Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy worldwide and its prognosis is poor (1). HCC tissue is composed of tumor cells and
blood vessels (2). Treatment of this
disease includes local ablation, chemoembolization and systemic
administration of chemotherapeutic agents (3,4); however,
this is limited by hepatotoxicity (5).
Glucose and pyruvate are important sources of energy
for cell survival (6,7). Galactose enters glycolysis as a
substrate for galactokinase (GALK), which is expressed in the liver
and kidney (8,9). Therefore, hepatocytes are expected to
survive in a galactose-containing medium, in the absence of glucose
or pyruvate (10,11).
Arginine, an essential amino acid, is produced from
ornithine by the action of ornithine carbamoyltransferase (OTC) in
the urea cycle (12). As the urea
cycle is exclusive to hepatocytes, these cells are able to survive
in a medium lacking glucose and arginine that is supplemented with
galactose and ornithine (13).
We previously developed a hepatocyte selection
medium (HSM) that lacks glucose and arginine but contains galactose
and ornithine (14). This medium was
demonstrated to purify primary human hepatocytes from co-culture
with human induced pluripotent stem cells (15). In the same manner, the current study
attempted to eliminate HCC cell lines using HSM. Additionally, a
co-culture of HCC cells with human umbilical vascular endothelial
cells (HUVECs) was studied as a model of HCC tissues.
Materials and methods
Cell lines and culture media
The human HCC cell lines HLE, HLF, PLC/PRL/5, Hep3B
and HepG2 were purchased from the Riken Bioresource Centre Cell
Bank (Tsukuba, Japan). Hepatoma cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA),
and supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA). HUVECs and their culture
medium, Endothelial Cell Growth Media BulletKit™ (EGM), were
purchased from Lonza Group Ltd. (Walkersville, MD, USA). Cell lines
were cultured with 5% carbon dioxide at 37°C in a humidified
chamber, and were observed using a CKX41N-31PHP microscope (Olympus
Corporation, Tokyo, Japan).
Co-culture of HLF or PLC/PRL/5 with
HUVECs
HUVECs were seeded onto a 24-well plate coated with
150 µl Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) per well,
at a density of 1.0×104 cells/well. After one day of
culture in EGM, the medium was discarded and the HLF cells were
added at a density of 1.0×104 cells/well. The cells were
cultured in DMEM supplemented with 10% fetal bovine serum.
Preparation of HSM
HSM was prepared from amino acid powders following
the formulation of Leibovitz's L-15 medium (Thermo Fisher
Scientific, Inc.), excluding arginine, tyrosine, glucose and sodium
pyruvate, but with the addition of galactose (900 mg/l), ornithine
(1 mM), glycerol (5 mM) and proline (260 mM), all obtained from
Wako Pure Chemical Industries, Ltd. (Osaka, Japan) (15). Proline (30 mg/l) was added as it is
essential for DNA synthesis (16).
KnockOut Serum Replacement (KSR) (Thermo Fisher Scientific, Inc.)
was added at a final concentration of 10%, and was used instead of
fetal calf serum (FCS) to establish defined xeno-free conditions.
Depending on the experiment, KSR was dialyzed against
phosphate-buffered saline (PBS) to remove the amino acids and
glucose.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were seeded in 6-well plates (Asahi Techno
Glass, Tokyo, Japan) and cultured in DMEM supplemented with 10%
fetal bovine serum. When the cultured cells reached 70% confluency,
the medium was changed to HSM. At 48 h after the media was changed
to HSM, total RNA (5 µg) was isolated using Isogen (Nippon Gene,
Co., Ltd., Tokyo, Japan). cDNA was synthesized using
SuperScript® III Reverse Transcriptase and oligo dT
primers, as per the manufacturer's instructions (Thermo Fisher
Scientific, Inc.). Human fetal liver total RNA and human adult
liver total RNA were purchased from Clontech Laboratories Inc.
(Mountain View, CA, USA). The PCR primers, GenBank accession
numbers and expected product sizes are listed in Table I. RT-qPCR was conducted with 25 µl of
Fast SYBR Green Master Mix (Thermo Fisher Scientific, Inc.) in
total volume of 50 µl for each reaction in the Mini Opticon system
(Bio Rad Laboratories, Inc., Hercules, CA, USA). PCR was performed
for 40 cycles, with denaturation (95°C) and annealing-extension
(60°C) times of 5 sec each. Ribosomal protein L19 was used as an
internal control. Expression levels of the genes involved were
automatically analyzed with this system. RPL19 was used as
an endogenous control to monitor the amount of mRNA, as it is
constitutively expressed housekeeping gene (17). The gene expression levels were
analyzed automatically using the Mini Opticon system based on the
delta-delta cycle threshold (ddCt) method (18). The relative expression was calculated
as the expression level of a specific gene divided by that of
RPL19.
 | Table I.List of primers used for polymerase
chain reaction, GenBank accession numbers and expected product
lengths. |
Table I.
List of primers used for polymerase
chain reaction, GenBank accession numbers and expected product
lengths.
| Primer | Sequence (5′-3′) | GenBank accession
number | Product size
(bp) |
|---|
| RPL19 forward |
CGAATGCCAGAGAAGGTCAC | BC000530 | 157 |
| RPL19 reverse |
CCATGAGAATCCGCTTGTTT |
|
|
| Albumin forward |
CCATGAGAATCCGCTTGTTT | NM_000477 | 114 |
| Albumin reverse |
GCAAAGCAGGTCTCCTTATCGTC |
|
|
| GALK1 forward |
GCTGCTTGAGAGAGGTAGAAGGTG | NM_000154 | 153 |
| GALK1 reverse |
TCACGACTTACTGGAGCAGGATG |
|
|
| GALK2 forward |
TCACGACTTACTGGAGCAGGATG | NM_002044 | 177 |
| GALK2 reverse |
CAAAACCAAAGCCCCACCTC |
|
|
| OTC forward |
GGACATTTTTACACTGCTTGCCC | BC107153 | 105 |
| OTC reverse |
TCCACTTTCTGTTTTCTGCCTCTG |
|
|
| PEPCK forward |
GGCTACAACTTCGGCAAATACCTG | NM_002591 | 167 |
| PEPCK reverse |
TTGAACATCCACTCCAGCACCCTG |
|
|
| G6P forward |
AACAGAGCCAGTCACAGCACCAAG | NM_000151 | 139 |
| G6P reverse |
CCTCAGGAAATCCATTGATACGG |
|
|
| Cyclin D1
forward |
AGAGGCGGAGGAGAACAAACAG | NM_053056 | 180 |
| Cyclin D1
reverse |
AGGCGGTAGTAGGACAGGAAGTTG |
|
|
Statistical analysis
One-way analysis of variance was performed with JMP
10.0.2 software (SAS Institute, Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
GALK1, GALK2 and OTC expression in HCC
cells
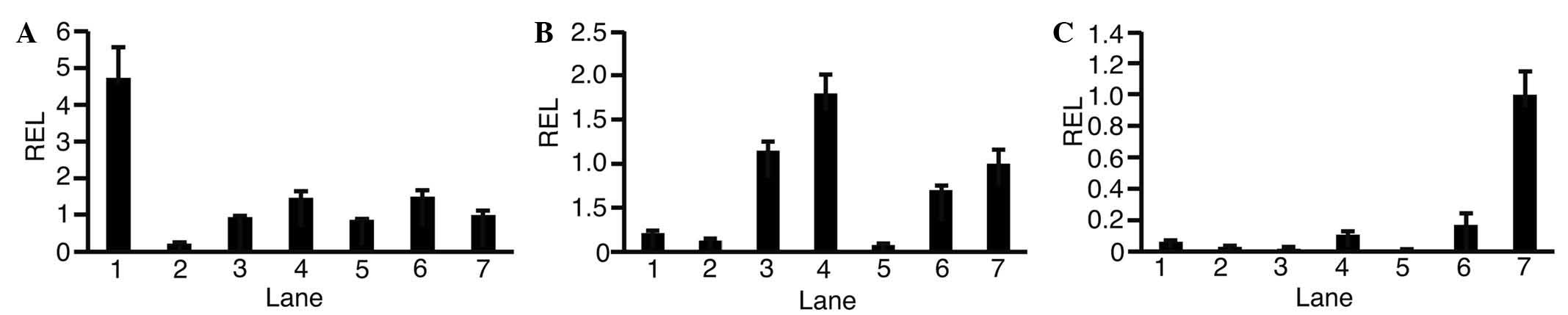
To observe the expression levels of GALK1 (Fig. 1A), GALK2 (Fig. 1B) and OTC (Fig. 1C) in each HCC cell line, RT-qPCR was
performed. This revealed that expression levels of GALK1 and GALK2
varied depending on the type of cell. Expression levels of OTC in
HCC cells were lower compared with in adult liver cells. In HLF
cells, expression levels of GALK1 (0.22±0.03; P<0.01), GALK2
(0.12±0.03; P<0.01) and OTC (0.03±0.00; P<0.01) were lower
compared with in adult liver cells.
Effect of HSM on HCC cells and
HUVECs
To address the possibility that HCC cells and HUVECs
would undergo cell death in HSM, cultures were established
(Fig. 2). Hep3B, HepG2 and HUVECs
were microscopically observed to have died after being cultured for
7 days in HSM, which is morphologically shown in Fig. 2. A small number of HLE, HLF and
PLC/PRL/5 cells survived on day 7. On day 11, all HLE, HLF and
PLC/PRL/5 cells died, as observed morphologically
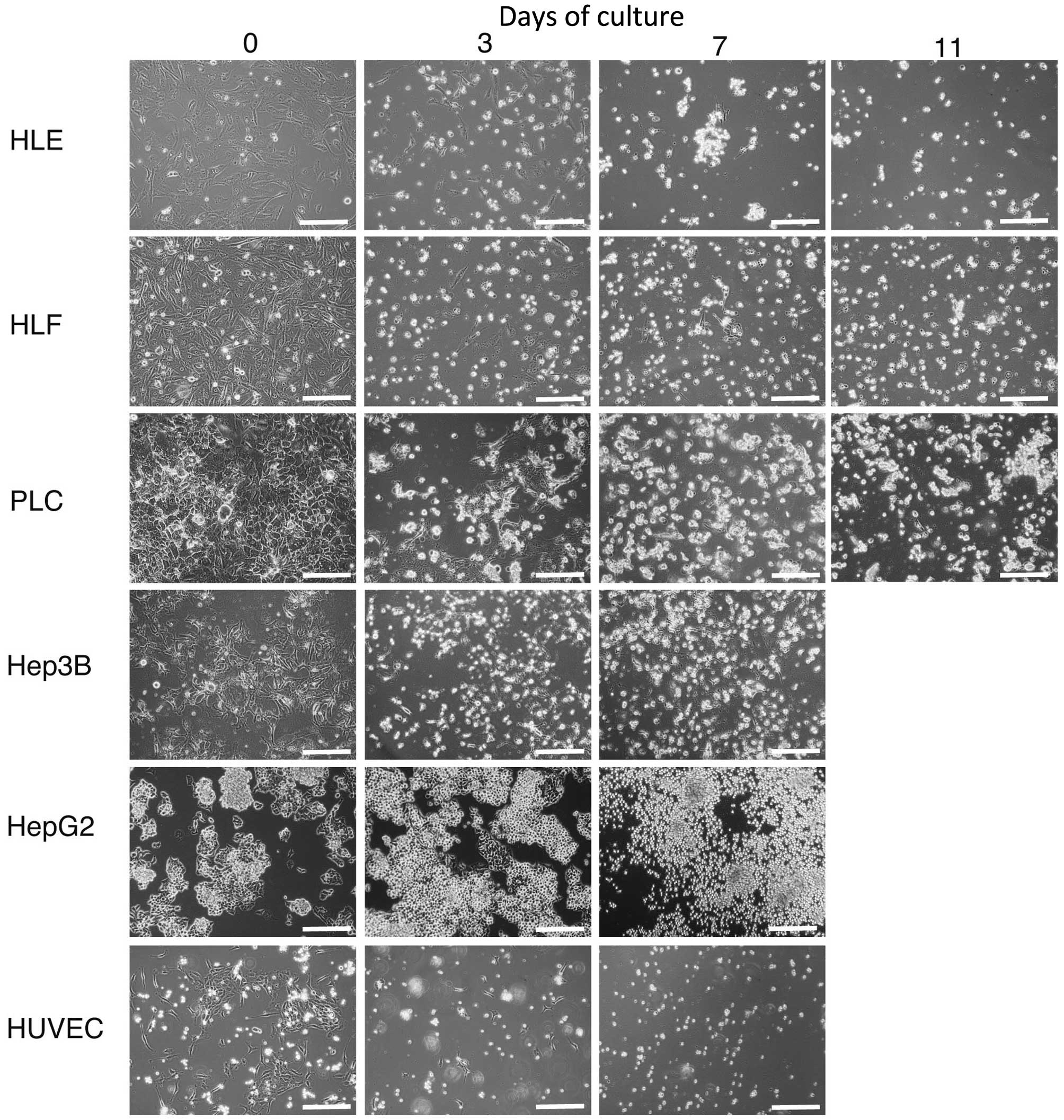 | Figure 2.Hepatoma cells, HLE, HLF, PLC/PRL/5,
Hep3B, HepG2 and HUVECs were cultured in hepatocyte selection
medium and observed by microscopy. A small number of HLE, HLF and
PLC/PRL/5 cells survived on day 7. On day 11, HLE, HLF and
PLC/PRL/5 cells died. Original magnification, ×100; scale bar, 50
µm. HUVEC, human umbilical vascular endothelial cell. |
Expression of metabolic genes and
cyclin D1 in HLF cells
The HLF cell line was selected for further analysis
of the change in expression levels of metabolic genes as its
expression levels of GALK1, GALK2 and OTC were lower compared with
those in adult liver; however, it was hypothesized that certain
genes involved in metabolism would be upregulated to promote
survival in HSM. Cyclin D1 expression was also analyzed as this
gene is involved in cell proliferation; it was hypothesized that
cell proliferation would be suppressed in HSM. RT-qPCR was
performed to investigate changes in expression levels of GALK1
(Fig. 3A), GALK2 (Fig. 3B), OTC (Fig.
3C), phosphoenolpyruvate carboxykinase (PEPCK) (Fig. 3D), glucose-6-phosphatase (G6P)
(Fig. 3E) and cyclin D1 (Fig. 3F). The expression levels of GALK1
changed from 0.21±0.04 (day 0) to 0.33±0.05 (day 7) (P=0.01). The
expression levels of GALK2 changed from 0.11±0.03 (day 0) to
0.18±0.02 (day 7) (P=0.03). The expression levels of OTC changed
from 0.06±0.01 (day 0) to 0.07±0.02 (day 7) (P=0.67). The
expression levels of PEPCK and G6P were 0.00±0.00 on day 0 and day
7. These data suggested that GALK1 and GALK2 were upregulated in
HSM. The expression levels of cyclin D1 changed from 1.00±0.33 to
0.18±0.09 (P<0.01). These results suggested that cyclin D1 was
downregulated as predicted.
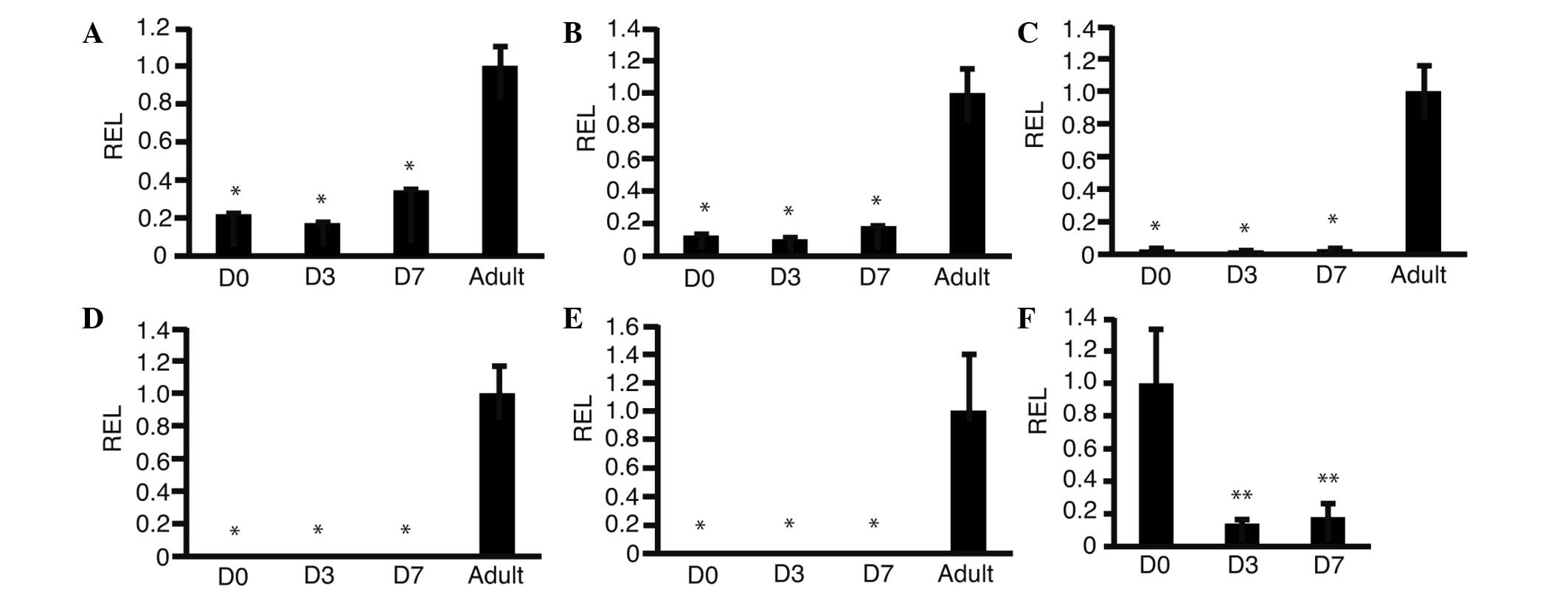 | Figure 3.Changes in expression levels of genes
involved in metabolism and cyclin D1. HLF cells were cultured in
HSM and RNA was isolated. Reverse transcription-quantitative
polymerase chain reaction was performed. Graphs show the REL,
normalized against human adult liver cells, of (A) galactokinase 1,
(B) galactokinase 2, (C) ornithine carbamoyltransferase, (D)
phosphoenolpyruvate carboxykinase, (E) glucose-6-phosphatase and
(F) cyclin D1; *P<0.05 as compared with adult, **P<0.05 as
compared with D0 (n=3). D0, day 0 after culture in HSM; D3, three
days after culture in HSM; D7, seven days after culture in HSM;
adult, human adult liver; HSM, hepatocyte selection medium; REL,
relative expression levels. |
Effect of HSM on HUVEC and HCC
co-culture
Co-culture of HLF cells with HUVECs was established
as an in vitro model of HCC tissues (Fig. 4A). HLF cells and HUVECs were cultured
in HSM and observed by microscopy. HLF and HUVECs began to die at
18 h (Fig. 4B), and almost all the
cells had died after 60 h (Fig. 4C)
of culture.
Discussion
Cellular energy deprivation is a potential treatment
for HCC (19). For example, HCC cells
do not survive ischemia, which causes deprivation of glucose
(20). However, such a lack of
glucose and oxygen also may damage normal hepatocytes. To treat HCC
with the deprivation of glucose, survival of normal hepatocytes
would be a major problem.
HCC cells can modify their mechanism to survive
under the deprivation of glucose (21). These literatures suggest that HCC
cells may not die without glucose alone. The HSM used in the
present study did not contain arginine or glucose. It was expected
that HSM would be more effective for death of HCC cells compared
with glucose deprivation alone.
Primary human hepatocytes are able to survive in HSM
(15). In the present study, all HCC
cells and HUVECs died within 7 or 11 days of culture. HSM also
caused the death of HLF cells and HUVECs in co-culture. The
co-culture of HLF and HUVECs could be used as a model for HCC
tissues, as HUVECs were used as a feeding artery. It was expected
that the feeding artery of HCC would also be damaged with HSM. If
HCC cells survived in HSM, the cells would not survive as the
feeding artery was damaged, and glucose and oxygen were not
supplied. Death of the two cell types in HSM indicated that HSM was
applicable for the treatment of HCC. Notably, normal hepatocytes
survive in HSM. These results suggested that HSM would be useful in
the treatment of HCC tissues, and safe for normal hepatocytes.
Arginine is synthesized from citrulline catalyzed by
argininosuccinate synthetase (22).
Arginine deprivation is, therefore, a treatment for a type of HCC
that lacks argininosuccinate synthetase (22). HSM would not be harmful to normal
hepatocytes as it contains no potentially toxic reagents.
One major limitation of the present study was the
method of application of HSM to HCC. It did not seem practical to
apply HSM intravenously. Transcatheter arterial chemoembolization
is an established method for the treatment of HCC (23). Balloon occlusion of the hepatic artery
is a novel technique (21). The
balloon occlusion uses a micro balloon to obstruct the hepatic
artery. HCC tissues are immersed with chemotherapeutic agents
following the occlusion. HSM could be used to immerse HCC tissues
instead of chemotherapeutic agents. With the occlusion technique,
it is possible that applying HSM may be more practical.
In conclusion, HCC cells and HUVECs died in a medium
without glucose or arginine, and supplemented with galactose and
arginine, namely HSM. HSM might be useful for the harmless
treatment of HCC as normal hepatocytes are able to survive in
HSM
References
|
1
|
Zhu RX, Seto WK, Lai CL and Yuen MF:
Epidemiology of hepatocellular carcinoma in the Asia-Pacific
region. Gut Liver. 10:332–339. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomizawa M, Kondo F and Kondo Y: Growth
patterns and interstitial invasion of small hepatocellular
carcinoma. Pathol Int. 45:352–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raoul JL, Gilabert M and Piana G: How to
define transarterial chemoembolization failure or refractoriness: A
European perspective. Liver Cancer. 3:119–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leffert HL and Paul D: Studies on primary
cultures of differentiated fetal liver cells. J Cell Biol.
52:559–568. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto K, Yamada K, Kohmura E,
Kinoshita A and Hayakawa T: Role of pyruvate in ischaemia-like
conditions on cultured neurons. Neurol Res. 16:460–464. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohira RH, Dipple KM, Zhang YH and McCabe
ER: Human and murine glycerol kinase: Influence of exon 18
alternative splicing on function. Biochem Biophys Res Commun.
331:239–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ai Y, Jenkins NA, Copeland NG, Gilbert DH,
Bergsma DJ and Stambolian D: Mouse galactokinase: Isolation,
characterization and location on chromosome 11. Genome Res.
5:53–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phillips JW, Jones ME and Berry MN:
Implications of the simultaneous occurrence of hepatic glycolysis
from glucose and gluconeogenesis from glycerol. Eur J Biochem.
269:792–797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sumida KD, Crandall SC, Chadha PL and
Qureshi T: Hepatic gluconeogenic capacity from various precursors
in young versus old rats. Metabolism. 51:876–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wheatley DN, Scott L, Lamb J and Smith S:
Single amino acid (arginine) restriction: Growth and death of
cultured HeLa and human diploid fibroblasts. Cell Physiol Biochem.
10:37–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walker V: Ammonia toxicity and its
prevention in inherited defects of the urea cycle. Diabetes Obes
Metab. 11:823–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomizawa M, Toyama Y, Ito C, Toshimori K,
Iwase K, Takiguchi M, Saisho H and Yokosuka O: Hepatoblast-like
cells enriched from mouse embryonic stem cells in medium without
glucose, pyruvate, arginine and tyrosine. Cell Tissue Res.
333:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8:e718972013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura T, Teramoto H, Tomita Y and
Ichihara A: L-proline is an essential amino acid for hepatocyte
growth in culture. Biochem Biophys Res Commun. 122:884–891. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tam S, Clavijo A, Engelhard EK and
Thurmond MC: Fluorescence-based multiplex real-time RT-PCR arrays
for the detection and serotype determination of foot-and-mouth
disease virus. J Virol Methods. 161:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simons AL, Mattson DM, Dornfeld K and
Spitz DR: Glucose deprivation-induced metabolic oxidative stress
and cancer therapy. J Cancer Res Ther. 5:(Suppl 1). S2–S6. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Módis K, Gerő D, Stangl R, Rosero O,
Szijártó A, Lotz G, Mohácsik P, Szoleczky P, Coletta C and Szabó C:
Adenosine and inosine exert cytoprotective effects in an in vitro
model of liver ischemia-reperfusion injury. Int J Mol Med.
31:437–446. 2013.PubMed/NCBI
|
|
21
|
Cai H, Jiang D, Qi F, Xu J, Yu L and Xiao
Q: HRP-3 protects the hepatoma cells from glucose
deprivation-induced apoptosis. Int J Clin Exp Pathol.
8:14383–14391. 2015.PubMed/NCBI
|
|
22
|
Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr
M, Spector S and Savaraj N: Arginine deprivation as a targeted
therapy for cancer. Curr Pharm Des. 14:1049–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ju W, Li S, Wang Z, Liu Y and Wang D:
Decorin protects human hepatocma HepG2 cells against oxygen-glucose
deprivation via modulating autophagy. Int J Clin Exp Med.
8:13347–13452. 2015.PubMed/NCBI
|