Introduction
Endometrial cancer (EC) has the highest incidence
rate of all malignant tumors of the female genital system in the
United States (1,2). EC-associated morbidity and mortality has
increased in recent years and its incidence is slightly below that
of breast, lung and bronchus, and colorectal cancer. According to
the American Cancer Society, in 2016 there were 60,050 newly
expected cases and 10,470 mortalities associated with EC in the
United States (2). Despite treatment
options including surgery, radiotherapy and chemotherapy, the
prognosis of poorly-differentiated EC is poor (3). Fatty acid and cholesterol synthesis is
important in the growth of cancer cells, with ectopic lipid
metabolism leading to tumorigenesis (4). Lipogenesis is usually upregulated and
obesity occurs in 40% of all EC cases (5).
FOXO1, which belongs to the FOX transcription factor
family, is typically regarded as a tumor suppressor and lies
downstream in the phosphoinositide 3-kinase (PI3K)/Akt signaling
pathway. This molecule performs various biological activities and
participates in energy metabolism (6,7),
cell-cycle progression (8),
apoptosis, cellular differentiation (9,10), wound
healing and stress response (11).
Through upregulation of adipose triglyceride lipase and lysosomal
acid lipase levels, FOXO1 is able to enhance fat catabolism
(12). FOXO1 is directly targeted by
insulin and highly expressed in insulin-sensitive tissues (7). Insulin inhibits the action of FOXO1 by
binding insulin growth factor (IGF)-1 and FOXO1 receptor, which
subsequently activates certain intracellular kinases involved in
the PI3K/Akt pathway. Activation of this signaling pathway results
in FOXO1 phosphorylation, which reduces FOXO1 nuclear
translocation, thereby inhibiting its transcriptional function
(12). Previous research has
identified that dysregulation of the PI3K/Akt pathway is
characteristic of EC (13).
Immunohistochemical (IHC) and RT-PCR studies from Ward et al
(14) and Goto et al (15) demonstrated that FOXO1 exhibited lower
expression levels in EC samples compared with normal endometrium
tissue. Loss of FOXO1 expression promotes uncontrolled EC cell
proliferation.
Sterol regulatory element-binding proteins (SREBPs)
regulate the expression of lipogenic genes and are members of the
basic helix-loop-helix leucine zipper family (3). The family has three different isoforms:
SREBP1a, SREBP1c and SRBEP2 (3). Each
isoforms has different effects; SREBP1 is the primary SREBP and it
selectively regulates intracellular lipid homeostasis by
controlling the synthesis of fatty acids and triglycerides
(16). High expression of SREBP1 is
directly correlated with tumorigenesis in several forms of cancer,
including prostate, breast and pancreatic cancer (17–19).
SREBP1 is also a target of insulin; insulin is able to activate
transcription of the gene encoding SREBP1 by increasing the
activity of liver X receptors (16).
Using IHC staining, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blotting, a previous study
observed a significant increase in SREBP1 protein expression in EC
samples and in poorly-differentiated EC cells (3). Similarly, in vitro and in
vivo research has demonstrated that SREBP1-knockdown is able to
reduce proliferation and induce apoptosis in EC cells (3).
Despite the importance of FOXO1 and SREBP1 in EC,
they are also important in lipogenesis and may be regulated by
insulin. However, the association between FOXO1 and SREBP1 in EC
remains unclear. Therefore, the present study aimed to elucidate
this association.
Materials and methods
Cell lines and reagents
A total of six human EC cells (Ishikawa, AN3 CA,
HEC-1-A, SPEC-2, RL95-2 and KLE) were purchased from the American
Type Culture Collection (ATCC; Rockville, MD, US). Fetal bovine
serum (FBS), Dulbecco's modified Eagle's medium (DMEM)/high glucose
medium, minimal essential medium (MEM), McCoy's 5A medium and
DMEM/F12 medium were purchased from Gibco, Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Anti-FOXO1 antibodies (no.
ab39670) were purchased from Abcam (Cambridge, UK), anti-SREBP1
antibodies (H-160; no. sc-8984) from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) and anti-GAPDH antibodies (no. G9545) from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Cell culture
All cells were incubated at 37°C in 5%
CO2. The Ishikawa and AN3 CA cells were cultured in
DMEM/high glucose medium, the RL95-2 and KLE cells in DMEM/F12
medium, the SPEC-2 cells in MEM and the HEC-1-A cells in McCoy's 5A
medium. All cell culture media were supplemented with FBS at a
concentration of 10%.
Cell transfection and
transduction
Lentiviruses expressing green fluorescent
(GFP)-tagged proteins with a human FOXO1 overexpression vector
(group named as LV-FOXO1) and lentiviruses with a control vector
(group named as LV-CON) were constructed and prepared for
transfection by GeneChem Co., Ltd. (Shanghai, China). The FOXO1
low-expression cell lines [Ishikawa (well-differentiated EC cells)
and AN3 CA (poorly-differentiated EC cells)] were screened for
transfection according to western blot analysis. Prior to
transduction, cells were incubated on 6-well plates for 24 h until
adherence. Lentiviruses at multiplicity of infection (MOI) 50 were
transduced into cells. At 10 h post-transduction, the culture
medium was replaced to normal medium. A total of 72 h were required
for stable cell transfection. The whole transduction was performed
in accordance with the manufacturer's protocol (GeneChem Co.,
Ltd.). After 72 h, a fluorescence microscope was used to observe
GFP-positive cells and western blotting was performed to determine
the transduction efficiency.
Western blot analysis
Six types of human EC cells (Ishikawa, AN3 CA,
HEC-1-A, SPEC-2, RL95-2 and KLE) were washed with 1X PBS, harvested
and lysed with radio immunoprecipitation assay buffer (RIPA buffer,
1% NP40, 1X PBS, 0.1% SDS, 5 mM EDTA, 1 mM sodium orthovanadate and
0.5% sodium deoxycholate) containing phenylmethanesulfonyl fluoride
(dilution, 1:100) as a protease inhibitor. The mixture was placed
on ice for 30 min for complete lyse, and was subsequently
centrifuged at 13,800 × g for 15 min at 4°C. The suspensions
were carefully collected and tested for protein concentration using
a BCA Protein assay kit (no. p0010; Beyotime Institute of
Biotechnology, Haimen, China) [containing reagent A, reagent B and
bovine serum albumin (BSA); reagent A contained sodium carbonate,
sodium bicarbonate, bicinchoninic acid and sodium tartrate in 0.1 M
sodium hydroxide; reagent B contained 4% cupric sulfate; BSA at 2.0
mg/ml in 0.9% saline and 0.05% sodium azide]. Proteins were
resolved with SDS-PAGE loading buffer and 30 µg each sample was
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with non-fat milk
(5%) for 2 h at room temperature and then incubated with the
primary antibodies against FOXO1 (dilution, 1:500), SREBP1
(dilution, 1:1,000) and GAPDH (dilution, 1:1,000) at 4°C overnight.
Next, the membranes were washed with TBST, and incubated with
horseradish peroxidase-coupled rabbit IgG secondary antibody
(dilution, 1:5,000; no. 074-1506; Kirkegaard & Perry
Laboratories, Inc., Gaithersburg, MD, USA) for 2 h at room
temperature. Membranes were washed with TBST and analyzed using
ImagemQuant™ LAS 4000 with enhanced chemiluminescence. The protein
signals were analyzed with ImageJ software (https://imagej.nih.gov/ij/) and protein levels were
compared with those of GAPDH.
Cell proliferation and clonogenic
assay
MTT assay was used to assess cell proliferation.
Ishikawa (LV-FOXO1 and LV-CON) and AN3 CA (LV-FOXO1 and LV-CON)
were seeded in 96-well plates at 4,000 and 3,000 cells/well
respectively, and attached overnight. Cells were subsequently
incubated at 37°C for 1–5 days, and 20 µl 5 mg/ml MTT was added
each day to each well at the specified time. Following further
incubation for 4 h at 37°C, the supernatants were carefully
discharged and replaced with 100 µl dimethyl sulfoxide.
Infinite® 200 PRO NanoQuant (Tecan Group Ltd.,
Männedorf, Switzerland) were used to read the absorbance values at
570 nm. To determine clonogenic ability, 400 cells of each group
were allowed to grow for 14 days on 6-well plates to form colonies.
When distinguished by the naked eye, crystal violet (2%, w/v;
Sigma-Aldrich; Merck Millipore) were used to stain colonies of
clone formation and clone numbers were subsequently counted under
an inverted microscope.
Migration and invasion assay
Transwell systems with polycarbonate membranes
(24-well, 8 mm size pore; Costar; Corning Incorporated, Corning,
NY, USA) were used to perform migration and invasion assay.
Matrigel (BD Biosciences, San Jose, CA, US) was also used to coat
the membranes in the invasion assay. A total of 200 µl medium free
from FBS with 1.5×105 cells were added to the upper
well, and 700 µl medium with 20% FBS was added to the lower
chamber. The cells were incubated for 24 h for the migration assay
and 48 h for the invasion assay. Cells that had adhered to the
lower well were stained with crystal violet and counted under an
inverted microscope for six random visual fields.
In vivo tumorigenesis
Two groups of stable transfected AN3 CA cells
(LV-FOXO1 and LV-CON) were used to perform in vivo
tumorigenesis. Once cells had grown to 70–80% density, they were
digested and counted. A total of 8×106 cells from each
group were suspended in 200 µl PBS (mixture with Matrigel at 3:1).
The cells were subsequently injected into the subcutaneous flank of
4–5-week-old BALB/c-nu/nu female mice (raised in a specific
pathogen-free laboratory; 18–20 g; 7 mice/group). Following
injection, the diameters of the transplanted tumors [length (L) and
width (W)] were measured every 4 days using a slide caliper. Tumor
volume was calculated as (L × W2)/2. At day 28
post-injection, the mice were sacrificed under anesthesia, and the
tumors were separated, collected and weighed. The Animal Care and
Use Committee of Shandong University (Jinan, China) approved all
animal experiments.
Statistical analysis
SPSS v17.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Data are presented as the mean ± standard
deviation, and each experiment was repeated three times. Student's
two-tailed t-test was used to determine statistical significance of
two groups, and P<0.05 was considered to indicate a
statistically significant difference.
Results
FOXO1 overexpression suppresses
Ishikawa and AN3 CA cell proliferation and colonigenic ability in
vitro
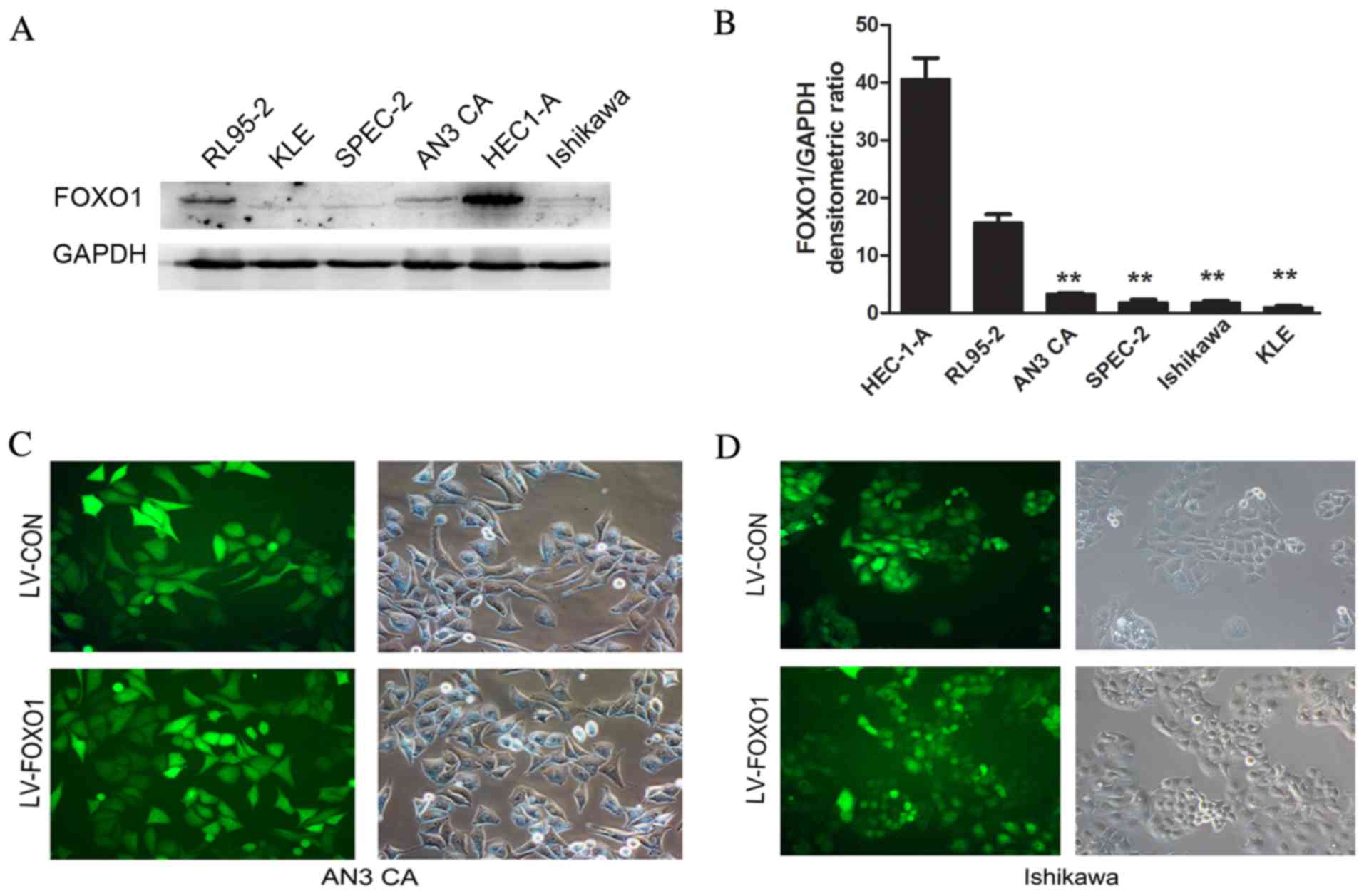
FOXO1 expression was analyzed in six different human
EC cells (Ishikawa, AN3 CA, HEC-1-A, SPEC-2, RL95-2 and KLE). From
western blot analysis, it was observed that the expression of FOXO1
in the AN3 CA, SPEC-2, Ishikawa and KLE cells was lower than that
observed in the HEC-1-A and RL95-2 cells (Fig. 1A and B). Thus, the two differentiated
cell lines (Ishikawa and AN3 CA) were selected for further
experiments. Following transfection, GFP-positive cells accounted
for >90% of the total cells observed by fluorescence microscopy
in the AN3 CA (Fig. 1C) and Ishikawa
(Fig. 1D) cells. This certified that
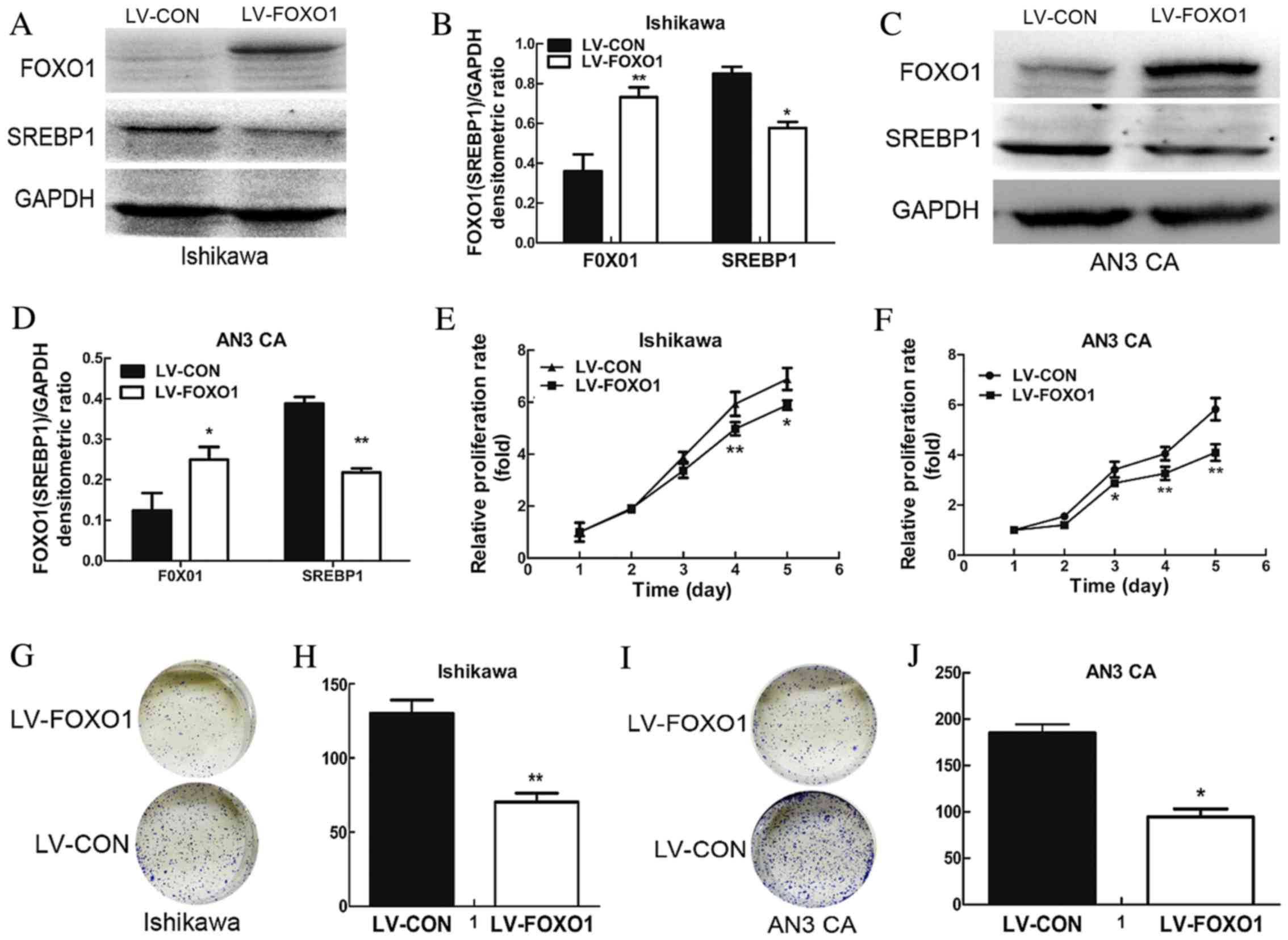
transfection was successful. To further detect the efficiency of
transduction, western blotting was performed. The results
demonstrated a significant increase in the expression of FOXO1
protein in the LV-FOXO1 group compared with the LV-CON group for
both the Ishikawa (P<0.01; Fig. 2A and
B) and AN3 CA cells (P<0.05; Fig.
2C and D).
A cell viability assay was performed using MTT to
examine the effect of FOXO1 on cell growth. The results
demonstrated that FOXO1 overexpression was able to significantly
suppress proliferation of the Ishikawa (day 4, P=0.004 and day 5,
P=0.034; Fig. 2E) and AN3 CA cells
(day 3, P=0.016, day 4, P=0.006 and day 5, P=0.001; Fig. 2F) when compared with the LV-CON group.
To further investigate this, colonigenic assay was performed to
evaluate the oncogenic potential of FOXO1. It was observed that the
colonigenic ability of the LV-FOXO1 group was significantly reduced
in the Ishikawa (P=0.005; Fig. 2G and
H) and AN3 CA (P=0.013; Fig. 2I and
J).
FOXO1 overexpression suppresses
Ishikawa and AN3 CA cell migration and invasion in vitro
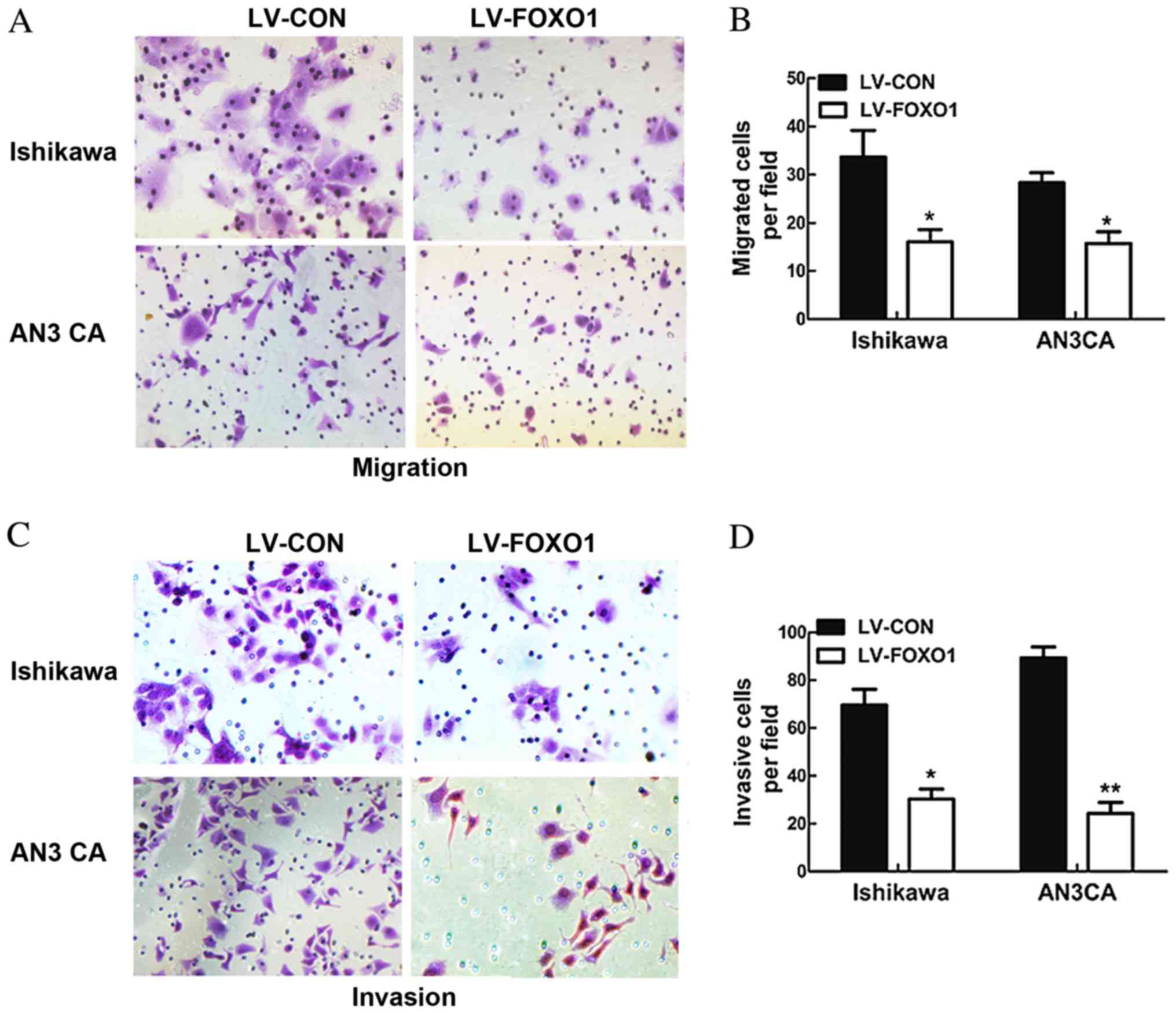
In order to observe the potential impact of FOXO1
overexpression on the migratory and invasive ability of Ishikawa
and AN3 CA cells, a Transwell assay was performed. The results
demonstrated that the migratory abilities of the LV-FOXO1 group
were significantly inhibited in the Ishikawa (P=0.034) and AN3 CA
(P=0.029; Fig. 3A and B) cells, and
the invasive abilities of the LV-FOXO1 group was also significantly
inhibited in the Ishikawa (P=0.019) and AN3 CA cells compared with
the LV-CON group (P=0.003; Fig. 3C and
D).
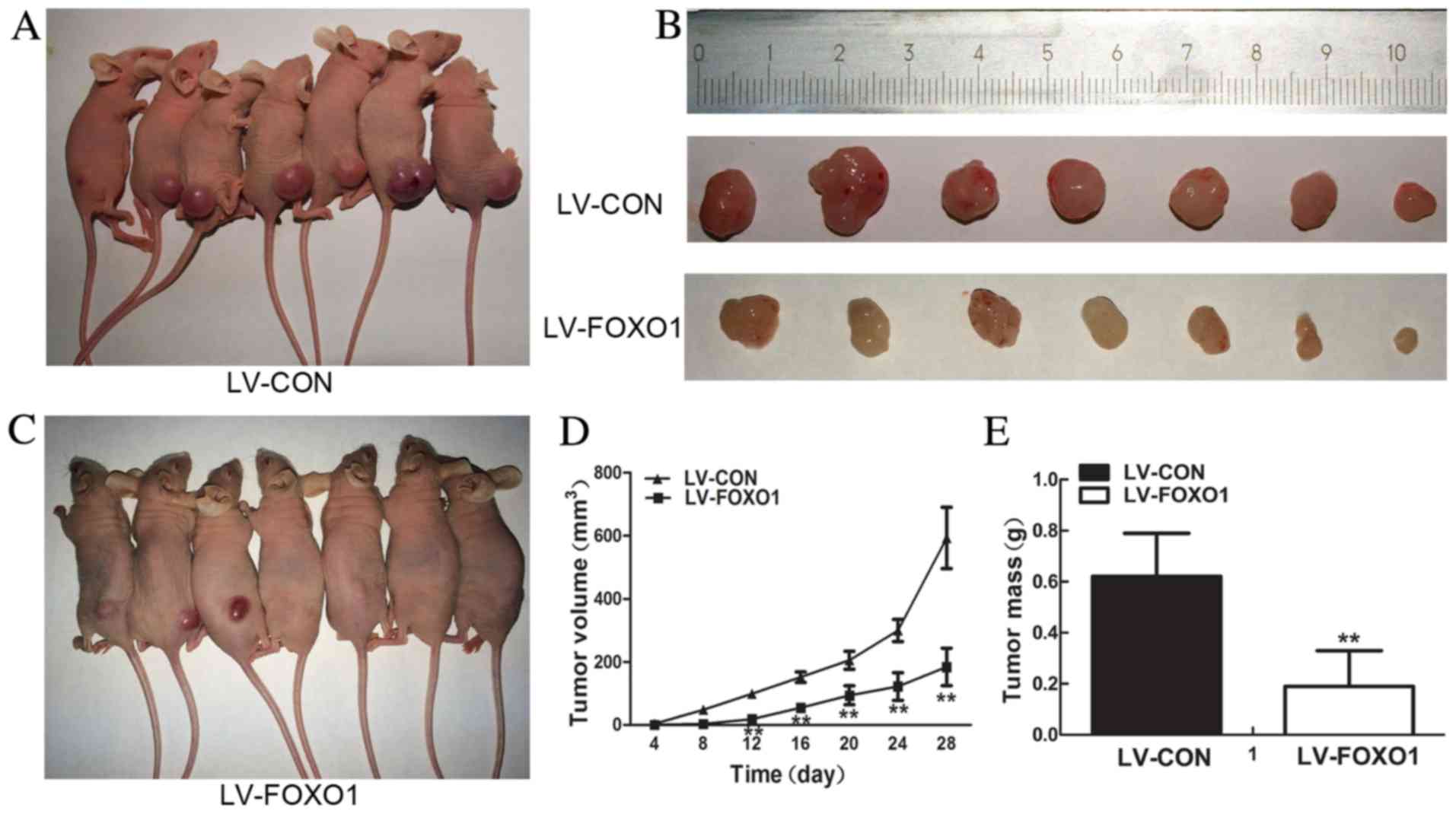
FOXO1 suppresses AN3 CA cell
tumorigenesis in a xenograft model
FOXO1 overexpression suppressed the proliferation,
and colonigenic abilities of AN3 CA cells in vivo. To
investigate the role of FOXO1 expression in cell proliferation, an
in vivo experiment was constructed. A xenograft model of
human EC was used. Stably transfected AN3 CA (LV-CON) were injected
subcutaneously into the flank of nude mice and day 28
post-injection tumors were separated (Fig. 4A and B), and stably transfected AN3 CA
(LV-FOXO1) were injected into the flank of nude mice and then
tumors were separated (Fig. 4B and
C). The results demonstrated that LV-FOXO1 group compared with
LV-CON group FOXO1 evidently inhibited tumor formation and growth
(tumor size at day 12, day 16, day 20, day 24 and day 28
post-injection, P=0.00; Fig. 4D;
tumor weight, P=0.00; Fig. 4E).
FOXO1 inhibits Ishikawa and AN3 CA
cell migration and invasion by targeting SREBP1
FOXO1 and SREBP1 are crucial in lipid metabolism
(7,19). The present study speculated that FOXO1
functions though cross-talk to SREBP1, therefore, western blot
analysis was performed to investigate the association between them.
It was concluded that SREBP1 protein expression was markedly
decreased in the LV-FOXO1 group in the Ishikawa (P=0.016; Fig. 2A and B) and AN3 CA (P=0.005; Fig. 2C and D). Therefore, overexpression of
FOXO1 is able to downregulate the expression of SREBP1.
Discussion
As a member of the forkhead box transcription factor
family, FOXO1 has gained increasing attention from researchers in
recent years. The transcription factor is a key regulator of cell
fate, regulating cell differentiation, cell cycle arrest, apoptosis
and defense responses against oxidative stress (20). Loss of FOXO1 expression may result in
uncontrolled cell proliferation and lead to tumorigenesis, which
has been reported in various forms of cancer, including ovarian
cancer (21), prostate cancer
(22,23), lung cancer (24), breast cancer (25–27) and
gastric cancer (28). Furthermore,
downregulation of FOXO1 serves an important role in EC
tumorigenesis (15,29,30).
FOXO1 is a downstream target of the phosphatase and
tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/Akt pathway
(31). Downregulation and dysfunction
of PTEN/PI3K/Akt signaling is a hallmark of EC (31). Research has demonstrated that a ~55%
decrease in PTEN expression may be observed in total endometrial
lesions and ~80% of PTEN is inactivated in EC cases (32). Low PTEN expression is considered to be
an early event of EC (33,34). Activation of the PTEN/PI3K/Akt pathway
leads to the Akt-dependent phosphorylation of FOXO1, which
subsequently promotes FOXO1 translocation from the nucleus to the
cytoplasm, resulting in the inhibition of FOXO1 transcription
activation (35). This process is
important for cell apoptosis and differentiation (36). Ward et al (14) demonstrated that FOXO1 exhibited lower
expression levels in EC samples compared with normal endometrium
tissue. Loss of FOXO1 expression promotes uncontrolled EC cell
proliferation. An additional study demonstrated that FOXO1
expression levels were high in HEC-1B cells and low in Ishikawa
cells (15), which is consistent with
the results of the present study. Furthermore, the present study
identified that in vitro overexpression of FOXO1 was able to
suppress the proliferative, colonigenic, migratory and invasive
ability of Ishikawa and AN3 CA cells, and in vivo FOXO1
overexpression was able to suppress AN3 CA cell proliferation.
These results were in line with previous studies investigating in
EC (14,15), supporting the notion that FOXO1 is a
tumor suppressor in EC.
Obesity is strongly associated with EC (37). One meta-analysis reported that when
body mass index increased per 5 kg/m2, the risk of a
woman developing EC increased by 59% (38). Ectopic lipid metabolism serves an
important role in the formation of endometrial cancer (38).
SREBP1 coded by SREBP1, also known as
adipocyte determination and differentiation dependent factor 1, is
a transcription factor that primarily regulates lipid homeostasis
by targeting genes in cholesterol and fatty acid synthesis
(16). Intracellular sterol level is
able to control the function of SREBP1. When sterol decreases
within the cell, inactive precursors of SREBP1 transport to the
Golgi apparatus where they are cleaved and become active; these are
then released into the nucleus where they target genes involved in
cholesterol biosynthesis (39). By
contrast, when the level of sterol is high, SREBP1 remains
inactive, thus maintaining a balance between sterol level and FA
metabolism (39,40). Several studies have demonstrated that
high SREBP1 expression may result in tumor formation, including
prostate (17), breast (18) and colon (41) cancer. In addition, a previous study
reported that SREBP1 was overexpressed in EC and resulted in
tumorigenesis (3). And another
research of my group found that SIRT1 can regulate the lipogenesis
by targeting the expression of SREBP1 in EC (42).
FOXO1 and SREBP1 are important in the lipogenesis
and tumorigenesis of EC, and are all targets of insulin; thus, the
present study speculated that there may be some relevance. Western
blot analysis was performed and the results demonstrated that the
protein level of SREBP1 in Ishikawa and AN3 CA transduced with
lentiviruses containing FOXO1 overexpression vectors was lower than
the control group. A previous study reported that FOXO1 is able to
directly repress SREBP-1 expression in hepatic lipogenesis
(43). In addition, the present study
supported the hypothesis that increased FOXO1 expression decreases
the level of SREBP1.
In conclusion, the present study demonstrated that
FOXO1 is important in EC progression. High expression of FOXO1 is
able to inhibit the capacity of EC proliferation in vitro
and in vivo, in addition to inhibiting migration and
invasion in vitro via SREBP1. This may possibly identify
novel therapeutic target in EC, with further studies required to
clarify the molecular mechanisms by which FOXO1 suppress SREBP1
expression in EC.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (nos. 81372808 and 81173614), the
Technology Development planning of Shandong (no. 2012G0021823) and
the Science and Technology Developing Planning of Jinan (no.
201303035).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li W, Tai Y, Zhou J, Gu W, Bai Z, Zhou T,
Zhong Z, McCue PA, Sang N, Ji JY, et al: Repression of endometrial
tumor growth by targeting SREBP1 and lipogenesis. Cell Cycle.
11:2348–2358. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipogenesis in cancer cells: New players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Modesitt SC, Hsu JY, Chowbina SR, Lawrence
RT and Hoehn KL: Not all fat is equal: Differential gene expression
and potential therapeutic targets in subcutaneous adipose, visceral
adipose, and endometrium of obese women with and without
endometrial cancer. Int J Gynecol Cancer. 22:732–741. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang W, Patil S, Chauhan B, Guo S, Powell
DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al: FoxO1
regulates multiple metabolic pathways in the liver: Effects on
gluconeogenic, glycolytic, and lipogenic gene expression. J Biol
Chem. 281:10105–10117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kousteni S: FoxO1, the transcriptional
chief of staff of energy metabolism. Bone. 50:437–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho KK, Myatt SS and Lam EW: Many forks in
the path: Cycling with FoxO. Oncogene. 27:2300–2311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen C, Xu T, Zhou J, Yan Y, Li W, Yu H,
Hu G, Ding X, Chen J and Lu Y: High cytoplasmic FOXO1 and pFOXO1
expression in astrocytomas are associated with worse surgical
outcome. PLoS One. 8:e692602013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berry E, Hardt JL, Clardy J, Lurain JR and
Kim JJ: Induction of apoptosis in endometrial cancer cells by
psammaplysene A involves FOXO1. Gynecol Oncol. 112:331–336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barbato D Lettieri, Aquilano K and Ciriolo
MR: FoxO1 at the nexus between fat catabolism and longevity
pathways. Biochim Biophys Acta. 1555–1560. 2014. View Article : Google Scholar
|
|
13
|
Pavlidou A and Vlahos NF: Molecular
alterations of PI3K/Akt/mTOR pathway: A therapeutic target in
endometrial cancer. Scientific World Journal. 709–736. 2014.
|
|
14
|
Ward EC, Hoekstra AV, Blok LJ,
Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC and
Kim JJ: The regulation and function of the forkhead transcription
factor, Forkhead box O1, is dependent on the progesterone receptor
in endometrial carcinoma. Endocrinology. 149:1942–1950. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goto T, Takano M, Albergaria A, Briese J,
Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M,
et al: Mechanism and functional consequences of loss of FOXO1
expression in endometrioid endometrial cancer cells. Oncogene.
27:9–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Liang G, Ou J, Goldstein JL and
Brown MS: Central role for liver X receptor in insulin-mediated
activation of Srebp-1c transcription and stimulation of fatty acid
synthesis in liver. Proc Natl Acad Sci USA. 101:11245–11250. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heemers H, Maes B, Foufelle F, Heyns W,
Verhoeven G and Swinnen JV: Androgens stimulate lipogenic gene
expression in prostate cancer cells by activation of the sterol
regulatory element-binding protein cleavage activating
protein/sterol regulatory element-binding protein pathway. Mol
Endocrinol. 15:1817–1828. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Morin PJ, Han WF, Chen T, Bornman
DM, Gabrielson EW and Pizer ES: Regulation of fatty acid synthase
expression in breast cancer by sterol regulatory element binding
protein-1c. Exp Cell Res. 282:132–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, He W, Luo M, Zhou Y, Chang G, Ren
W, Wu K, Li X, Shen J, Zhao X and Hu Y: SREBP1 regulates
tumorigenesis and prognosis of pancreatic cancer through targeting
lipid metabolism. Tumour Biol. 36:4133–4141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao E and Graves DT: Impact of diabetes
on the protective role of FOXO1 in wound healing. J Dent Res.
94:1025–1026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Yang H, Li W, Xu H, Yang X and Gan
L: Thioredoxin 1 upregulates FOXO1 transcriptional activity in drug
resistance in ovarian cancer cells. Biochim Biophys Acta.
1852:395–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Y, Tindall DJ and Huang H: Modulation
of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer.
Int J Biol Sci. 10:614–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu JJ, Wu YX, Zhao FJ and Xia SJ: miR-96
promotes cell proliferation and clonogenicity by down-regulating of
FOXO1 in prostate cancer cells. Med Oncol. 31:9102014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao JG, Ren KM and Tang J: Zinc finger
protein ZBTB20 promotes cell proliferation in non-small cell lung
cancer through repression of FoxO1. FEBS Lett. 588:4536–4542. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu F, Jin L, Yang G, Ji L, Wang F and Lu
Z: Post-transcriptional repression of FOXO1 by QKI results in low
levels of FOXO1 expression in breast cancer cells. Oncol. Rep.
31:1459–1465. 2014.PubMed/NCBI
|
|
26
|
Yang J, Li T, Gao C, Lv X, Liu K, Song H,
Xing Y and Xi T: FOXO1 3′UTR functions as a ceRNA in repressing the
metastases of breast cancer cells via regulating miRNA activity.
FEBS Lett. 588:3218–3224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Lin C, Zhao X, Liu A, Zhu J, Li X
and Song L: Acylglycerol kinase promotes cell proliferation and
tumorigenicity in breast cancer via suppression of the FOXO1
transcription factor. Mol Cancer. 13:1062014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu DA, Yoon J, Ko YS, Park J, Kim SY, Kim
MA, Kim JH, Jung J, Cheon Y, Lee HS, et al: Forkhead transcription
factor FOXO1 inhibits nuclear factor-κB in gastric cancer. APMIS.
122:848–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Myatt SS, Wang J, Monteiro LJ, Christian
M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S and Lam EW:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korani M, Fallah S, Tehranian A,
Nourbakhsh M, Samadikuchaksaraei A, Pour MS and Maleki J: The
evaluation of the FOXO1, KLF9 and YT521 genes expression in human
endometrial cancer. Clin Lab. 59:483–489. 2013.PubMed/NCBI
|
|
31
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers, and the implications for
pathogenesis, classification and targeted therapies. Cancer
Control. 16:8–13. 2009.PubMed/NCBI
|
|
32
|
Ali IU: Gatekeeper for endometrium: The
PTEN tumor suppressor gene. J Natl Cancer Inst. 92:861–863. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: The PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mutter GL, Lin MC, Fitzgerald JT, Kum JB,
Baak JP, Lees JA, Weng LP and Eng C: Altered PTEN expression as a
diagnostic marker for the earliest endometrial precancers. J Natl
Cancer Inst. 92:924–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Modur V, Nagarajan R, Evers BM and
Milbrandt J: FOXO proteins regulate tumor necrosis factor-related
apoptosis inducing ligand expression. Implications for PTEN
mutation in prostate cancer. J Biol Chem. 277:47928–47937. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmandt RE, Iglesias DA, Co NN and Lu KH:
Understanding obesity and endometrial cancer risk: Opportunities
for prevention. Am J Obstet Gynecol. 205:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shimano H: Sterol regulatory
element-binding proteins (SREBPs): Transcriptional regulators of
lipid synthetic genes. Prog Lipid Res. 40:439–452. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Horton JD and Shimomura I: Sterol
regulatory element-binding proteins: Activators of cholesterol and
fatty acid biosynthesis. Curr Opin Lipidol. 10:143–150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schønberg SA, Lundemo AG, Fladvad T,
Holmgren K, Bremseth H, Nilsen A, Gederaas O, Tvedt KE, Egeberg KW
and Krokan HE: Closely related colon cancer cell lines display
different sensitivity to polyunsaturated fatty acids, accumulate
different lipid classes and downregulate sterol regulatory
element-binding protein 1. FEBS J. 273:2749–2765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin L, Zheng X, Qiu C, Dongol S, Lv Q,
Jiang J, Kong B and Wang C: SIRT1 promotes endometrial tumor growth
by targeting SREBP1 and lipogenesis. Oncol Rep. 32:2831–2835.
2014.PubMed/NCBI
|
|
43
|
Deng X, Zhang W, O-Sullivan I, Williams
JB, Dong Q, Park EA, Raghow R, Unterman TG and Elam MB: FoxO1
inhibits sterol regulatory element-binding protein-1c (SREBP-1c)
gene expression via transcription factors Sp1 and SREBP-1c. J Biol
Chem. 287:20132–20143. 2012. View Article : Google Scholar : PubMed/NCBI
|