Introduction
Gastric cancer is the fourth most common neoplasia
and the second highest cause of mortality associated with cancer
worldwide (1). It is reported that
gastric cancer is also the third most common type of cancer and the
second highest cause of mortality in patients with cancer in China
(1). Complete excision of the gastric
tumor is the best option of curative treatment (2). In patients diagnosed at advanced stages,
although chemotherapy effectively improves the survival quality and
time, the median survival time of patients with advanced gastric
cancer remains poor (1,2). The metastatic ability of gastric cancer
is the leading cause of poor prognosis and high mortality (3). Therefore, cancer metastasis is still a
big challenge in the clinic for oncologists.
Accumulating data have demonstrated that degradation
of extracellular matrix (ECM) proteins is the antecedent condition
of malignant tumor metastasis (1).
Matrix metalloproteinases (MMPs) are part of the endopeptidase
family and can cleave the majority of components of the ECM,
including fibronectin, collagen, elastin, proteoglycan and laminin,
thus playing critical roles in the development, progression and
metastasis of malignant tumors (3,4).
Upregulation of MMPs and altered expression of their tissue
inhibitors [known as tissue inhibitors of metalloproteinases
(TIMPs)] are associated with the invasiveness and metastasis of
cancer cells (4). Among them, MMP-2
and MMP-9 are the main enzymes for the degradation of basement
membranes in cancer cells and/or stromal cells (4,5). TIMPs are
natural inhibitors of MMPs, and TIMP2 specifically inhibits MMP-2,
while TIMP1 inhibits MMP-9 (3).
Fangchinoline (FCL) is a bisbenzylisoquinoline
alkaloid in Stephania tetrandra S. Moore (Fen fang ji)
(6). It is reported that FCL can
inhibit histamine release (7), lower
blood pressure as a non-specific calcium channel antagonist
(8) and inhibit glutamate release
from rat cortical synaptosomes (9).
In addition, FCL exerts anti-cancer activities in several types of
malignant tumors, including breast cancer (10), prostate carcinoma (11), hepatocellular carcinoma (12) and lung cancer (13). However, the effects of FCL on the
metastasis of gastric cancer and its underlying mechanisms remain
poorly understood.
In the present study, the anti-metastatic activity
of FCL (Fig. 1) in gastric cancer
cells and its molecular mechanism of action were explored. Our data
revealed that FCL inhibited the phosphorylation of AKT and
upregulated TIMP2/1, leading to reduced expression of MMP-2/9 and
inhibition of gastric cancer cell invasion in vitro.
Materials and methods
Chemicals and antibodies
FCL (purity >98%) was purchased from Shanghai
Standard Technology Co., Ltd. (Shanghai, China). Cell culture
materials were purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Specific antibodies against GAPDH
(1:1,000, sc-25778), MMP-2 (1:500, sc-53630), MMP-9 (1:500,
sc-21733), phosphorylated (p)-AKT (1:500, sc-135650) and AKT
(1:500, sc-5298) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Cell culture
Human gastric cancer AGS cells (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China)
were cultured in high glucose-Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS; both HyClone; GE
Healthcare Life Sciences, Chalfont, UK) and 200 mM glutamine at
37°C under a humidified 95% air/5% CO2 mixture
(v/v).
Cell viability assay
Cells were seeded at a density of 5×103
cells/well in a 96-well microplate containing DMEM with 10% FBS,
and incubated for 24 h. Next, cells were incubated with different
concentrations of FCL for 24 h. The proliferation rate was
determined with an MTT assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). The results were expressed as the
absorbance at 570 nm, as previously reported (11).
Cell adhesion assay
After pre-treating the cells with or without FCL (2,
4 and 8 µM) for 24 h, AGS cells were trypsinized and suspended at a
final concentration of 2×105 cells/ml in serum-free
medium. A total of 100 µl cell suspension was seeded to each well
in a 96-well plate coated with 20 µg/ml fibronectin overnight. The
cells were incubated for 30 min at 37°C, and the non-adherent cells
were removed by washing with PBS. MTT assay was used to determine
the number of remaining adherent cells.
Wound healing assay
AGS cells were seeded on a 6-well plate and
incubated in serum-free medium for 24 h. A transverse scratch wound
on each monolayer of cells was created using a sterilized 200-µl
pipette tip. The scratch wounded cell monolayers were then
stimulated with various concentrations of FCL (0, 2, 4 and 8 µM) in
5% FBS for an additional 36 h, at which point, the cells that had
migrated into the wound were photographed and analyzed.
Transwell chamber invasion assay
Transwell chamber invasion assay was conducted as
described by Magee et al (14). In brief, the transwell chambers (EMD
Millipore, Billerica, MA, USA) were pre-coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) for 1 h, and the upper
chambers were washed using serum-free medium. Following incubation
of AGS cells with FCL (0, 2, 4 and 8 µM) for 24 h, the cells were
trypsinized and suspended at a density of 2×105 cells/ml
in serum-free medium. A total of 200 µl cells were placed in the
upper chambers, and medium with 10% FBS was placed in the lower
chambers. After 24-h incubation at 37°C, the non-invaded cells were
removed using a cotton swab, while the invaded cells were fixed
with 100% methanol and stained with hematoxylin and eosin (Nanjing
Jiancheng Biotechnology Institute Co., Ltd., Nanjing, China). The
invaded cells on the lower surface of the membrane filter were
counted under an inverted microscope. The data are presented as the
mean number of cells attached to the bottom surface from six
randomly selected fields.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from AGS cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Synthesis of complementary DNA was
performed using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China), and the PCR conditions
were as follows: Initial denaturation at 95°C for 5 min, followed
by 24–33 cycles of denaturation at 94°C for 30 sec, annealing at
54°C for 30 sec and extension at 72°C for 45 sec. The primer
sequences were as follows: MMP-2 (forward
5′-TGGATGATGCCTTTGCTCGT-3′ and reverse 5′-AAACTTGCAGGGCTGTCCTT-3′);
MMP-9 (forward 5′-GGACAAGCTCTTCGGCTTCT-3′ and reverse
5′-TTCAGGGCGAGGACCATAGA-3′); TIMP1 (forward
5′-CTCGTCATCAGGGCCAAGTT-3′ and reverse 5′-GTAGGTCTTGGTGAAGCCCC-3′);
TIMP2 (forward 5′-TAGTGATCAGGGCCAAAGCG-3′ and reverse
5′-CAGGCTCTTCTTCTGGGTGG-3′); and GAPDH (forward
5′-GAGAAGGCTGGGGCTCATTT-3′ and reverse 5′-GTCAGGTCCACCACTGACAC-3′).
The expression levels of MMP-2, MMP-9, TIMP1 and TIMP2 were
normalized to those of GAPDH, which served as an internal
control.
Western blot analysis
To analyze the level of protein expression, cell
lysates were prepared, as described previously (15), and separated by 10% SDS-PAGE and then
transferred to polyvinylidene difluoride membranes for 2 h. The
membrane was treated with blocking solution (5% bovine serum
albumin in TBS) at room temperature for 1 h, and then incubated
overnight at 4°C with antibodies against MMP-2, MMP-9, AKT, p-AKT
or GAPDH. The membranes were washed with Tris-buffered saline
containing 0.5% Tween-20 (Beyotime Institute of Biotechnology), and
then incubated for 1 h at room temperature with an
anti-immunoglobulin G secondary antibody conjugated to horseradish
peroxidase (1:1,000; A0208; Beyotime Institute of Biotechnology).
The expression levels of the proteins were analyzed via
chemiluminescence (Beyotime Institute of Biotechnology) and
quantified using Quantity One software, version 4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The expression levels of
MMP-2, MMP-9, p-AKT and AKT were normalized to those of the
internal control GAPDH.
Statistical analysis
The results are expressed as the mean ± standard
deviation from ≥3 independent experiments. The statistical
differences between the experimental groups were assessed using a
Student's t-test in SPSS version 13.0 (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
FCL decreases cell proliferation in
gastric cancer AGS cells
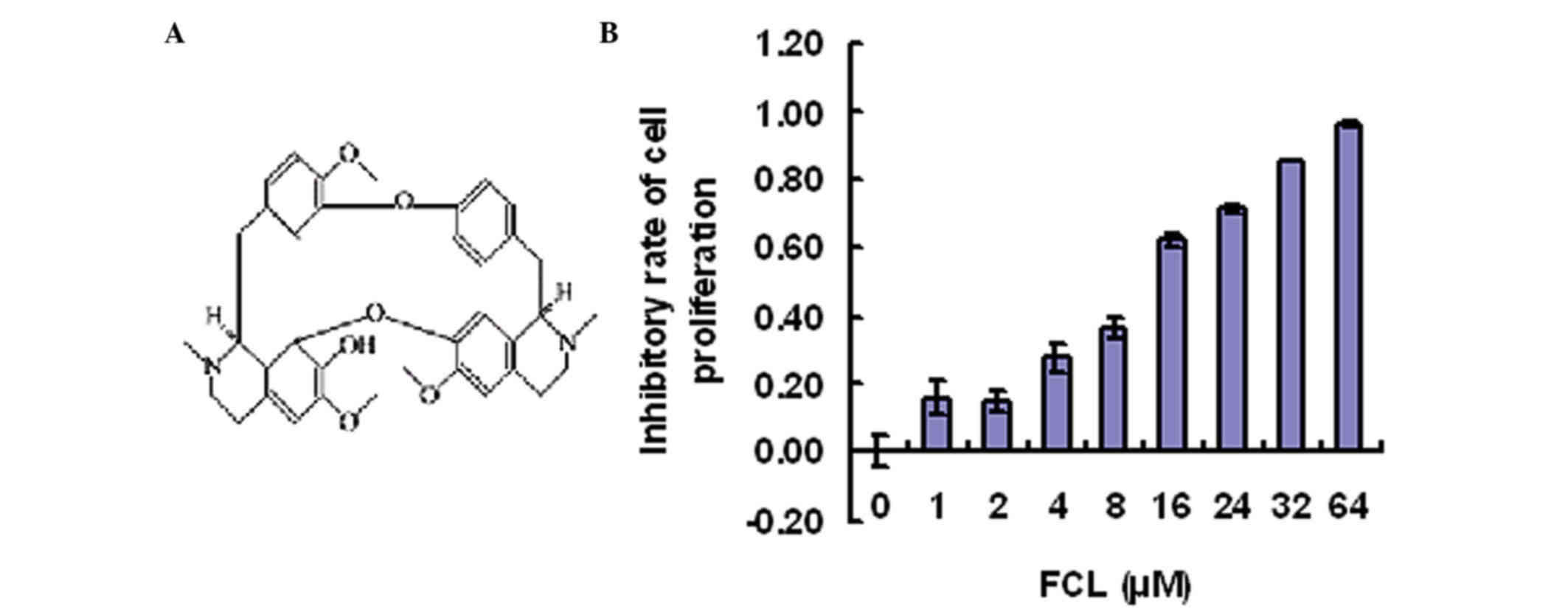
To determine the inhibitory effect of FCL (Fig. 1A) on gastric cancer cell
proliferation, an MTT assay was used. AGS cells were pre-treated
with FCL (0–64 µM) for 24 h. As shown in Fig. 1B, gastric cancer cell proliferation
was inhibited by FCL in a dose-dependent manner, while FCL at doses
<8 µM exhibited little effect on the cell viability of gastric
cancer AGS cells (Fig. 1B).
Therefore, these doses (0, 2, 4 and 8 µM) were used in the
following experiments.
FCL inhibits the attachment of gastric
cancer cells to fibronectin
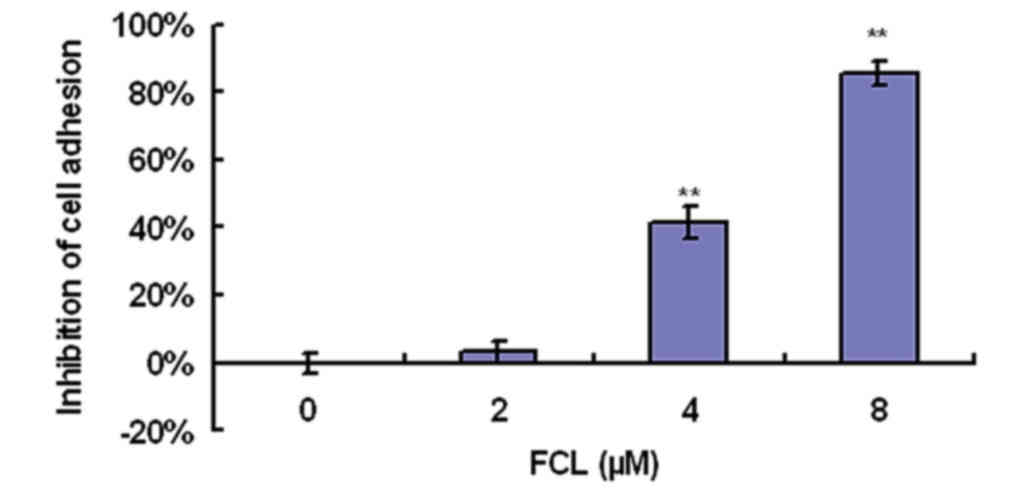
To determine the effect of FCL on cell adhesion to
the ECM, an adhesion assay was conducted in gastric cancer AGS
cells. As shown in Fig. 2, FCL
greatly inhibited cell adhesion ability in a dose-dependent manner,
as demonstrated by cell attachment assay. Cell adhesion was
inhibited by 41.53±4.85% for the 4-µM dose of FCL and by
85.63±3.51% for 8 µM FCL (Fig.
2).
FCL reduces the migration of gastric
cancer cells
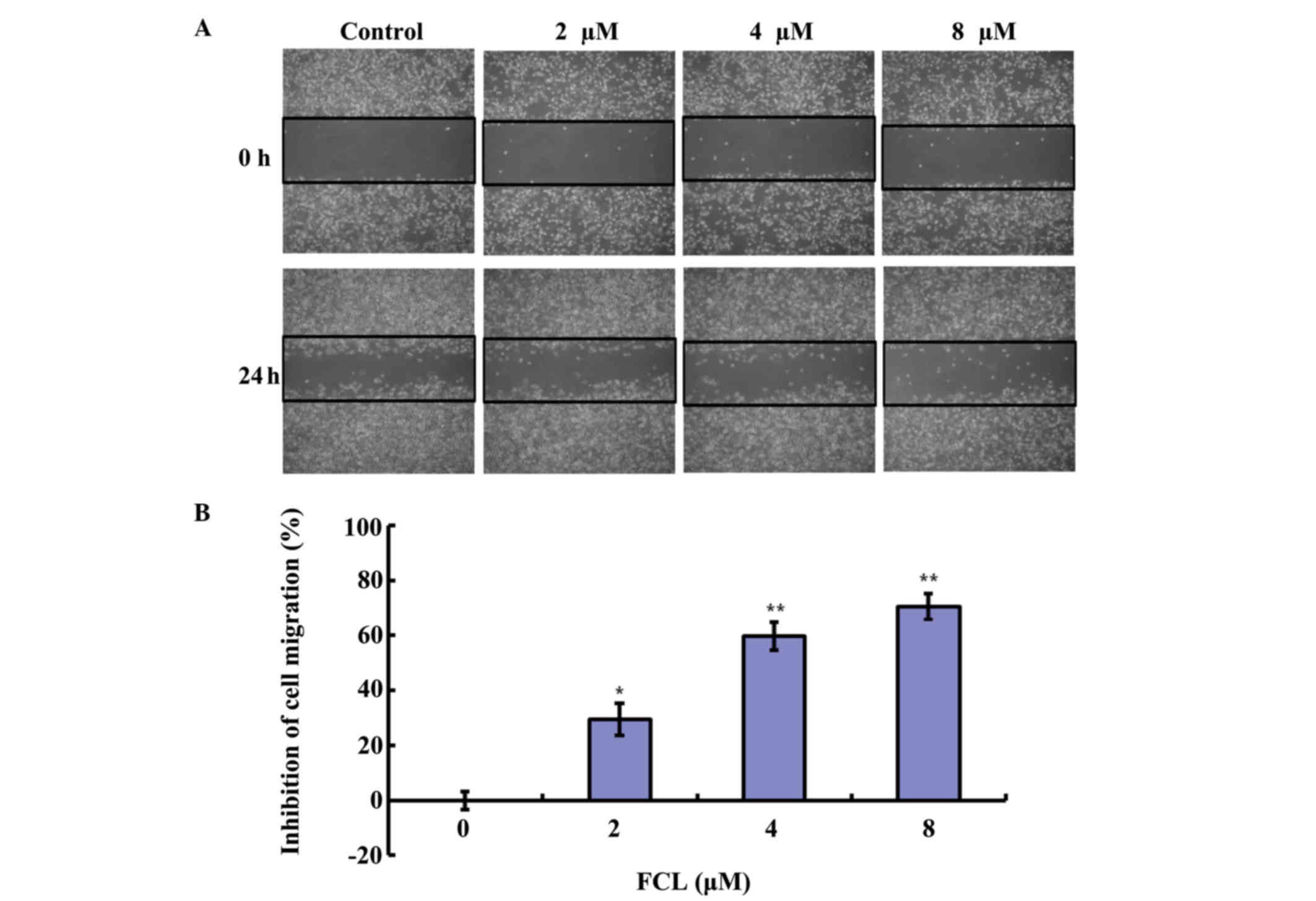
Next, the inhibitory effect of FCL on cell migration
was assessed in the artificial scratch wound of gastric cancer AGS
cells. As shown in Fig. 3A, FCL
obviously reduced cell numbers in the scratch wound area in gastric
cancer AGS cells in a dose-dependent manner. The inhibitory rates
of cell migration were 29.55±5.74, 64.65±4.99 and 76.68±4.82% for
2, 4 and 8 µM FCL, respectively (Fig.
3B).
FCL suppresses cell invasion in
gastric cancer cells
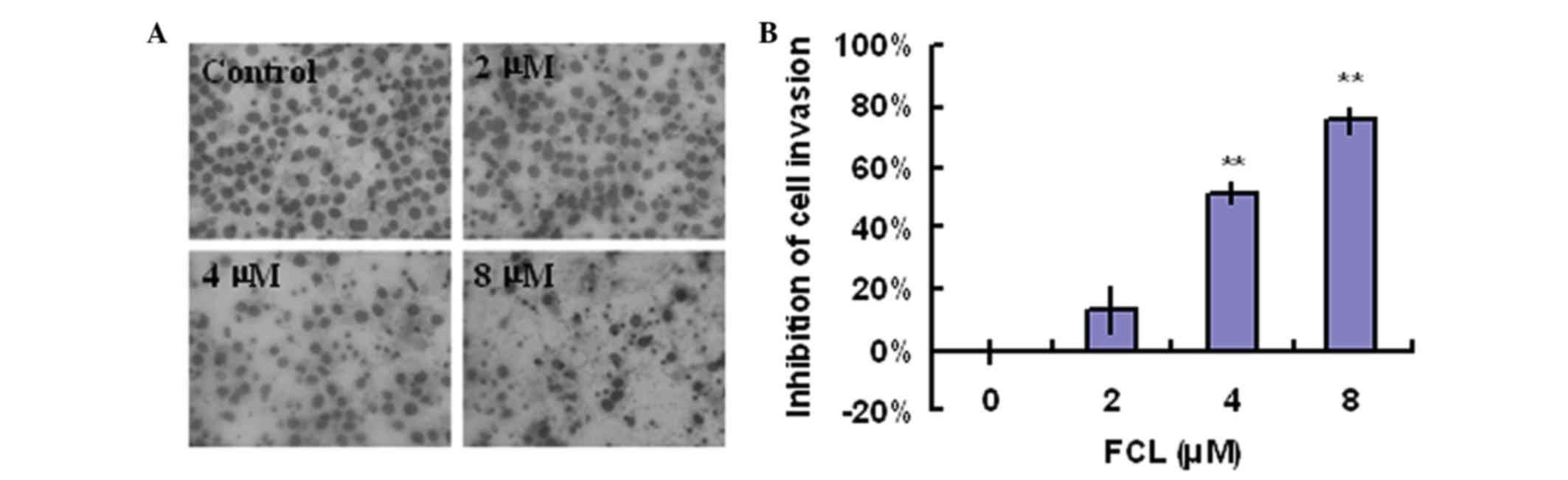
A Boyden chamber assay was used to examine whether
FCL could suppress the cell invasion of gastric cancer cells. As
shown in Fig. 4A, exposure to FCL
greatly decreased the invasive ability of gastric cancer cells in a
concentration-dependent manner. The inhibitory rates of FCL on
gastric cancer cells invasion were 13.47±7.30, 51.93±3.56 and
75.97±4.30% for 2, 4 and 8 µM FCL, respectively (Fig. 4B).
Effects of FCL on the expression of
MMP-2, MMP-9, TIMP2 and TIMP1 genes
Accumulating evidence has demonstrated that MMPs,
particularly MMP-2/MMP-9, and their inhibitors TIMP2/TIMP1
participated in the invasiveness and metastasis of malignant tumors
via stimulating the degradation of ECM and cell migration (3,5). To verify
the possible anti-metastatic molecular mechanism of FCL on gastric
cancer cells, the expression of MMP-2/MMP-9 and TIMP2/TIMP1 genes
was detected in FCL-treated gastric cancer AGS cells. The
expression of MMP-2 and MMP-9 messenger (m) RNAs was obviously
decreased in a concentration-dependent manner, with a maximum
decrease of 31.23±4.16 and 63.83±4.64% for MMP-2 and MMP-9,
respectively, following exposure to 8 µM FCL (Fig. 5). In addition, the mRNA levels of
their specific inhibitors TIMP2 and TIMP1 were significantly
increased in a dose-dependent manner, with a maximum elevated rate
of 1.96±0.08-fold and 1.50±0.06-fold for TIMP2 and TIMP1,
respectively, upon exposure to 8 µM FCL (Fig. 5). Additionally, the expression levels
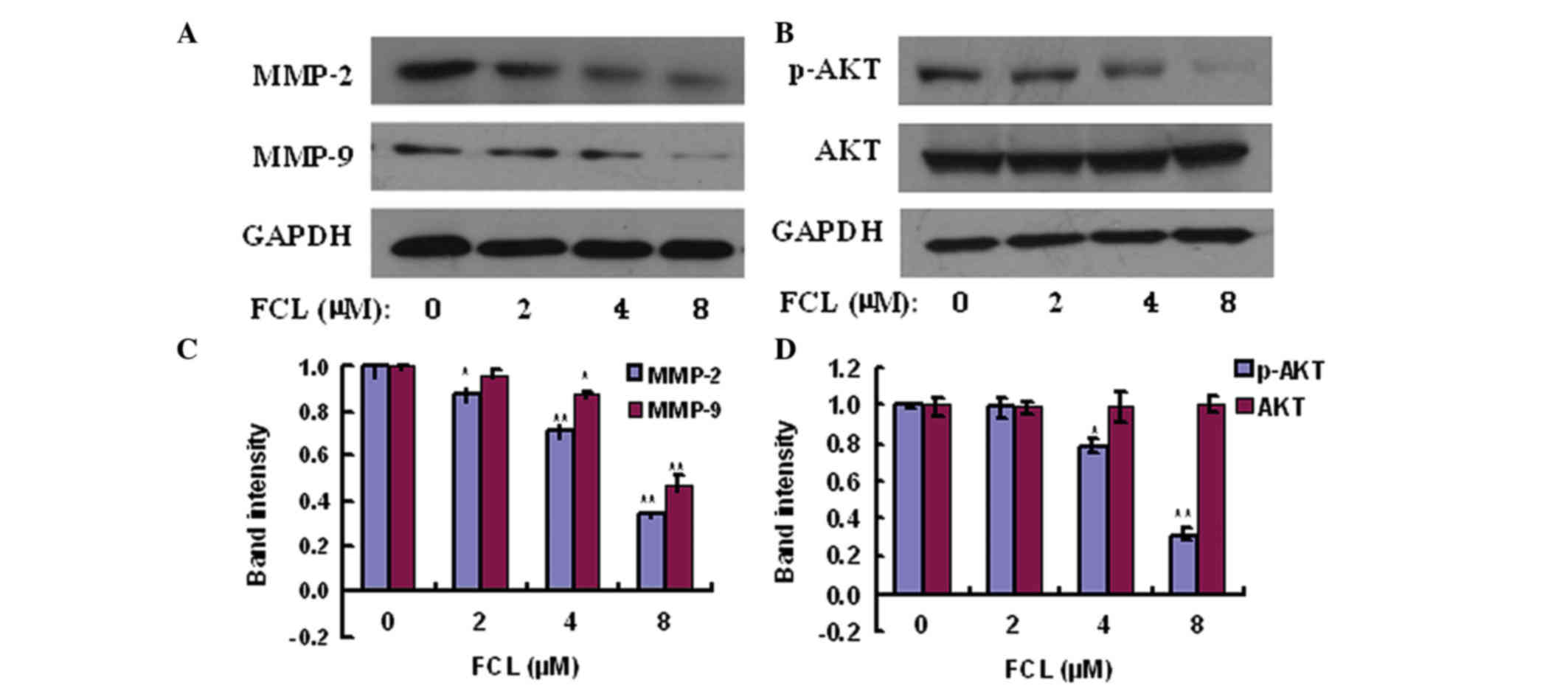
of MMP-2 and MMP-9 were also confirmed by western blot assay, and
the results demonstrated that FCL also significantly inhibited the
expression of MMP-2 and MMP-9 proteins in a similar pattern
(Fig. 6). The above data suggested
that FCL inhibited the invasiveness of gastric cancer AGS cells via
downregulation of MMP-2/MMP-9 and upregulation of TIMP2/TIMP1.
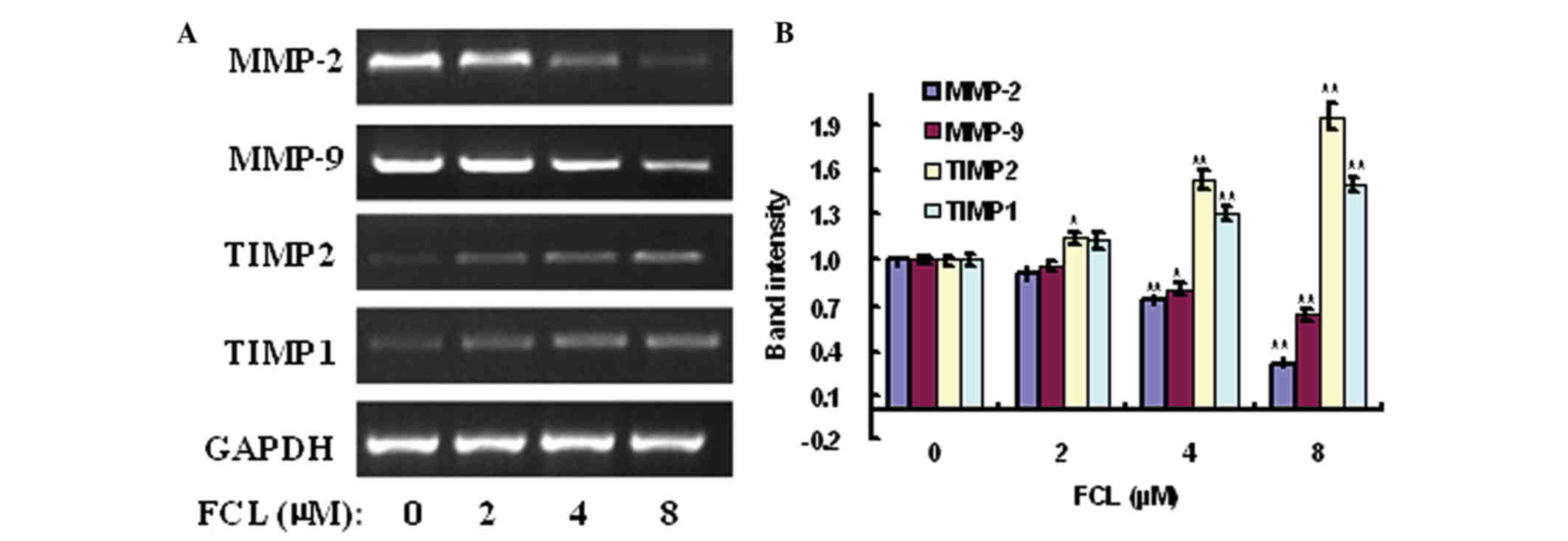 | Figure 5.Effects of FCL on the expression of
MMP-2, MMP-9, TIMP1 and TIMP2 mRNAs. (A) Gastric cancer cells were
treated with or without FCL (2–8 µM) for 24 h. Total RNA was
isolated, and reverse transcription-polymerase chain reaction was
performed to detect the expression of MMP-2, MMP-9, TIMP1 and TIMP2
mRNAs. (B) The densitometry of the bands was normalized to the
expression of GAPDH and analyzed. *P<0.05, **P<0.01 compared
with the control. Data are presented as the mean ± standard
deviation of three separate experiments. FCL, fangchinoline; mRNA,
messenger RNA; MMP, matrix metalloproteinase; TIMP, tissue
inhibitor of metalloproteinase. |
FCL reduces the phosphorylation of AKT
in gastric cancer cells
Since our data revealed that FCL exhibited
inhibitory effects on cell adhesion, migration, invasion and
proteinases, the effects of FCL on the expression of the components
of the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT
pathway were explored by western blotting to elucidate the
underlying molecular mechanisms. As shown in Fig. 6, the phosphorylation of AKT was
significantly reduced in a dose-dependent manner to 79.6±4.2 and
31.8±3.5% upon treatment with 4 and 8 µM FCL, respectively, while
the total AKT levels were not significantly affected upon exposure
to FCL.
Discussion
As an active compound of Stephania tetrandra
S. Moore, FCL has been reported to exhibit anti-cancer effects,
which are associated with cell apoptosis promotion, autophagic
death induction, cell cycle arrest and multidrug resistance
(6–8);
however, the anti-metastatic activity of FCL has not been clarified
yet, particularly in gastric cancer. The present study attempted to
identify novel functions of FCL, and our data revealed that FCL
inhibited cell adhesion, migration and invasion in human gastric
cancer AGS cells without obvious cytotoxicity. In addition, the
anti-metastatic function of FCL was associated with downregulation
of MMP-2/9, upregulation of TIMP2/1 and decreased phosphorylation
of AKT.
The invasion and metastasis of malignant tumors are
the leading causes of poor prognosis in patients with gastric
cancer, and these processes are usually accompanied by the
destruction of basement membrane components in the ECM, which is
mainly controlled by a number of proteolytic enzymes such as MMPs
and their endogenous inhibitors TIMP2/1 (1,5).
Immunohistochemistry analysis revealed that expression of MMP-2 and
MMP-9 was detected in 94 and 70% of gastric cancer specimens,
respectively, and it was associated with poor overall survival in
patients with gastric cancer (16,17).
Overexpression of MMP-2/9 was observed to accelerate the invasion
of tumor cells in vitro and in vivo (18,19), and
metastasis in MMP-2 and MMP-9-null mice was greatly suppressed
compared with that in wild-type mice, suggesting that the elevated
expression of MMP-2/9 promotes the metastatic potential of
malignant tumor cells (3,5). Therefore, suppression of cancer
migration and invasion mediated by MMP-2/9 provides a new direction
for drug research in cancer metastasis. In the current study,
exposure of human gastric cancer AGS cells to non-toxic doses of
FCL inhibited cell adhesion, migration and invasion, compared with
control cells, in a concentration-dependent manner. In addition,
FCL was observed to inhibit the expression of MMP-2/9 mRNAs as well
as their protein levels, while it increased the expression of
TIMP2/1 genes, implying that FCL exhibited an ability to inhibit
matrix degradation proteases, thus limiting the adhesive and
invasive capabilities of gastric cancer cells.
A considerable number of studies have shown that
PI3K/AKT signaling serves a critical role in regulating the
expression of MMPs; therefore, suppression of PI3K/AKT signaling
may inhibit proliferation, invasion, angiogenesis and metastasis in
various malignant tumors (15). In
addition, inhibition of PI3K/AKT signaling could result in
decreased expression of MMP-2/9 (20–22).
Considering that FCL at non-toxic doses (0–8 µM) could greatly
suppress cell adhesion, migration and invasion via inhibition of
MMP-2/9 mRNAs and proteins, the present authors hypothesized
whether PI3K/AKT signaling was involved in this process. Our
results demonstrated that FCL suppressed the phosphorylation of AKT
in gastric cancer cells, suggesting that the inhibition of cell
adhesion, migration and invasion by FCL could be mediated by
suppression of MMP-2/9 and increase of TIMP2/1, in part, via
decreasing the phosphorylation of AKT.
In summary, our current study has shown that FCL
prevented the metastatic potential of human gastric cancer AGS
cells via inhibition of MMP-2/9 and increase of TIMP2/1 through
downregulation of PI3K/AKT signaling, thus highlighting the
potential role of FCL in the therapy of gastric cancer
metastasis.
Acknowledgements
The present study was supported by a Youth
Development Fund from the Health Authorities of Suzhou (Suzhou,
China; grant no. 201017).
References
|
1
|
Qiu MZ and Xu RH: The progress of targeted
therapy in advanced gastric cancer. Biomark Res. 1:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong H and Yau T: Targeted therapy in the
management of advanced gastric cancer: Are we making progress in
the era of personalized medicine. Oncologist. 17:346–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray GI, Duncan ME, Arbuckle E, Melvin
WT and Fothergill JE: Matrix metalloproteinases and their
inhibitors in gastric cancer. Gut. 43:791–797. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pereira AL, Veras SS, Silveira EJ, Seabra
FR, Pinto LP, Souza LB and Freitas RA: The role of matrix
extracellular proteins and metalloproteinases in head and neck
carcinomas: An updated review. Braz J Otorhinolaryngol. 71:81–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Wang Y, Yamamoto G and Tachikawa
T: Expression of matrix metalloproteinases MMP-2, MMP-9 and their
tissue inhibitors TIMP-1 and TIMP-2 in the epithelium and stroma of
salivary gland pleomorphic adenomas. Histopathology. 55:250–260.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desgrouas C, Taudon N, Bun SS, Baghdikian
B, Bory S, Parzy D and Ollivier E: Ethnobotany, phytochemistry and
pharmacology of Stephania rotunda Lour. J Ethnopharmacol.
154:537–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang LH, Zhang YH and Ku BS: Fangchinoline
inhibited the antinociceptive effect of morphine in mice.
Phytomedicine. 12:183–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HS, Zhang YH, Oh KW and Ahn HY:
Vasodilating and hypotensive effects of fangchinoline and
tetrandrine on the rat aorta and the stroke-prone spontaneously
hypertensive rat. J Ethnopharmacol. 58:117–123. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin TY, Lu CW, Tien LT, Chuang SH, Wang
YR, Chang WH and Wang SJ: Fangchinoline inhibits glutamate release
from rat cerebral cortex nerve terminals (synaptosomes). Neurochem
Int. 54:506–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing Z, Zhang Y, Zhang X, Yang Y, Ma Y and
Pang D: Fangchinoline induces G1 arrest in breast cancer cells
through cell-cycle regulation. Phytother Res. 27:1790–1794. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang CD, Huang JG, Gao X, Li Y, Zhou SY,
Yan X, Zou A, Chang JL, Wang YS, Yang GX and He GY: Fangchinoline
induced G1/S arrest by modulating expression of p27, PCNA, and
cyclin D in human prostate carcinoma cancer PC3 cells and tumor
xenograft. Biosci Biotechnol Biochem. 74:488–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang N, Pan W, Zhu M, Zhang M, Hao X,
Liang G and Feng Y: Fangchinoline induces autophagic cell death via
p53/sestrin2/AMPK signalling in human hepatocellular carcinoma
cells. Br J Pharmacol. 164:731–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo B, Su J, Zhang T, Wang K and Li X:
Fangchinoline as a kinase inhibitor targets FAK and suppresses
FAK-mediated signaling pathway in A549. J Drug Target. 23:266–274.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magee PJ, Allsopp P, Samaletdin A and
Rowland IR: Daidzein, R-(+)equol and S-(−)equol inhibit the
invasion of MDA-MB-231 breast cancer cells potentially via the
down-regulation of matrix metalloproteinase-2. Eur J Nutr.
53:345–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PN, Hsieh YS, Chiou HL and Chu SC:
Silibinin inhibits cell invasion through inactivation of both
PI3K-Akt and MAPK signaling pathways. Chem Biol Interact.
156:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sampieri CL, León-Córdoba K and
Remes-Troche JM: Matrix metalloproteinases and their tissue
inhibitors in gastric cancer as molecular markers. J Cancer Res
Ther. 9:356–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sier CF, Kubben FJ, Ganesh S, Heerding MM,
Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB and Verspaget
HW: Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are
related to the overall survival of patients with gastric carcinoma.
Br J Cancer. 74:413–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beliën AT, Paganetti PA and Schwab ME:
Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive
migration of glioma cells in central nervous system white matter. J
Cell Biol. 144:373–384. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deryugina EI, Luo GX, Reisfeld RA, Bourdon
MA and Strongin A: Tumor cell invasion through matrigel is
regulated by activated matrix metalloproteinase-2. Anticancer Res.
17:3201–3210. 1997.PubMed/NCBI
|
|
20
|
Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya
S, Lin YC and Yang JS: Phenethyl isothiocyanate suppresses
EGF-stimulated SAS human oral squamous carcinoma cell invasion by
targeting EGF receptor signaling. Int J Oncol. 43:629–637.
2013.PubMed/NCBI
|
|
21
|
Lu KH, Yang HW, Su CW, Lue KH, Yang SF and
Hsieh YS: Phyllanthus urinaria suppresses human osteosarcoma cell
invasion and migration by transcriptionally inhibiting u-PA via ERK
and Akt signaling pathways. Food Chem Toxicol. 52:193–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang SF, Chen MK, Hsieh YS, Yang JS,
Zavras AI, Hsieh YH, Su SC, Kao TY, Chen PN and Chu SC:
Antimetastatic effects of Terminalia catappa L. on oral cancer via
a down-regulation of metastasis-associated proteases. Food Chem
Toxicol. 48:1052–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|