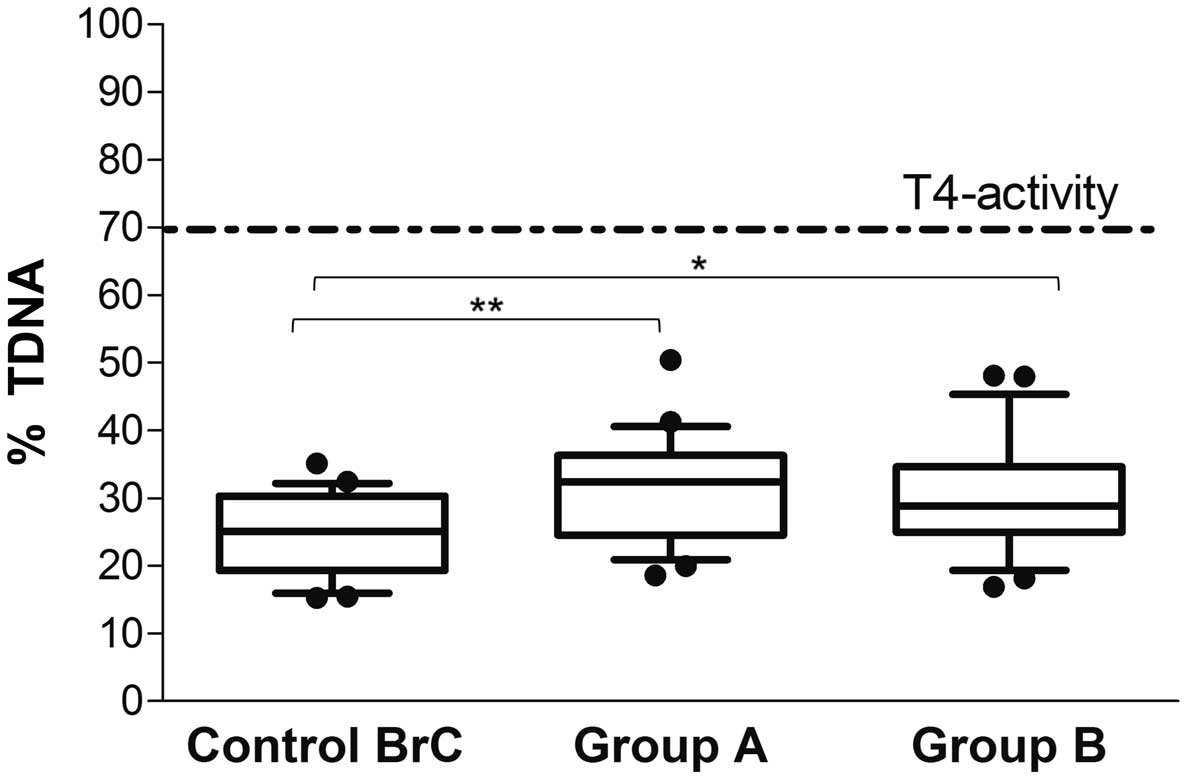 | Figure 3.Nucleotide excision repair capability
in breast cancer survivors. Naked DNA from ultraviolet C-irradiated
human umbilical vein endothelial cells were incubated in gel with
buffer, or T4 endonuclease V, or lymphocyte extracts from each
subject. Following 45 min of incubation, DNA breaks introduced by
repair endonuclease activity in the lymphocyte extracts were
measured by comet assay and expressed as % TDNA, i.e. the
percentage of DNA in the comet Tail. For each group, the line in
the middle of the box represents the median, the black dash
represents the mean value, and the lower and the upper edges of the
box represent the 1st and 3rd quartiles, respectively. Whiskers
represent the minimum and maximum values; observations denoted as
black circles are considered outliers. The dotted line represents
the calculated % TDNA (percentage of DNA in the comet tail) from T4
endonuclease V, which was considered as reference value for
evaluating patients repair capability. **P<0.01; *P<0.05.
Group A, dragon boat racing group; Group B, walking group; Control
BrC, breast cancer survivors at rest. |
Discussion
One adverse outcome of surgical or radiologic breast
cancer treatment is the risk of lymphedema. Onset may occur months
or years following treatment for breast cancer; survivors remain at
risk for life so to prevent postoperative development, vigorous
repetitive movements of the upper limbs was strongly discouraged
until ~10 years ago (23,24). However, more recently doctors have
encouraged the practice of physical activity at least six months
post-treatment, for blocking lymphedema development, in addition to
beneficial effects that the practice of stable and diversified
physical activity exhibits in preventing carcinogenesis, in
ameliorating various symptoms associated with chemotherapeutic
treatment, improving the quality of life and resilience of
survivors, and decreasing levels of distress (24,25).
However, to the best of our knowledge, no useful data have been
reported regarding the biochemical changes that underlie the
improved health of cancer survivors who participate in upper body
exercise programs such as dragon boating.
Numerous previous studies have documented an
interference in ‘redox regulation’ associated with carcinogenesis,
tumour progression and/or chemotherapeutic efficacy, including in
breast cancer (2,26). In order to investigate the association
between physical activity and oxidative stress-related biochemical
parameters in breast cancer survivors, the present study assessed
two groups of breast cancer patients involved in different physical
activities twice per week: dragon boat racing (Group A) and walking
(Group B).
Current opinions regarding the various indices that
may be used to measure the oxidative status of patients are
controversial. Such indices include total antioxidant capability
(TAS or BAP test), total oxidative status (TOS), and the TAS/TOS
ratio, which is expressed as the oxidative stress index. The latter
of these indices is considered as the best approach for determining
the net oxidative stress condition, at a diagnostic and/or
therapeutic level (27).
In the present study, various oxidative stress
biomarkers were evaluated. First, measurements of ROS levels
(d-ROMs test, which is similar to TOS) and antioxidant capability
(BAP test, which is similar to TAS) were taken in patient groups
BST and AST. Secondly, following the physical training programs, in
addition to the aforementioned parameters, the plasmatic LPO and
GSH levels and the enzymatic activities of SOD and GPx were
examined in the breast cancer survivors. Furthermore, DNA status
and repair capability in the experimental groups were explored. The
present data demonstrated that enhanced oxidative stress was
present at time of diagnosis in all enrolled subjects, which is in
agreement with data from a number of previous studies (28,29),
affecting both components of SOS. Certain authors have reported
that the activities of all the studied antioxidant enzymes (SOD,
catalase, GPx and glutathione S-transferases) and the levels of
reduced GSH were significantly increased in breast cancer patients
compared with their healthy control group (30); whereas others have reported reduced
SOD and GPx activities in breast cancer patients (31). In the present study, the oxidative
stress level was improved marginally following surgical treatment,
and was positively affected by physical activities, but in
different ways in the two survivor groups who undertook training
programs. Enzymatic and non-enzymatic antioxidants, as well as
lipid peroxidation, have been reported to be altered among various
tissues types and individual breast cancer patients (32,33). In
the present study, in the presence of elevated levels of ROS (based
on the d-ROMs test), high levels of LPO levels were observed
following physical training. This ROS increase positively
influences the plasmatic antioxidant component (BAP), and may be
considered a direct reaction to a physical activity-induced
oxidative environment.
In the present study, despite the high ROS
production, the total plasmatic thiol group levels, comprising
mainly GSH, were not depleted but were maintained within the
reference normal range, being higher in Group A than in Group B.
This data demonstrates the benefits of physical activity, as
previously reported in other studies (34,35). We
consider that the high level of GSH, as well as the GPx and SOD
activity, may be generated to counteract the effects of increased
oxidative stress and lipoperoxidation, as an adaptive response to
the increase in circulating ROS; this was also reported by Carter
et al (36). Such an adaptive
response, mediated by a physical activity-induced oxidative
environment, may involve changes in antioxidant gene expression via
the antioxidant-responsive elements, which may be consistent with
the enhanced enzyme activities observed as previously reported by
Kobayashi et al (37).
Furthermore, in our opinion, the maintenance of high antioxidant
levels observed in the studied patients is the result of the diet
rich in vegetables/fruit and the physical activity, as reported in
a previous study (38).
Certain previous studies have reported the presence
of higher baseline DNA damage in breast cancer patients without
physical training (39,40). The present results regarding
double-strand break DNA damage (neutral comet assay) indicated that
the % TDNA values were similar in all breast cancer patients and
were comparable with healthy control values. A similar finding was
observed in the majority of women in both groups that underwent
training programs when using the alkaline comet assay, although
some patients exhibited values higher than that of the reference
control. These data may be associated with possible persistent DNA
damage and non-functional/inefficient DNA repair systems.
It is well-known that most damage is removed by
repair enzymes before it is able to interfere with DNA replication
and introduce mutations. Individual variation in DNA repair
capacity is therefore likely to be an important factor in
determining cancer risk. In the present study, the DNA-NER
capability, represented by the activity of lymphocyte extracts, was
less than the T4 enzyme-treated control in all of the studied
women. In particular, the specific lesions that occur following UVC
radiation exposure, which are recognised and excised by T4
endonuclease (1 unit), produced an increase in % TDNA equal to 70%
over time, reflecting the maximum DNA repair activity. By contrast,
the lymphocyte extract of the breast cancer patients exhibited
repair capacities equal to 31.04 and 30.15% for Groups A and B,
respectively. This data demonstrates that almost half of
radiation-induced damage was not repaired by the enzyme activity of
the lymphocyte extracts, and remained as persistent lesions.
Shahidi et al (41), who used
a kinetic repair approach, demonstrated the radiation-induced DNA
damage is not completely repaired compared with control subjects
after 3 h, leading us to hypothesise that deficient radio-induced
damage repair may promote the onset of late harmful irradiation
effects in breast cancer patients.
One limitation of this in vitro assay is that
the lymphocytes are not directly irradiated, and the NER system is
perhaps not sufficiently activated, which may have produced the
deficient repair activity observed. Similarly to Gaivão et
al (22), who performed an
NER-assay on healthy subjects, inter-individual variability of
repair activity in cancer survivors was observed in the present
study; this may arise from genetic polymorphisms and epigenetic
factors, thus influencing individual susceptibility to cancer
development. In our opinion, the present data is of interest,
although the experimental approach must be repeated and extended to
a larger number of samples, particularly because this data may be
useful in the context of individual radiosensitivity in women with
breast cancer for whom radiotherapy is chosen as a treatment.
Considering this data on DNA repair, we hypothesise that dragon
boating and continuity of physical activity, inducing ROS
production, may stimulate an increase in DNA repair ability over
time, among the possible adaptive responses; this is also in
agreement with Mao et al (42).
In conclusion, the present data indicates that the
measurement of different blood redox biomarkers may be a useful
approach in defining an individual antioxidant therapy to support
and/or reinforce the efficacy of primary treatments; that the
monitoring of DNA repair capacity (in particular the NER system)
may be useful in defining an eventual radiotherapeutic plan; and
that dragon boating is beneficial for breast cancer survivors,
leading us to suggest the large scale adoption of this activity,
since it may also lead to considerable savings in costs associated
with physiotherapy.
Acknowledgements
The authors would like to thank Mr. David Shanahan,
an independent native English translator, for proofreading the
manuscript in English.
References
|
1
|
Carlson RW, Allred DC, Anderson BO,
Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ,
Gradishar WJ, et al: Breast cancer. Clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 7:122–192. 2009.PubMed/NCBI
|
|
2
|
Acharya A, Das I, Chandhok D and Saha T:
Redox regulation in cancer: A double-edged sword with therapeutic
potential. Oxid Med Cell Longev. 3:23–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin KR and Barrett JC: Reactive oxygen
species as double-edged swords in cellular processes: Low-dose cell
signaling versus high-dose toxicity. Hum Exp Toxicol. 21:71–75.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooke MS, Evans MD, Dizdaroglu M and Lunec
J: Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB
J. 17:1195–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klaunig JE, Kamendulis LM and Hocevar BA:
Oxidative stress and oxidative damage in carcinogenesis. Toxicol
Pathol. 38:96–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scott TL, Rangaswamy S, Wicker CA and
Izumi T: Repair of oxidative DNA damage and cancer: Recent progress
in DNA base excision repair. Antioxid Redox Signal. 20:708–726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCausland LL: Dragon boat racing: Life
after breast cancer treatment. Am J Nurs. 110:48–54. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hull MM: Lymphedema in women treated for
breast cancer. Semin Oncol Nurs. 16:226–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bouillet T, Bigard X, Brami C, Chouahnia
K, Copel L, Dauchy S, Delcambre C, Descotes JM, Joly F, Lepeu G, et
al: Role of physical activity and sport in oncology: Scientific
commission of the National Federation Sport and Cancer CAMI. Crit
Rev Oncol Hematol. 94:74–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knop K, Schwan R, Bongartz M, Bloch W,
Brixius K and Baumann F: Sport and oxidative stress in oncological
patients. Int J Sports Med. 32:960–964. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCullough LE, Santella RM, Cleveland RJ,
Bradshaw PT, Millikan RC, North KE, Olshan AF, Eng SM, Ambrosone
CB, Ahn J, et al: Polymorphisms in oxidative stress genes, physical
activity, and breast cancer risk. Cancer Causes Control.
23:1949–958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
NLN Medical Advisory Committee, . Position
statement of the National Lymphedema Network: exercise. San
Francisco: National Lymphedema Network; 2013
|
|
13
|
Trotti R, Carratelli M and Barbieri M:
Performance and clinical application of a new, fast method for the
detection of hydroperoxides in serum. Panminerva Med. 44:37–40.
2002.PubMed/NCBI
|
|
14
|
Benzie IF and Strain JJ: The ferric
reducing ability of plasma (FRAP) as a measure of ‘antioxidant
power’: The FRAP assay. Anal Biochem. 239:70–76. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Giacomo C, Acquaviva R, Sorrenti V,
Vanella A, Grasso S, Barcellona ML, Galvano F, Vanella L and Renis
M: Oxidative and antioxidant status in plasma of runners: Effect of
oral supplementation with natural antioxidants. J Med Food.
12:145–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
17
|
Sandström J, Nilsson P, Karlsson K and
Marklund SL: 10-fold increase in human plasma extracellular
superoxide dismutase content caused by a mutation in
heparin-binding domain. J Biol Chem. 269:19163–19166.
1994.PubMed/NCBI
|
|
18
|
Tomasello B, Grasso S, Malfa G, Stella S,
Favetta M and Renis M: Double-face activity of resveratrol in
voluntary runners: Assessment of DNA damage by comet assay. J Med
Food. 15:441–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
European Standards Committee on Oxidative
DNA Damage (ESCODD), . Measurement of DNA oxidation in human cells
by chromatographic and enzymic methods. Free Radic Biol Med.
34:1089–1099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russo A, Palumbo M, Aliano C, Lempereur L,
Scoto G and Renis M: Red wine micronutrients as protective agents
in Alzheimer-like induced insult. Life Sci. 72:2369–2379. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collins AR, Dusinská M, Horváthová E,
Munro E, Savio M and Stĕtina R: Inter-individual differences in
repair of DNA base oxidation, measured in vitro with the comet
assay. Mutagenesis. 16:297–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaivão I, Piasek A, Brevik A, Shaposhnikov
S and Collins AR: Comet assay-based methods for measuring DNA
repair in vitro; estimates of inter- and intra-individual
variation. Cell Biol Toxicol. 25:45–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harris SR and Niesen-Vertommen SL:
Challenging the myth of exercise-induced lymphedema following
breast cancer: A series of case reports. J Surg Oncol. 74:95–99.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu MR, Ridner SH and Armer J: Post-breast
cancer. Lymphedema: Part 1. Am J Nurs. 109:48–54; quiz 55. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mandelblatt JS, Luta G, Kwan ML, Makgoeng
SB, Ergas IJ, Roh JM, Sternfeld B, Adams-Campbell LL and Kushi LH:
Associations of physical activity with quality of life and
functional ability in breast cancer patients during active adjuvant
treatment: The Pathways Study. Breast Cancer Res Treat.
129:521–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vera-Ramirez L, Sanchez-Rovira P,
Ramirez-Tortosa MC, Ramirez-Tortosa CL, Granados-Principal S,
Lorente JA and Quiles JL: Free radicals in breast carcinogenesis,
breast cancer progression and cancer stem cells. Biological bases
to develop oxidative-based therapies. Crit Rev Oncol Hematol.
80:347–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aslan R, Kutlu R, Civi S and Tasyurek E:
The correlation of the total antioxidant status (TAS), total
oxidant status (TOS) and paraoxonase activity (PON1) with smoking.
Clin Biochem. 47:393–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen J, Gammon MD, Terry MB, Wang Q,
Bradshaw P, Teitelbaum SL, Neugut AI and Santella RM: Telomere
length, oxidative damage, antioxidants and breast cancer risk. Int
J Cancer. 124:1637–1643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fortner RT, Tworoger SS, Wu T and Eliassen
AH: Plasma florescent oxidation products and breast cancer risk:
Repeated measures in the Nurses' Health Study. Breast Cancer Res
Treat. 141:307–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajneesh CP, Manimaran A, Sasikala KR and
Adaikappan P: Lipid peroxidation and antioxidant status in patients
with breast cancer. Singapore Med J. 49:640–643. 2008.PubMed/NCBI
|
|
31
|
Abiaka C, Al-Awadi F, Al-Sayer H, Gulshan
S, Behbehani A and Farghally M: Activities of erythrocyte
antioxidant enzymes in cancer patients. J Clin Lab Anal.
16:167–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kasapović J, Pejić S, Todorović A,
Stojiljković V and Pajović SB: Antioxidant status and lipid
peroxidation in the blood of breast cancer patients of different
ages. Cell Biochem Funct. 26:723–730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hasan HR, Mathkor TH and Al-Habal MH:
Superoxide dismutase isoenzyme activities in plasma and tissues of
Iraqi patients with breast cancer. Asian Pac J Cancer Prev.
13:2571–2576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gago-Dominguez M, Jiang X and Castelao J
Esteban: Lipid peroxidation and the protective effect of physical
exercise on breast cancer. Med Hypotheses. 68:1138–1143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balneaves LG, Van Patten C, Truant TL,
Kelly MT, Neil SE and Campbell KL: Breast cancer survivors'
perspectives on a weight loss and physical activity lifestyle
intervention. Support Care Cancer. 22:2057–65. 2014.PubMed/NCBI
|
|
36
|
Carter CL, Onicescu G, Cartmell KB, Sterba
KR, Tomsic J, Fox T, Dunmeyer E and Alberg AJ: Factors associated
with cancer survivors' selection between two group physical
activity programs. J Cancer Surviv. 4:388–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carayol M, Romieu G, Bleuse JP, Senesse P,
Gourgou-Bourgade S, Sari C, Jacot W, Sancho-Garnier H, Janiszewski
C, Launay S, et al: Adapted physical activity and diet (APAD)
during adjuvant breast cancer therapy: Design and implementation of
a prospective randomized controlled trial. Contemp Clin Trials.
36:531–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sánchez P, Peñarroja R, Gallegos F, Bravo
JL, Rojas E and Benítez-Bribiesca L: DNA damage in peripheral
lymphocytes of untreated breast cancer patients. Arch Med Res.
35:480–483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hussien MM, McNulty H, Armstrong N,
Johnston PG, Spence RA and Barnett Y: Investigation of systemic
folate status, impact of alcohol intake and levels of DNA damage in
mononuclear cells of breast cancer patients. Br J Cancer.
92:1524–1530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shahidi M, Mozdarani H and Bryant PE:
Radiation sensitivity of leukocytes from healthy individuals and
breast cancer patients as measured by the alkaline and neutral
comet assay. Cancer Lett. 257:263–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mao Z, Hine C, Tian X, Van Meter M, Au M,
Vaidya A, Seluanov A and Gorbunova V: SIRT6 promotes DNA repair
under stress by activating PARP1. Science. 332:1443–1446. 2011.
View Article : Google Scholar : PubMed/NCBI
|