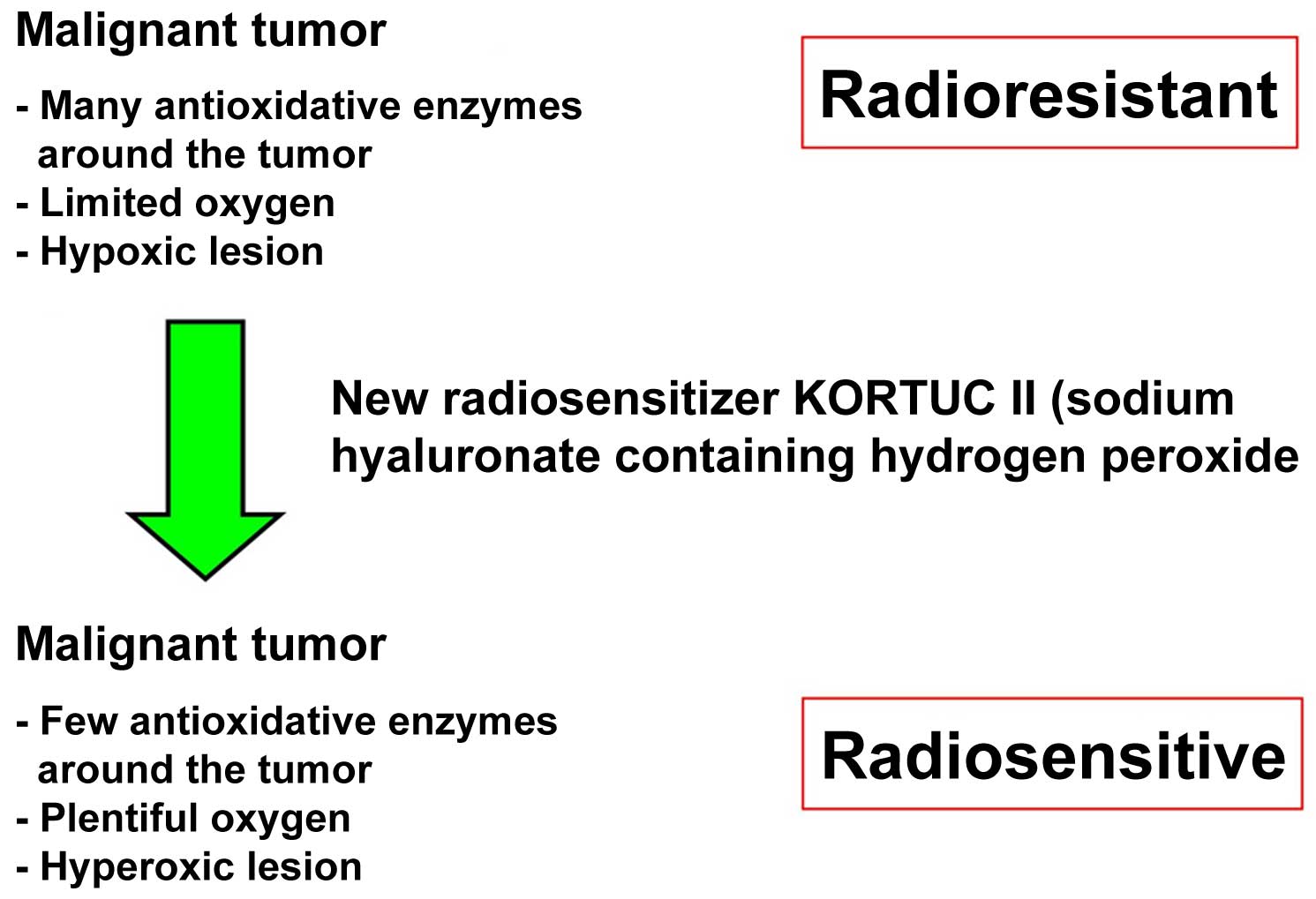
Introduction
Previously metastasis was a predictor of patient
mortality; however, advances in the current understanding of cancer
biology have enabled the cure or control of various malignancies
and improved the prognosis and quality of life for patients
(1,2).
Surgery and radiotherapy are used for local control and
chemotherapy is used for systemic control (1–3). Through
the application of these treatments, certain types of cancer may be
cured; however, various specific types of cancer contain numerous
hypoxic cancer cells and antioxidant enzymes that may confer
resistance to radiotherapy (1–3). In
addition to increasing tissue oxygen concentrations, it is
important to inactivate antioxidant enzymes, including peroxidase
and catalase, which protect cancer cells from oxidative stress
(1–10). Hydrogen peroxide is able to inactivate
antioxidant enzymes and produce oxygen when applied topically to
cancer tissues (1–3). By adding hyaluronic acid to hydrogen
peroxide the decomposition of hydrogen peroxide is delayed, and a
high oxygen concentration is maintained in the cancer tissues for
over 24 h (1–3). As reported in our previous studies
(5,9,10), a novel
radiosensitizer, Kochi Oxydol Radiation Therapy for Unresectable
Carcinomas II (KORTUC II), was developed for the treatment of
cancers that contain numerous hypoxic cancer cells and antioxidant
enzymes. The components of KORTUC II are sodium hyaluronate 0.83%
w/v and hydrogen peroxide 0.5% w/v. The concept of KORTUC II is to
transform radioresistant cancer into radiosensitive cancer
(9,10). Following KORTUC II therapy, hypoxic
and radioresistant cancer cells become hyperoxic and radiosensitive
(Figure 1). In our previous studies,
the efficacy of KORTUC II in the treatment of supraclavicular lymph
node metastates and recurrent breast cancers was demonstrated in
humans (9,10). The aim of the current study is to
evaluate the safety and effectiveness of KORTUC II in patients with
stage IV primary breast cancer, following induction chemotherapy
with epirubicin combined with cyclophosphamide and paclitaxel or
docetaxel.
Materials and methods
KORTUC II formulation
Hyaluronic acid preparation (2.5 ml) with 1% w/v
sodium hyaluronate (ARTZ Dispo®; Seikagaku Corporation,
Tokyo, Japan) was present in each dose. This was composed of 25 mg
sodium hyaluronate, 2.5 mg L-methionine (Kaken Pharmaceutical Co.,
Ltd., Tokyo, Japan), sodium chloride, potassium phosphate,
crystalline sodium dihydrogen phosphate and an isotonizing agent,
sodium hyaluronate (Kaken Pharmaceutical Co., Ltd.). The
preparation was a colorless, transparent, viscous and aqueous
solution with a pH of 6.8–7.8, a specific osmotic pressure of
1.0–1.2 (relative to physiological saline) and an average molecular
weight of 0.6–1.2 million. Immediately prior to administration, 0.5
ml hydrogen peroxide 3% w/v solution (Oxydol; Ken-ei Pharmaceutical
Co., Ltd., Osaka, Japan) was added to the solution. The final
solution of the radiosensitizer had a sodium hyaluronate
concentration of ~0.83% and a hydrogen peroxide concentration of
~0.5%. The radiosensitizer solution was the same as the solution
administered for the treatment of chemotherapy-resistant
supraclavicular lymph node metastates and recurrent breast cancers
in our previous studies (9,10).
The recruitment of patients for the present study
was conducted between November 2006 and January 2011 at the Kochi
Medical School Hospital (Kochi, Japan). The institutional ethics
committee of Kochi University approved the use of KORTUC II for the
treatment of patients with stage IV primary breast cancer. Seven
patients with stage IV primary breast cancer were enrolled in the
current study and provided written, informed consent. The criteria
for case selection included a patient age of 20–80 years, and the
patient and their families had opted for the KORTUC II treatment.
The ages of the patients ranged from 36–65 years (mean average age,
56 years). Localized management of the lesions was determined to be
problematic using regular chemotherapy or radiotherapy. Prior to
and following KORTUC II treatment, chemotherapy including
epirubicin (Nippon Kayaku, Tokyo, Japan), cyclophosphamide
(Shionogi & Co., Ltd., Osaka, Japan), fluorouracil (Kyowa Hakko
Kirin Co., Ltd., Tokyo, Japan), epirubicin combined with
cyclophosphamide, or docetaxel hydrate (Elmed Eisai Co., Ltd.,
Tokyo, Japan) was administered to prevent disease progression. The
numbers of cycles of chemotherapy prior to KORTUC II therapy are
shown in Table I. Combinations of
systemic chemotherapy were selected according to updated guidelines
for breast cancer treatment.
 | Table I.Patient clinical and therapeutic
data. |
Table I.
Patient clinical and therapeutic
data.
| Pt. | Age (y) | Metastatic area | Induction
chemotherapy | Treatment area
(KORTUC II field) | FDG accumulation | Tumor size (mm) | Therapeutic
effect |
|---|
| 1 | 63 | Bone, lung and lymph
node | PTX × 15 | Left breast | 5.6→Da | 14→10 | PR |
| 2 | 57 | Lymph node and
skin | FEC × 8 | Left breast | 7.7→Da | 28→Da | CRb |
| 3 | 65 | Bone, lung and lymph
node | EC × 6 | Left breast | 11.2→Da | 15→3 | CRb |
| 4 | 58 | Lymph node and
skin | EC × 3 | Right breast | 8.6→Da | 28→14 | CRb |
| 5 | 55 | Lung | EC × 3 | Left breast | 6.2→2.2 | 28→22 | PR |
| 6 | 36 | Lymph node | FEC × 4 and PTX ×
12 | Right breast | No data→2.5 | No
data→Da | CRb |
| 7 | 51 | Bone | EC × 9 and TXT ×
6 | Left breast | 5.1→Da | 23→Da | CRb |
KORTUC II treatment was administered for the local
control of breast cancer and chemotherapy was performed for
systemic control. The radiotherapy regimen consisted of 2.75
Gy/fraction, 5 fractions/week for a total of 16–18 fractions and a
total radiation dose of 44–49.5 Gy. For two patients (designated 6
and 7), boost irradiation (3 Gy/fraction, 3 fractions) was
administered to control the site of the primary lesions;
irradiation by X-ray for the target lesion was performed by
tangential irradiation. The energy level of the X-ray was 4 MV, and
the energy level of the electron beam was 15 MeV. The KORTUC II
agent was composed of 2.5 ml 1% w/v sodium hyaluronate and 0.5 ml
3% w/v hydrogen peroxide. The administration of 3–6 ml KORTUC II
was initiated following the sixth radiation fraction and performed
twice a week under ultrasonographic guidance in order to maintain a
high oxygen concentration in the cancerous tissues for 48 h based
on our previous experimental and studies (1–10). The
volume of the KORTUC II administered to each patient was adjusted
according to the respective tumor diameter evaluated based on
imaging modalities such as ultrasonographic studies and/or computed
tomography (5). The total number of
injections/patient was 5–6. The clinical features and therapeutic
effects observed in each patient are presented in Table I.
Assessment of the therapeutic response
to KORTUC II treatment
The majority of the patients included in the current
study underwent positron emission tomography-computed tomography
(PET-CT) examinations prior to, and 1–7 months following, KORTUC II
treatment. The therapeutic effects were evaluated by comparison of
the pre-treatment and post-treatment PET-CT scans of the local
tissues subjected to KORTUC II therapy. The patients subsequently
underwent PET-CT examinations every six months when possible for at
least three years, following the conclusion of KORTUC II treatment.
The final therapeutic response of the lesion was assessed according
to the Revised Response Evaluation Criteria in Solid Tumors
guidelines (version 1.1) and patient follow-ups and tumor
assessments were performed once a month following KORTUC II
therapy. Each patient was assigned a toxicity grade using the
National Institutes of Health Common Toxicity Criteria (9,10). To
evaluate the feasibility of KORTUC II treatment, all
treatment-associated complications were assessed according to the
Common Terminology Criteria for Adverse Events (CTCAE; version 4.0)
(9,10). Patient follow-ups were conducted for
≥15 months (1–3).
Results
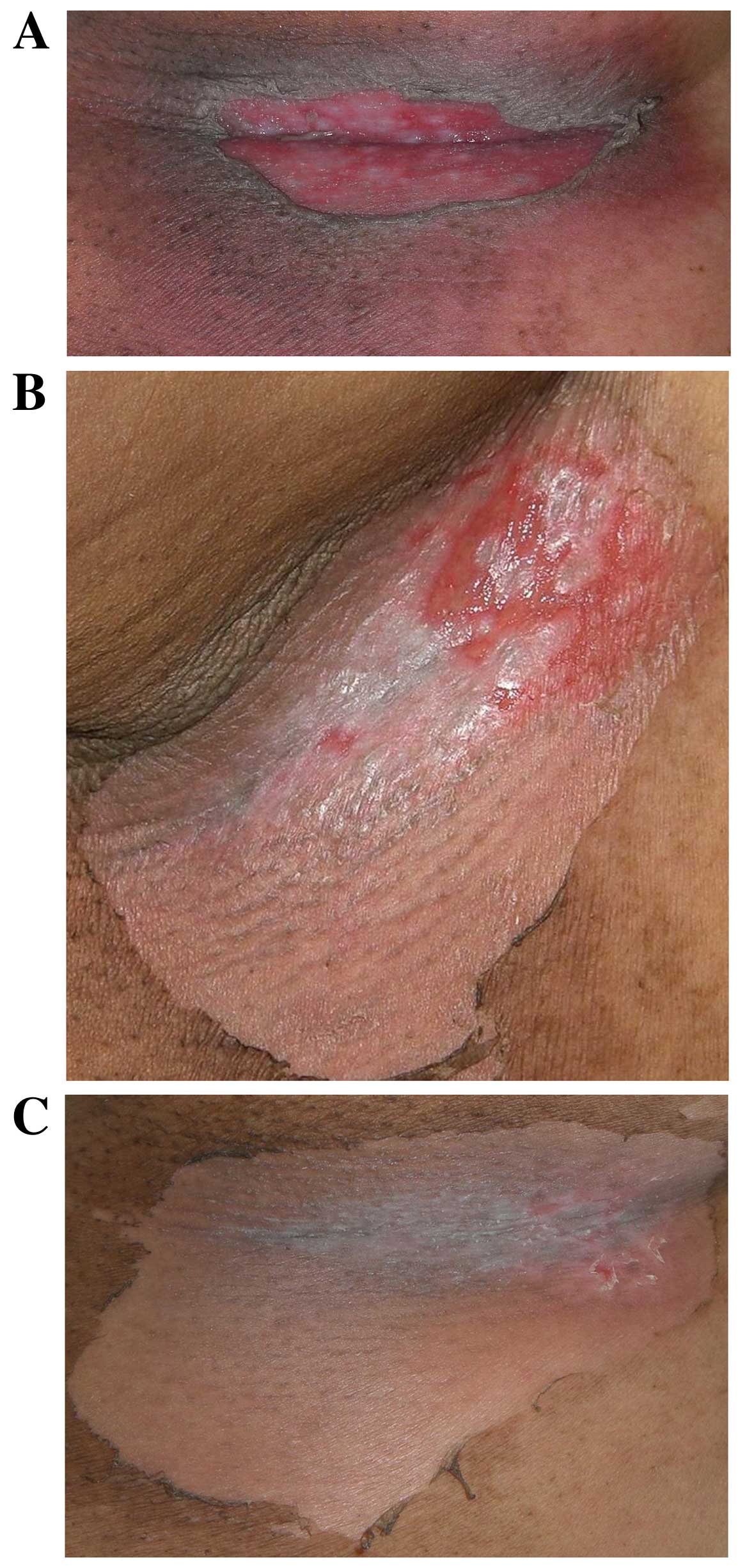
KORTUC II treatment was completed with tolerable
adverse effects. Four patients (57%) exhibited the acute phase
complications of local erosion or ulceration in the treated tissues
and required professional dermatological treatment [patient (Pt.)2,
Pt.3, Pt.4 and Pt.5]. These complications were considered to be
causally associated with tumor shrinkage induced by KORTUC II
treatment. The aforementioned patients were determined to have
CTCAE grade IV complications. Three patients (43%) experienced
soreness and depilation in the treated tissues (Pt.1, Pt.6 and
Pt.7) and were determined to have CTCAE grade II complications. All
the complications exhibited by the patients were cured by local
treatment and are presented in Fig.
2. None of the patients were observed to develop chronic
complications during the present study.
Of the seven patients, five (71%) exhibited a
complete response, two (29%) demonstrated a partial response and
none of the patients exhibited stable or progressive disease; thus,
the response rate was 100%. The overall survival rate was 100% at 1
year, 86% (6/7) at 2 years and 71% (5/7) at 3 years. The local
control rate was 100% at 1 year (7/7), 2 years (6/6) and 3 years
(5/5). The disease-free survival rate was 86% (6/7) at 1 year, 50%
(3/6) at 2 years and 40% (2/5) at 3 years (Table II).
 | Table II.Patient treatment outcomes. |
Table II.
Patient treatment outcomes.
| Outcome | 1 year | 2 years | 3 years |
|---|
| Overall survival
rate | 100% (7/7) | 86%
(6/7) | 71%
(5/7) |
| Local control
rate | 100% (7/7) | 100% (6/6) | 100% (5/5) |
| Disease-free survival
rate | 86%
(6/7) | 50%
(3/6) | 40%
(2/5) |
The treatment outcomes were satisfactory and the
adverse effects were determined to be within an acceptable range.
Representative PET-CT examinations are presented in Fig. 3. Although new metastatic lesions were
detected in four patients during follow-up, overall the lesions
were ameliorated by the appropriate treatment. The mean follow-up
period by December 2014 was 51 months; four patients have succumbed
to the disease at the time of writing.
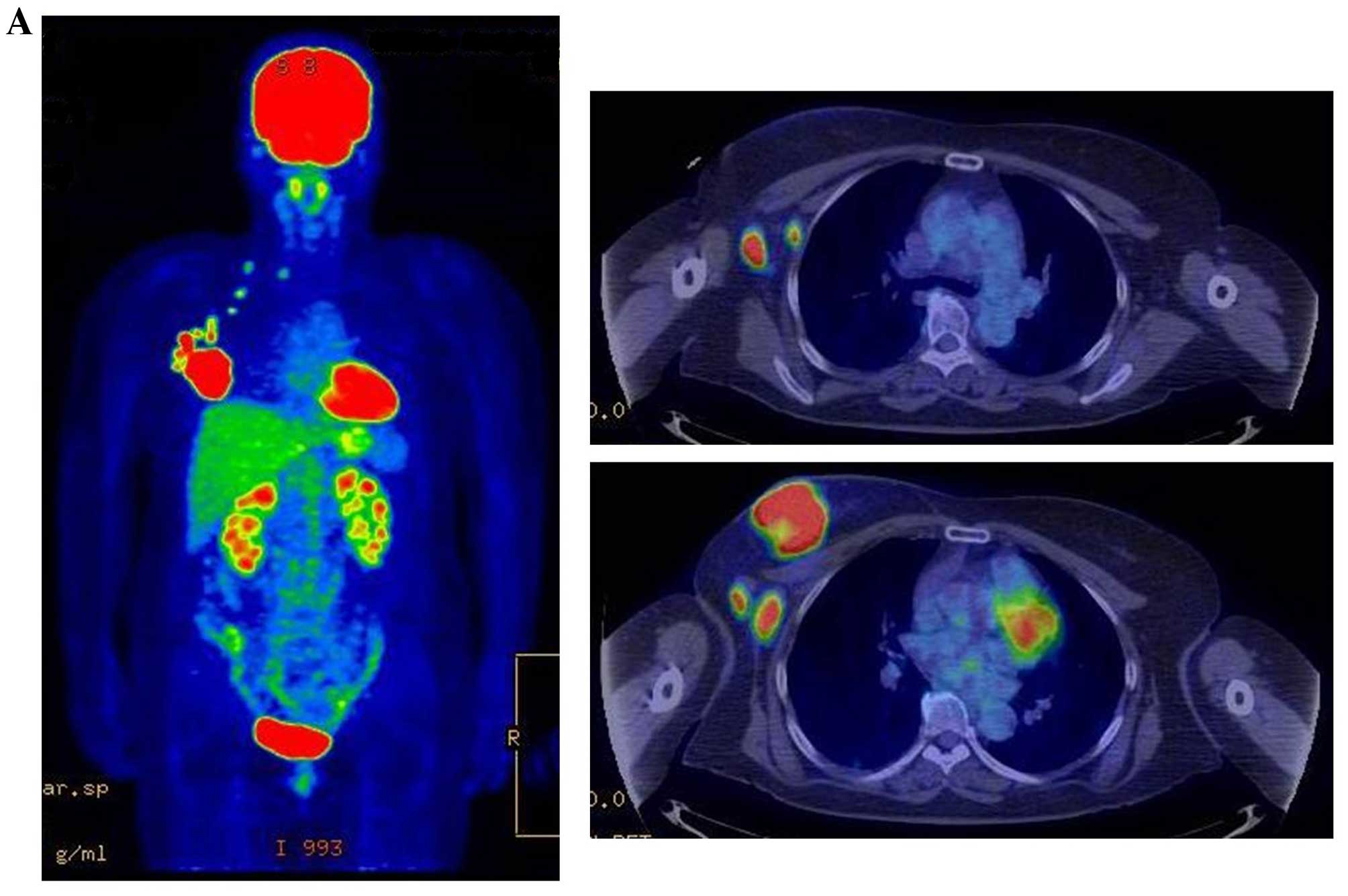 | Figure 3.Therapeutic response following KORTUC
II treatment as evaluated by PET-CT examination of patient number 4
(58-year-old female with stage IV primary breast cancer). (A)
PET-CT prior to induction chemotherapy with epirubicin and
cyclophosphamide. Numerous lymph node and skin metastases are
depicted. (B) PET-CT prior to KORTUC II treatment. With induction
chemotherapy, FDG accumulation in the right breast and right axilla
improved and the skin metastases disappeared. KORTUC II, Kochi
Oxydol Radiation Therapy for Unresectable Carcinomas II; PET-CT,
positron emission tomography-computed tomography; FDG,
fluorodeoxyglucose. (C) Four months following KORTUC II treatment.
FDG accumulation in the left breast and in the chest disappeared.
(D) PET-CT seven months following KORTUC II treatment. FDG
accumulation had not recurred on the PET-CT scan conducted (E) 16
months following KORTUC II treatment. KORTUC II, Kochi Oxydol
Radiation Therapy for Unresectable Carcinomas II; PET-CT, positron
emission tomography-computed tomography; FDG, fluorodeoxyglucose.
PET-CT scans conducted (F) 21 months, (G) 26 months and (H) 32
months following KORTUC II treatment. KORTUC II, Kochi Oxydol
Radiation Therapy for Unresectable Carcinomas II; PET-CT, positron
emission tomography-computed tomography. PET-CT scans conducted (I)
38 months, (J) 49 months and (K) 64 months following KORTUC II
treatment. KORTUC II, Kochi Oxydol Radiation Therapy for
Unresectable Carcinomas II; PET-CT, positron emission
tomography-computed tomography. PET-CT scan conducted (L) 76 months
following KORTUC II treatment. FDG accumulation and recurrence of
metastasis were not observed during follow-up. Prior to treatment,
the size of the right breast tumor was 28 mm and the SUVmax of FDG
accumulation was 8.6. The target lesion and the FDG accumulation
had disappeared four months following KORTUC II treatment, as
determined by a PET-CT scan. The therapeutic effect was determined
to be a complete response in patient number 4. KORTUC II, Kochi
Oxydol Radiation Therapy for Unresectable Carcinomas II; PET-CT,
positron emission tomography-computed tomography; FDG,
fluorodeoxyglucose; SUVmax, max value of the standardized uptake
value. |
Discussion
Improvements in diagnostic methods have facilitated
the earlier detection of numerous types of primary cancer,
recurrent cancer and metastases, and previous advances in
chemotherapy have been notable. For metastatic or unresectable
cancer, combination chemotherapy and radiotherapy
(chemoradiotherapy) is typically administered with good therapeutic
outcomes (11–21). The results of our previous studies
(9,10)
regarding the use of KORTUC II for the treatment of supraclavicular
lymph node metastates and recurrent breast cancers indicated
comparable efficacy to treatment with chemoradiotherapy. Although
the present study utilized alternative evaluation and timing
methods, the effect of KORTUC II for the treatment of local lesions
in patients with stage IV breast cancer, following induction
chemotherapy, revealed similar treatment outcomes compared with
chemoradiotherapy (11–21). The response rate (100%) and the
overall survival rate at 1 year (100%), 2 years (85%) and 3 years
(71%) were good, and the treatment regimen was well tolerated.
Certain patients exhibited severe side effects in the treated
tissues, which was possibly due to tumor shrinkage induced by
KORTUC II. Although metastasis was observed during patient
examinations following the conclusion of the treatment, the
majority of the lesions were improved. The results indicate that
KORTUC II treatment outcomes are satisfactory with adverse effects
within an acceptable range, particularly for stage IV primary
breast cancer following induction chemotherapy. Though the numbers
of the patients treated with KORTUC II in the individual studies
are small, good therapeutic effects of KORTUC II have also been
reported in other studies (1–8). Therefore, KORTUC II may be a novel
therapeutic treatment for patients with breast cancer (3,4,6,8). KORTUC II
consists of sodium hyaluronate 0.83% w/v and hydrogen peroxide 0.5%
w/v, which are inexpensive and readily available. The mechanism of
action of KORTUC II is simple (9,10),
involving inactivation of peroxidase/catalase and production of
oxygen in the target tumor tissue simultaneously, and deep lesions
are accessible to treatment through the use of a Cathelin needle to
administer the radiosensitizer under ultrasonographic guidance.
Therefore, the novel radiosensitizer KORTUC II may
be used for the treatment of certain types of cancer. However, the
number of patients currently and previously treated with KORTUC II,
and the respective follow-up periods, is too small for a definitive
conclusion. Further randomized clinical trials with a larger sample
size are required to establish the therapeutic efficacy of KORTUC
II.
Acknowledgements
The authors would like to thank Forte Science
Communications, Tokyo, Japan, for their editorial assistance. This
study was partially supported by a Grant-in-Aid for Scientific
Research from the Japanese Ministry of Education, Culture, Sports,
Science and Technology (grant no. 25461916).
References
|
1
|
Ogawa Y, Kubota K, Ue H, Nishioka A,
Kariya S, Yokota N, Sasaki T, Suzuki K, Nakatani K, Yamanishi T, et
al: Development and clinical application of a new radio sensitizer
containing hydrogen peroxide and hyaluronic acid sodium for topical
tumor injection - a new enzyme-targeting radiosensitization
treatment, KORTUC II (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas, Type II). Strahlenther Onkol. 183:100–101.
2007.
|
|
2
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et
al: New radiosensitization treatment (KORTUC I) using hydrogen
peroxide solution-soaked gauze bolus for unresectable and
superficially exposed neoplasms. Oncol Rep. 19:1389–1394.
2008.PubMed/NCBI
|
|
3
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
et al: Phase I study of a new radiosensitizer containing hydrogen
peroxide and sodium hyaluronate for topical tumor injection: A new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II). Int J Oncol. 34:609–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyatake K, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical care for locally advanced
breast cancer: Radiologically assessed therapeutic outcome of a new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II) with systemic chemotherapy. Oncol Rep. 24:1161–1168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a novel
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer-radioresistant neoplasms. Oncol Lett. 1:1025–1028.
2010.PubMed/NCBI
|
|
6
|
Hitomi J, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical therapy and radiologic
assessment of stage I breast cancer treatment with novel
enzyme-targeting radiosensitization: Kochi Oxydol-Radiation Therapy
for Unresectable Carcinomas, type II (KORTUC II). Exp Ther Med.
1:769–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radiosensitization treatment (KORTC II) for intratumoral injection
for low-LET radioresistant tumors. Int J Oncol. 39:553–560.
2011.PubMed/NCBI
|
|
8
|
Tsuzuki A, Ogawa Y, Kubota K, Tokuhiro S,
Akima R, Yaogawa S, Itoh K, Yamada Y, Sasaki T, Onogawa M, et al:
Evaluation of changes in tumor shadows and Microcalcifications on
mammography following KORTUC II, a new radiosensitization treatment
without any surgical procedure for elderly patients with Stage I
and II breast cancer. Cancers (Basel). 3:3496–3505. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoyama N, Ogawa Y, Kubota K, Ohgi K,
Kataoka Y, Miyatake K, Tadokoro M, Yamanishi T, Ohnishi T, Hamada
N, et al: Therapeutic response to a new enzyme-targeting
radiosensitization treatment (KORTUC-SC) for patients with
chemotherapy-resistant supraclavicular lymph node metastasis. J
Cancer Res Ther. 1:215–219. 2013. View Article : Google Scholar
|
|
10
|
Aoyama N, Ogawa Y, Kubota K, Yasuoka M,
Takahashi M, Iwasa H, Miyatake K, Yamanishi T, Hamada N, Tamura T,
et al: Therapeutic response to a novel enzyme-targeting
radiosensitization treatment (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas) in patients with recurrent breast cancer.
Oncol Lett. 12:29–34. 2016.PubMed/NCBI
|
|
11
|
Danforth DN Jr, Zujewski J, O'Shaughnessy
J, Riseberg D, Steinberg SM, McAtee N, Noone M, Chow C, Chaudhry U,
Lippman M, et al: Selection of local therapy after neoadjuvant
chemotherapy in patients with Stage IIIA, B breast cancer. Ann Surg
Oncol. 5:150–158. 1988. View Article : Google Scholar
|
|
12
|
Lorvidhaya V, Kamnerdsupaphon P,
Chitapanarux I, Sukthomya V and Tonusin A: Cisplatin and
gemcitabine in patients with metastatic cervical cancer. Gan To
Kagaku Ryoho. 31:1057–1062. 2004.PubMed/NCBI
|
|
13
|
Hu XC, Zhang J, Xu BH, Cai L, Ragaz J,
Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, et al: Cisplatin plus
gemcitabine versus paclitaxel plus gemcitabine as first-line
therapy for metastatic triple-negative breast cancer (CBCSG006): A
randomized, open-label, multicenter, phase 3 trial. Lancet Oncol.
16:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karasawa K, Saito M, Hirowatari H, Izawa
H, Furuya T, Ozawa S, Ito K, Suzuki T and Mitsuhashi N: The role of
chemoradiotherapy in patients with unresectable T4 breast tumors.
Breast Cancer. 20:254–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson CJ, Graff R, Moran P, Cariou C and
Bordeaux S: Breast cancer stage, surgery, and survival statistics
for Idaho's national breast and cervical cancer early detection
program population, 2004–2012. Prev Chronic Dis. 12:E362015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukai H, Watanabe T, Mitsumori M, Tsuda H,
Nakamura S, Masuda N, Yamamoto N, Shibata T, Sato A, Iwata H and
Aogi K: Final results of a safety and efficacy trial of
preoperative sequential chemoradiation therapy for the nonsurgical
treatment of early breast cancer: Japan Clinical Oncology Group
Study JCORG306. Oncology. 85:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin Y, Zhang P, Xu BH, Zhang BL, Li Q,
Yuan P, Cai RG, Wang JY, Wang X and Xu XZ: Unfavorable pathological
complete response rate of neoadjuvant chemotherapy epirubicin plus
taxanes for locally advanced triple-negative breast cancer. J
Huazhong Univ Sci Technolog Med Sci. 33:262–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matuschek C, Bölke E, Roth S, Orth K, Lang
I, Bojar H, Janni JW, Audretsch W, Nestle-Kraemling C, Lammering G,
et al: Long-term outcome after neoadjuvant radiochemotherapy in
locally advanced noninflammatory breast cancer and predictive
factors for pathologic complete remission: Results of a
multivariate analysis. Strahlenther Onkol. 188:777–781. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Genet D, Lejeune C, Bonnier P, Aubard Y,
Venat-Bouvet L, Adjadj DJ, Martin J, Labourey JL, Benyoub A,
Clavère P, et al: Concomitant intensive chemoradiotherapy induction
in non-metastatic inflammatory breast cancer: Long-term follow-up.
Br J Cancer. 97:883–887. 2007.PubMed/NCBI
|
|
20
|
Bollet MA, Sigal-Zafrani B, Gambotti L,
Extra JM, Meunier M, Nos C, Dendale R, Campana F, Kirova YM, Diéras
V, et al: Pathological response to preoperative concurrent
chemo-radiotherapy for breast cancer: Result of phase II study. Eur
J Cancer. 42:2286–2295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaughnessy JN, Meena RA, Dunlap NE, Jain
D, Riley EC, Quillo AR and Dragun AE: Efficacy of concurrent
chemoradiotherapy for patients with locally recurrent or advanced
inoperable breast cancer. Clin Breast Cancer. 15:135–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|