Introduction
Renal cell carcinoma (RCC) is a common urological
malignancy, and local invasion and metastasis are detected
frequently at diagnosis or following radical surgery (1). While radical nephrectomy is typically
performed during the organ-confined clinical tumor stages, disease
relapse develops in approximately 10% of patients following surgery
(2). In addition, a previous study
has demonstrated that the median survival of patients with
metastatic RCC is ~13 months (3). In
recent years, various molecular targeting agents have been used in
the treatment of patients with advanced RCC (4). The anti-cancer effects of these agents,
including prolonged survival, are more effective compared with
those of other forms of therapy, such as immunotherapy (4,5). However,
the effective period of these molecular targeting therapies is
short, and the frequency and severity of adverse reactions are
relatively high (5). Thus, further
research into the underlying molecular mechanisms of RCC cell
invasion and metastasis is required for the development of
observational and therapeutic strategies.
The malignant characteristics of RCC are known to be
regulated by multiple molecules and signaling pathways. Increased
expression of Fps/Fes related (Fer) in cancer cells was previously
reported to be associated with high malignant aggressiveness and
poor survival in patients with RCC (6). Fer was originally isolated as a
tyrosine-protein kinase, which belongs to the subgroup IV of the
non-receptor tyrosine-protein kinase family, and is ubiquitously
expressed in the cytoplasm and nucleus of a number of mammalian
cells (7). Notably, it is well
established that Fer is expressed in hematopoietic cells, immune
cells and endothelial cells, where it regulates their biological
functions (8–10). Fer has been demonstrated to regulate
cell proliferation, migration and adhesion, in fibroblasts and
various types of immune cells (9,11–13). Conversely, Fer is known to be
associated with malignant aggressiveness in several types of
cancer, including increased cell proliferation, invasion and
metastasis (14–16).
Numerous studies have examined the role of
cancer-associated genes, messenger RNAs and proteins in cancer
cells, to determine their pathological characteristics, prognostic
value and potential for use in targeted therapy (4,17). In
recent years, the surrounding cancer-associated stromal cells have
also been implicated in tumor development and progression (18). While the pathological and prognostic
significance of Fer expression in cancer cells has been
investigated, the role of Fer expression in RCC tumor-associated
stromal cells, including fibroblasts and immune cells, has not been
studied thus far. Therefore, the primary aim of the present study
is to determine the association between cancer-associated stromal
cell Fer expression, and the pathological features, malignant
potential and survival rate of patients with RCC. Furthermore, the
association between stromal cell Fer expression and cell
proliferation, apoptosis, angiogenesis, and macrophage and natural
killer (NK) cell density, was investigated in human RCC tissue
samples.
Materials and methods
Patients
Formalin-fixed and paraffin-embedded sections were
obtained from surgical specimens from Nagasaki University Hospital
(Nagasaki, Japan), between January 1991 and December 2007.
Consecutive specimens were used in the present study; however,
certain specimens were not analyzed due the low number of cancer
cells (<500) resulting from their use in previous investigations
(6,19,20).
Patients who received neo-adjuvant therapy, including immunotherapy
and molecular targeting therapy, were excluded. Tissue samples from
152 patients with RCC, comprising 110 males and 42 females, were
analyzed. The mean ± standard deviation (SD) and median ages at
diagnosis were 60.7±12.2 and 61 years, respectively. All patients
were evaluated using chest X-ray, ultrasonography and computed
tomography (CT). In addition, CT of the lung or brain, and magnetic
resonance imaging was performed when metastasis is suspected.
Tumors were staged according to the 2002 Tumor-Node-Metastasis
Classification of Malignant Tumours (21), and the grade was determined using the
criteria set out by Fuhrman et al (22). In the present study, tumors were
categorized into the following groups for statistical analysis:
Low-[pathological tumor (pT) stages 1 and 2] and high-stage (pT3
and 4), or low-(grades 1 and 2) and high-grade (grades 3 and 4).
The present study comprised 129 patients with conventional RCC, 11
with chromophobe RCC and 12 with papillary RCC. The mean ± SD
follow-up period was 43.3 (39.7) months, and 40 patients (26.3%)
succumbed to disease-specific causes. In addition, 30 wild-type
kidney tissue samples obtained from patients with transitional cell
carcinoma of the ureter were examined. The present study protocol
approved by the ethical standards of the Human Ethics Review
Committee of Nagasaki University School of Medicine (Nagasaki,
Japan; approval no. 12052899-2). Written informed consent was
obtained from all patients prior to enrollment.
Immunohistochemistry
The following antibodies were used in the
immunohistochemical staining: Anti-Fer (1:80; no. HPA007641;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany),
anti-proliferation marker protein Ki-67 (1:100; no. 7240; Dako,
Glostrup, Denmark), anti-cleaved caspase-3 (1:100; no. MAB835;
R&D Systems Europe, Ltd., Abingdon, UK), anti-cluster of
differentiation (CD) 31 (1:60: no. NCL-CD31-1A10P; Leica
Microsystems, Ltd., Milton Keynes, UK), anti-CD68 (1:100; no.
NCL-CD68; Leica Microsystems, Ltd.) and anti-CD57 (1:200; no.
MS-136; Lab Vision Corporation, Fremont, CA, USA). The 5-µm-thick
sections were stepwise deparaffinized in xylene and rehydrated in
graded ethanol solutions. With the exception of the anti-Ki-67
antibody, antigen retrieval was performed at 95°C for 40 min. For
the anti-Ki-67 antibody, antigen retrieval was performed at 121°C
for 15 min in 0.01 M sodium citrate buffer (pH 6.0). All sections
were subsequently immersed in 3% hydrogen peroxide for 30 min at
room temperature to block endogenous peroxidase activity. The
sections were next incubated overnight with the primary antibody at
4°C and subsequently washed in 0.05% Tween-20 in PBS. The sections
were then incubated at room temperature with peroxidase, according
to the manufacturer's labeled polymer method, using Dako EnVision+™
Peroxidase (Dako) for 60 min. The peroxidase reaction was
visualized using the Pierce DAB Substrate kit (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The sections were
counterstained using hematoxylin, dehydrated stepwise with graded
alcohol solutions and washed in xylene, prior to mounting with
Poly-Mount® (Polysciences, Inc., Warminster, PA, USA). A
number of specimens, previously confirmed to be Ki-67, CD57, CD68
(all tonsil), cleaved caspase-3 (prostate cancer tissue following
hormone therapy), and CD31 and Fer (kidney) immunoreactive were
used as positive controls. To detect apoptotic cells, in
situ apoptotic cell labeling was performed as previously
described (23). The
ApopTag® In Situ Apoptosis Detection kit
(Intergen Company, L.P., Purchase, NY, USA), which is based on the
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) method, was used according to the manufacturer's protocol.
As positive control for the TUNEL method, prostate cancer tissue
following hormone therapy was used. These prostate specimens were
obtained from Nagasaki University Graduate School of Biomedical
Sciences (Nagasaki, Japan) and their reliability was confirmed in
our previous study (24). Positive
and negative control sections were prepared. The negative control
consisted of a consecutive section from each sample processed
without the primary antibody. Positive and negative controls were
set up for each set of experiments.
Evaluation
The expression of all molecules was assessed
semi-quantitatively using the percentage of positively stained
cancer cells in randomly selected 200 high-power fields (HPFs).
Similarly, the densities of positively stained vessels and stromal
cells were examined in five HPFs within the tumor area. When the
stromal area was small, ≤10 fields were evaluated. In the present
study, Fer expression in stromal cells was divided into the
following three groups according to the area of positively stained
stromal cells: Low (<25%), middle (25–50%) and high (>50%).
Fer expression was evaluated in cancer cells as described
previously (3). The proliferative
index (PI) represented the percentage of Ki-67-positive cells. The
apoptotic index (AI) was estimated using the percentage of
TUNEL-positive cells, and was confirmed by the proportion of
cleaved caspase-3-positive cells. The microvessel density (MVD) was
defined as the number of positively stained vessels/mm2.
The number of positively stained cells/mm2 defined the
densities of CD57- and CD68-stained cells. All slides were examined
using an E-400 microscope (Nikon Corporation, Tokyo, Japan).
Furthermore, a computer-aided image analysis system (Win ROOF
version 5.0; Mitani Corporation, Fukui, Japan) was used to
calculate the statistical variables. Each slide was evaluated twice
by three independent investigators.
Statistical analysis
Values are expressed as the mean ± SD. The Student's
t-test was used to compare the continuous variables. The
χ2 test was used for categorical comparison of the data,
while the Scheffé's method was used for multiple comparisons of the
data. Survival comparisons were performed using the Kaplan-Meier
estimator and the log-rank test. In the survival analysis, a
multivariate analysis using the Cox proportional hazards model
including all pathological features was conducted. The results are
described as the hazard ratio (HR), 95% confidence interval (CI)
and P-value. All statistical analyses were two-sided and were
performed using the statistical package StatView for Windows
version 5.0 (Abacus Concepts, Berkeley, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Fer expression
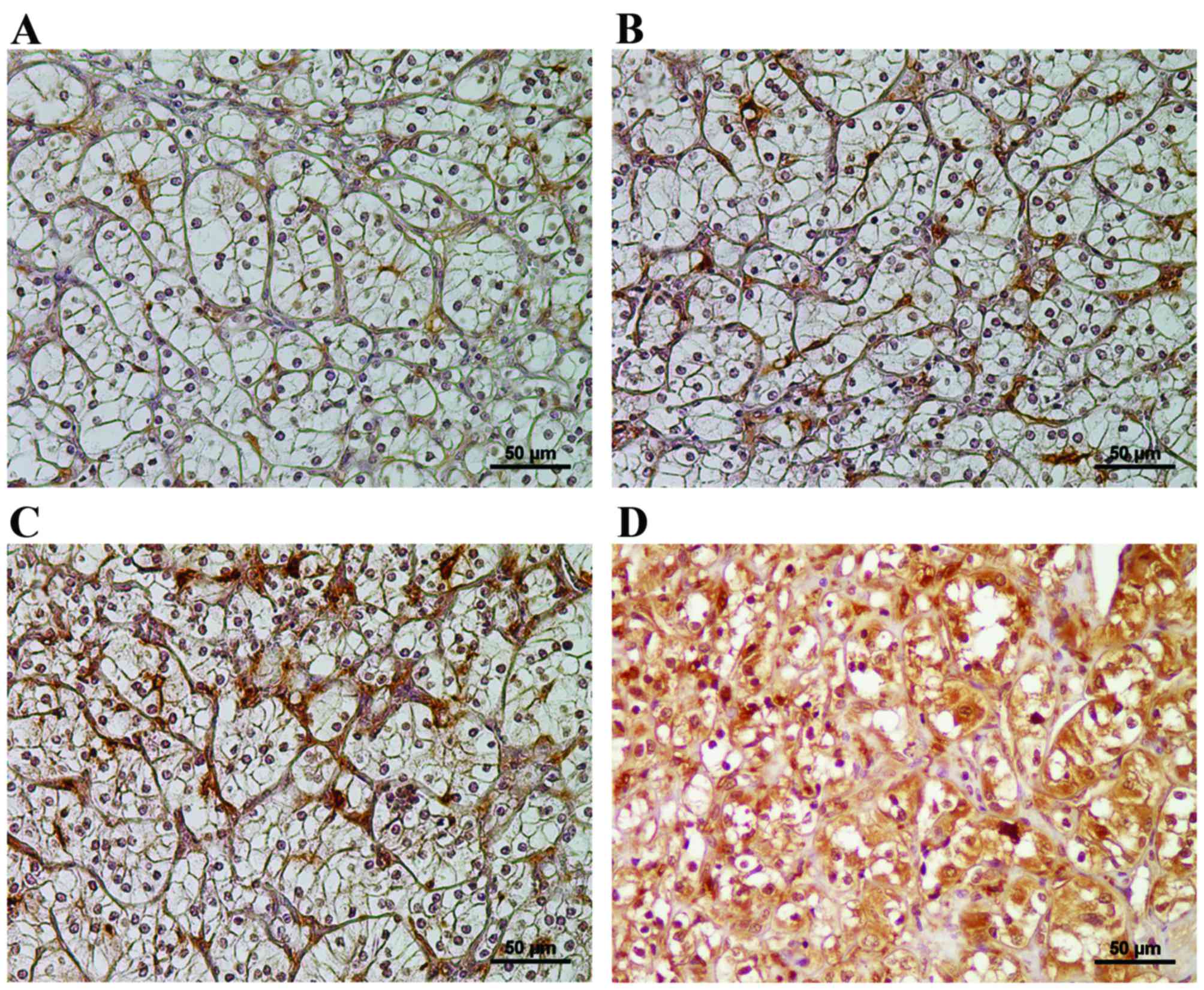
Representative images of low, middle and high Fer
expression in RCC stromal cells are shown in Fig. 1A-C, respectively. Fer-stained immune
and fibroblast-like cells were observed in the RCC-associated
stromal tissue. A total of 49 (32.2%), 51 (33.6%) and 52 (34.2%)
patients were considered to be in the low, middle and high
Fer-expressing groups, respectively. All specimens contained
Fer-expressing stromal cells, while conversely, Fer-stained
endothelial cells were rare and identification of Fer-stained
vessels was difficult. In addition to stromal tissues, Fer
expression was detected in the cytoplasm of cancer cells (Fig. 1D). A total of 38/49 patients (74.5%)
in the low stromal Fer-expressing group exhibited high Fer
expression in their cancer cells. By contrast, 33/52 patients
(63.5%) exhibited high stromal Fer expression and low cancer cell
Fer expression, thus suggesting a significant negative association
between Fer expression in stromal tissue and cancer tissue
(P<0.001).
Association between Fer expression and
pathological characteristics
As presented in Table
I, increased Fer expression in stromal cells was significantly
associated with increased Fuhrman grade, pT stage, and lymph node
and distal metastasis (P<0.001). In the present study, a total
of 30 patients exhibited metastatic (lymph node and/or distal) RCC
tumors. High stromal Fer expression was detected in 1/30 patients
(3.3%) with metastatic RCC (Table I).
No significant difference was observed between stromal Fer
expression and the RCC pathological subtype (P=0.804; Table I).
 | Table I.Association between stromal Fer
expression and pathological characteristics of renal cell carcinoma
tissue samples. |
Table I.
Association between stromal Fer
expression and pathological characteristics of renal cell carcinoma
tissue samples.
|
|
| Stromal Fer
expression |
|
|---|
|
|
|
|
|
|---|
| Pathological
characteristic | Patients, n | Low, n (%)
(n=51) | Middle, n (%)
(n=52) | High, n (%)
(n=49) | P-value |
|---|
| Pathological
type |
|
|
|
| 0.804 |
| Conventional | 129 | 43 (33.6) | 45 (35.2) | 40 (31.3) |
|
|
Papillary | 12 | 4 (30.8) | 5 (38.5) | 4 (30.8) |
|
|
Chromophobe | 11 | 4 (36.4) | 2 (18.2) | 5 (45.5) |
|
| pT stage |
|
|
|
|
|
|
pT1 | 88 | 13 (14.8) | 34 (38.6) | 41 (46.6) |
|
|
pT2 | 18 | 12 (66.7) | 4 (22.2) | 2 (11.1) |
|
|
pT3 | 40 | 21 (52.5) | 14 (35.0) | 5 (12.5) |
|
|
pT4 | 6 | 5 (83.3) | 0 (0.0) | 1 (16.7) |
|
| Low
(pT1/2) | 106 | 25 (23.6) | 38 (35.8) | 14 (40.6) | <0.001 |
| High
(pT3/4) | 46 | 26 (56.5) | 14 (30.4) | 6 (13.0) |
|
| LN metastasis |
|
|
|
|
|
|
Absence | 137 | 40 (29.2) | 48 (35.0) | 49 (35.8) | 0.001 |
|
Presence | 15 | 11 (73.3) | 4 (26.7) | 0 (0.0) |
|
| Distant
metastasis |
|
|
|
|
|
|
Absence | 126 | 31 (24.6) | 47 (37.3) | 48 (38.1) | <0.001 |
|
Presence | 26 | 20 (76.9) | 5 (19.2) | 1 (3.8) |
|
| Metastasis |
|
|
|
|
|
|
Absence | 122 | 29 (23.8) | 45 (36.9) | 48 (39.3) | <0.001 |
|
Presence | 30 | 22 (73.3) | 7 (23.3) | 1 (3.3) |
|
| Grade |
|
|
|
|
|
| 1 | 57 | 12 (21.1) | 17 (29.8) | 28 (49.1) |
|
| 2 | 64 | 17 (26.6) | 29 (45.3) | 18 (28.1) |
|
| Low
(1/2) | 121 | 29 (24.0) | 46 (38.0) | 46 (38.0) | <0.001 |
| High
(3/4) | 31 | 22 (71.0) | 6 (19.4) | 3 (9.7) |
|
Association between Fer expression,
malignancy and survival
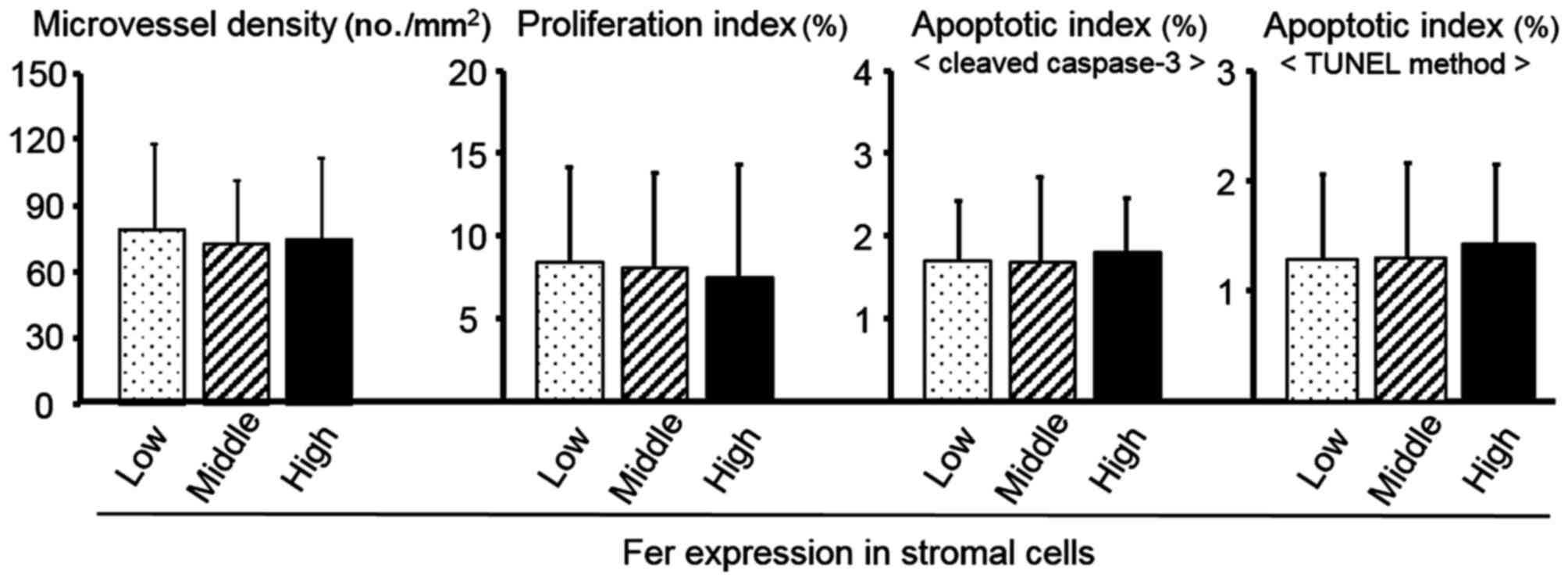
As shown in Fig. 2,
stromal Fer expression was not significantly associated wPI, AI or
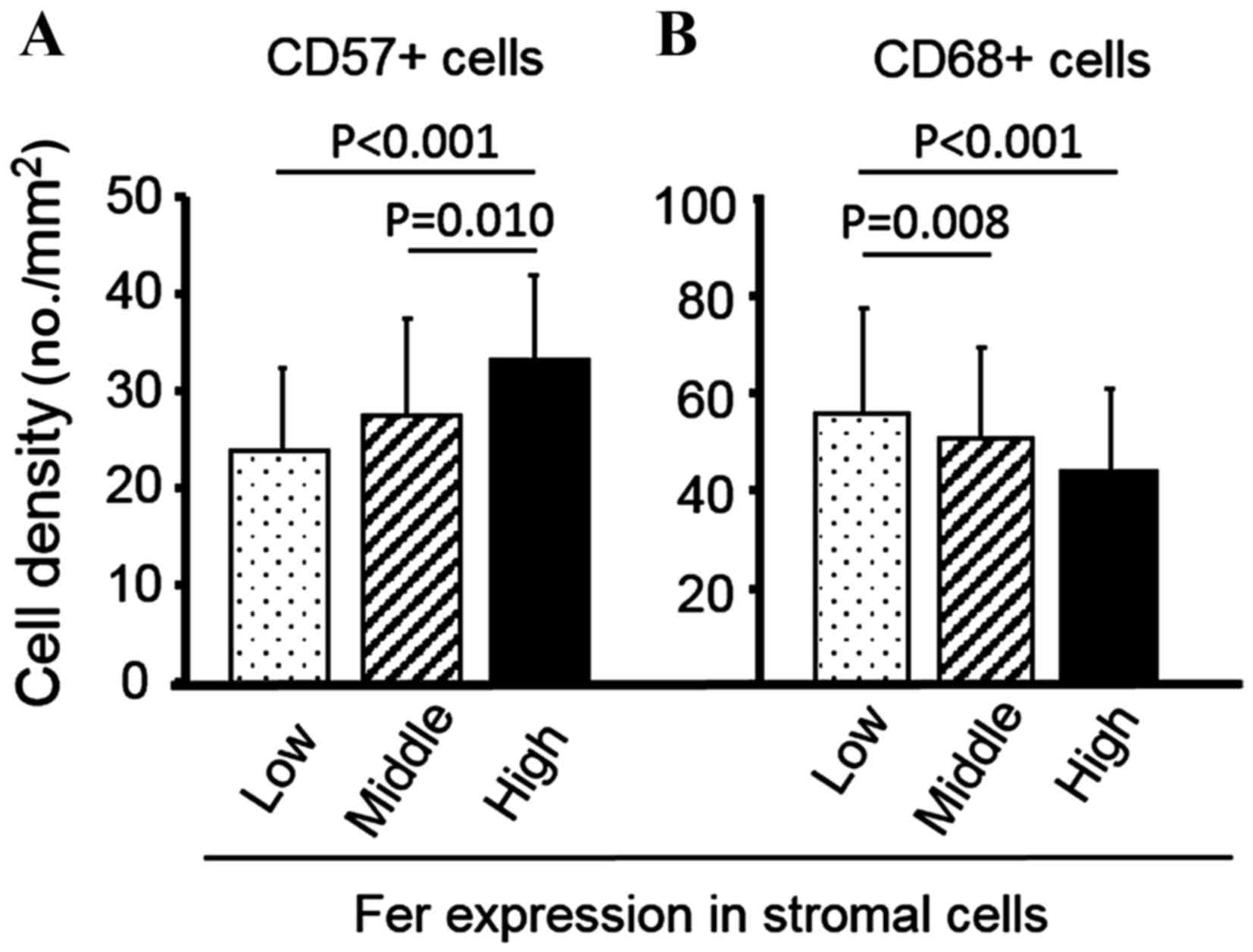
MVD. As shown in Fig. 3A, increased
CD57+ NK cell density was significantly associated with
high stromal Fer expression group (P=0.010 vs. middle group;
P<0.001 vs. low group). By contrast, increased CD68+
macrophage density was significantly associated with the low
stromal Fer expression group (P=0.008 vs. middle group; P<0.001
vs. high group; Fig. 3B).
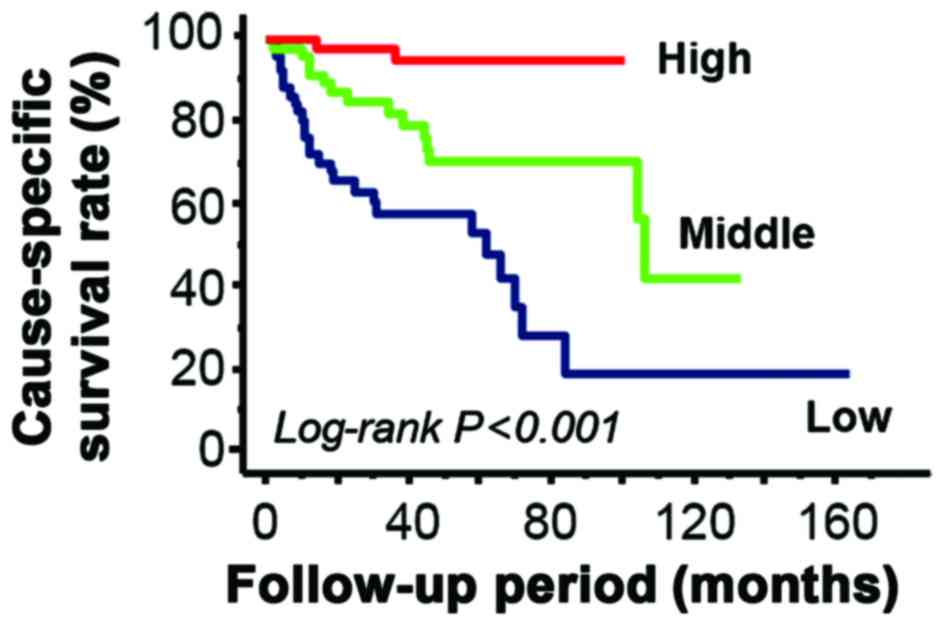
Kaplan-Meier estimators demonstrated that low stromal Fer
expression was significantly associated with a decreased
cause-specific survival rate (P<0.001; Fig. 4). In addition, univariate Cox
proportional hazard models demonstrated that increased grade
(HR=6.39, 95% CI=3.33–12.27, P<0.001), pT stage (6.54, 95%
CI=3.40–12.60, P<0.001) and metastasis (HR=9.17, 95%
CI=4.82–17.44, P<0.001) were predictors of decreased
cause-specific survival. Following multivariate analysis, the
cause-specific survival rate of patients with low stromal Fer
expression was significantly decreased compared with patients with
high stromal Fer expression (HR=7.41; 95% CI=1.67–33.03; P=0.009;
Table II). In addition, moderate
stromal Fer expression, high tumor grade and presence of metastasis
were identified to be independent predictors of significantly
decreased cause-specific survival (Table
II).
 | Table II.Association between the pathological
characteristics of renal cell carcinoma tissue samples and
cause-specific survival. |
Table II.
Association between the pathological
characteristics of renal cell carcinoma tissue samples and
cause-specific survival.
|
| Multivariate
analyses |
|---|
|
|
|
|---|
| Pathological
characteristic | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| pT stage |
|
|
|
| Low
(pT1/2) | 1.00 | – | – |
| High
(pT3/4) | 1.87 | 0.83–4.26 | 0.133 |
| Metastasis |
|
|
|
|
Absence | 1.00 | – | – |
|
Presence | 3.73 | 1.69–8.22 | 0.001 |
| Grade |
|
|
|
| Low
(1/2) | 1.00 | – | – |
| High
(3/4) | 2.70 | 1.27–5.78 | 0.010 |
| Stromal Fer
expression |
|
|
|
|
High | 1.00 | – | – |
|
Middle | 4.55 | 1.01–20.58 | 0.049 |
|
Low | 7.41 | 1.67–33.03 | 0.009 |
Discussion
The results of the present study demonstrated that
low Fer expression in stromal cells was associated with an
increased pT stage and tumor grade, and the presence of metastasis
in patients with RCC. Multivariate analysis also identified
decreased stromal Fer expression to be indicative of decreased
survival. Cancer-associated fibroblasts and infiltrating immune
cells within intratumoral areas are known to be important in
malignant tumor progression (25).
Therefore, an increased understanding of the pathological
significance and activity of RCC-associated stromal cells is
essential for the development of novel observational and treatment
strategies.
Stromal Fer expression in patients with RCC was
demonstrated to be inversely associated with Fer expression in
cancer cells. Increased Fer expression in RCC cells has previously
been demonstrated to be positively associated with tumor growth and
progression in patients with RCC (6).
A similar phenomenon has been reported in other malignancies,
including breast and lung cancer (16,26).
However, the association between stromal Fer expression, tumor
malignancy and survival remains to be elucidated.
In the present study, all stromal tissue
Fer-positive cells were evaluated collectively, and individual
Fer-positive cells were not identified. However, certain
Fer-positive cells appeared to be fibroblasts due to their
morphological characteristics. In addition, certain Fer-positive
cells were considered to be immune cells due to the important role
served by the infiltration of immune cells into stromal tissue in
RCC. Fibroblasts have been reported to be important in tumor
growth, cell invasion and metastasis (25,27).
Furthermore, Fer is known to be expressed in fibroblasts, and to be
associated with their biological and pathological characteristics
(9,28,29). There
is, therefore, a possibility that Fer expression in
cancer-associated fibroblasts is associated with tumor aggression
in various types of cancer, including RCC. The pathological
interaction between fibroblasts and tumor cells is regulated by a
number of complex mechanisms. For example, wild-type fibroblasts
upregulate the secretion of matrix metalloproteinase (MMP)-7 by
cervical cancer cells, whereas MMP-2 is produced primarily by
cancer-associated fibroblasts (30).
Furthermore, cancer-associated fibroblasts affect the migration of
glioma cells but not their proliferation (31). Therefore, further investigation into
the pathological significance of Fer expression in
cancer-associated fibroblast stromal tissue is necessary.
The present study demonstrated that stromal Fer
expression was negatively associated with CD68+ cell
density. CD68 is frequently used to identify macrophages in human
tissue. Increased macrophage density in the tumoral area has been
reported to be associated with increased malignant potential and a
poor prognosis in multiple types of cancer, including RCC (31,32). Low
Fer expression in stromal cells was expected to be associated with
decreased aggressiveness and increased survival, due to decreased
macrophage density in human RCC tissue. Conversely, stromal Fer
expression was negatively associated with intra-tumoral macrophage
density. Intra-tumoral CD68+ cell density was considered
to reflect the following differences in pathological status:
Fer-expressing macrophages do not infiltrate cancer-associated
stromal tissue, and/or chemokines and growth factors produced by
Fer-expressing stromal cells inhibit the growth and migration of
macrophages.
Infiltrating macrophages are known to be recruited
to sites of disease under various pathological conditions (12). However, the association between Fer
expression and immune cell (macrophage) migration in cancer tissues
is complex and unclear. Although Fer increases the recruitment and
activity of leukocytes and neutrophils in response to various
stimuli (33,34), it has also been reported to be an
inhibitory factor of neutrophil chemotaxis (35). In addition, it has been reported that
Fer expression is associated with the production of vascular
endothelial growth factor in myoblast cells (36). However, there is a little information
regarding the recruitment of macrophages through Fer-expressing
stromal cell-mediated chemokine and growth factor production.
Conversely, CD57+ cell density was
positively associated with Fer expression in stromal cells. CD57 is
frequently used to detect NK cells in human tissue, which have been
recognized to have anti-tumoral properties, including increased
survival time, in various malignancies (37–39). This
is consistent with the hypothesis that increased stromal Fer
expression is associated with decreased malignant potential and
improved prognosis in RCC. However, the underlying molecular
mechanism of Fer-expressing stromal cell-mediated NK cell
recruitment and activation remains to be elucidated. Therefore,
further in vitro studies are necessary to understand the
biological and pathological role of Fer in NK cell recruitment in
RCC.
The results of the present study suggest that Fer
expression in cancer-associated stromal tissue serves an
anti-oncogenic role in patients with RCC. In a Fer-deficient model,
the tumor-free rates in mice targeted with a knock-in
Fer-inactivating mutation were increased compared with those of
Fer+/+ mice (40). Previously, an inhibitor of Fes was
identified and used to inhibit the differentiation of osteoclasts
(41). Fer and Fes share a high
structural homology (8), Therefore,
it has been suggested that a subset of Fes inhibitors are likely
also to be Fer inhibitors, and may be used in future preclinical
cancer models (41,42). However, further investigation into the
pathological significance and prognostic value of stromal Fer
expression in patients with RCC is required.
In conclusion, the present study demonstrated that
stromal Fer expression was negatively associated with
aggressiveness in RCC tissue. Additionally, low Fer expression was
associated with an improved prognosis in patients with RCC. The
increase in CD57+ NK cells and the decrease in
CD68+ macrophages are considered to be regulated by
Fer-expressing cancer-associated stromal cells. However, the
individual types of Fer-expressing stromal cells could not be
identified in the present study. Nevertheless, the results of the
present study support the hypothesis that stromal Fer expression in
RCC acts as a suppressor of tumor progression. In addition, the
present results suggest that combined expression of Fer in stromal
and cancer tissue is an effective prognostic biomarker in patients
with RCC. Therefore, further studies are required to identify the
pathological significance and prognostic value of stromal Fer
expression in patients with RCC.
Acknowledgements
The authors of the present study thank Mr. Takumi
Shimogama (Nagasaki University Hospital, Nagasaki, Japan) for his
support. The present study was supported by a Grant-in-Aid from the
Japan Society for the Promotion of Science (grant no. 25462487).
The authors presented the summary of the current manuscript at the
29th Annual European Association of Urology Congress in April 2014
(European Urology Supplements, 13: e195, 2014).
References
|
1
|
Rini BI, Rathmell WK and Godley P: Renal
cell carcinoma. Curr Opin Oncol. 20:300–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stephenson AJ, Chetner MP, Rourke K,
Gleave ME, Signaevsky M, Palmer B, Kuan J, Brock GB and Tanguay S:
Guidelines for the surveillance of localized renal cell carcinoma
based on the patterns of relapse after nephrectomy. J Urol.
172:58–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. New Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lambea J, Anido U, Etxániz O, Flores L,
Montesa Á, Sepúlveda JM and Esteban E: The Wide Experience of the
Sequential Therapy for Patients with Metastatic Renal Cell
Carcinoma. Curr Oncol Rep. 18:662016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calvo E, Grünwald V and Bellmunt J:
Controversies in renal cell carcinoma: Treatment choice after
progression on vascular endothelial growth factor-targeted therapy.
Eur J Cancer. 50:1321–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyata Y, Kanda S, Sakai H and Greer PA:
Feline sarcoma-related protein expression correlates with malignant
aggressiveness and poor prognosis in renal cell carcinoma. Cancer
Sci. 104:681–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ben-Dor I, Bern O, Tennenbaum T and Nir U:
Cell cycle-dependent nuclear accumulation of the p94fer tyrosine
kinase is regulated by its NH2 terminus and is affected by kinase
domain integrity an. Cell Growth Differ. 10:113–129.
1999.PubMed/NCBI
|
|
8
|
Greer P: Closing in on the biological
functions of FPS/FES and FER. Nat Rev Mol Cell Biol. 3:278–289.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sangrar W, Gao Y, Scott M, Truesdell P and
Greer PA: Fer-mediated cortactin phosphorylation is associated with
efficient fibroblast migration and is dependent on reactive oxygen
species generation during integrin-mediated cell adhesion. Mol Cell
Biol. 27:6140–6152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Craig AW: FES/FER kinase signaling in
hematopoietic cells and leukemias. Front Biosci (Landmark Ed).
17:861–875. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim L and Wong TW: Growth factor-dependent
phosphorylation of the actin-binding protein cortactin is mediated
by the cytoplasmic tyrosine kinase FER. J Biol Chem.
273:23542–23548. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayun R, Shpungin S, Malovani H, Albeck M,
Okun E, Nir U and Sredni B: Novel involvement of the
immunomodulator AS101 in IL-10 signaling, via the tyrosine kinase
Fer. Ann N Y Acad Sci. 1095:240–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoneyama T, Angata K, Bao X, Courtneidge
S, Chanda SK and Fukuda M: Fer kinase regulates cell migration
though α-dystroglycan glycosylation. Mol Biol Cell. 23:771–780.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allard P, Zoubeidi A, Nguyen LT, Tessier
S, Tanguay S, Chevrette M, Aprikian A and Chevalier S: Links
between Fer tyrosine kinase expression levels and prostate cell
proliferation. Mol Cell Endocrinol. 159:63–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Ren Z, Kang X, Zhang L, Li X, Wang
Y, Xue T, Shen Y and Liu Y: Identification of
tyrosine-phosphorylated proteins associated with metastasis and
functional analysis of FER in human hepatocellular carcinoma cells.
BMC Cancer. 9:3662009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ivanova IA, Vermeulen JF, Ercan C,
Houthuijzen JM, Saig FA, Vlug EJ, van der Wall E, van Diest PJ,
Vooijs M and Derksen PW: FER kinase promotes breast cancer
metastasis by regulating α6- and β1-integrin-dependent cell
adhesion and anoikis resitance. Oncogene. 32:5582–5592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vitale MG and Cartenì G: Clinical
management of metastatic kidney cancer: The role of new molecular
drugs. Future Oncol. 12:83–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hashimoto O, Yoshida M, Koma Y, Yanai T,
Hasegawa D, Kosaka Y, Nishimura N and Yokozaki H: Collaboration of
cancer-associated fibroblasts and tumour-associated macrophages for
neuroblastoma development. J Pathol. 240:211–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe S, Miyata Y, Matsuo T, Mochizuki
Y, Nishikido M, Hayashi T and Sakai H: High density of
tryptase-positive mast cells in patients with renal cell carcinoma
on hemodialysis: Correlation with expression of stem cell factor
and protease activated receptor-2. Hum Pathol. 43:888–897. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuo T, Miyata Y, Watanabe S, Ohba K,
Hayashi T, Kanda S and Sakai H: Pathologic significance and
prognostic value of phosphorylated cortactin expression in patients
with sarcomatoid renal cell carcinoma. Urology. 476:e9–15.
2011.
|
|
21
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Manual. 6th.
Springer-Verlag; New York, NY: pp. 157–164. 2002
|
|
22
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyata Y, Watanabe S, Kanetake H and Sakai
H: Thrombospondin-1-derived 4N1K peptide expression is negatively
associated with malignant aggressiveness and prognosis in
urothelial carcinoma of the upper urinary tract. BMC Cancer.
12:3722012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe S, Miyata Y, Kanda S, Iwata T,
Hayashi T, Kanetake H and Sakai H: Expression of X-linked inhibitor
of apoptosis protein in human prostate cancer specimens with and
without neo-adjuvant hormonal therapy. J Cancer Res Clin Oncol.
136:787–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn J, Truesdell P, Meens J, Kadish C,
Yang X, Boag AH and Craig AW: Fer protein-tyrosine kinase promotes
lung adenocarcinoma cell invasion and tumor metastasis. Mol Cancer
Res. 11:952–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim L and Wong TW: The cytoplasmic
tyrosine kinase FER is associated with the catenin-like substrate
pp120 and is activated by growth factors. Mol Cell Biol.
15:4553–4561. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosato R, Veltmaat JM, Groffen J and
Heisterkamp N: Involvement of the tyrosine kinease fer in cell
adhesion. Mol Cell Biol. 18:5762–5770. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fullár A, Dudás J, Oláh L, Hollósi P, Papp
Z, Sobel G, Karászi K, Paku S, Baghy K and Kovalszky I: Remodeling
of extracellular matrix by normal and tumor-associated fibroblasts
promotes cervical cancer progression. BMC Cancer. 15:2562015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trylcova J, Busek P, Smetana K Jr,
Balaziova E, Dvorankova B, Mifkova A and Sedo A: Effect of
cancer-associated fibroblasts on the migration of glioma cells in
vitro. Tumour Biol. 36:5873–5879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohba K, Miyata Y, Kanda S, Koga S, Hayashi
T and Kanetake H: Expression of urokinase-type plasminogen
activator, urokinase-type plasminogen activator receptor and
plasminogen activator inhibitors in patients with renal cell
carcinoma: Correlation with tumor associated macrophage and
prognosis. J Urol. 174:461–465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tchou J, Zhang PJ, Bi Y, Satija C,
Marjumdar R, Stephen TL, Lo A, Chen H, Mies C, June CH, et al:
Fibroblast activation protein expression by stromal cells and
tumor-associated macrophages in human breast cancer. Hum Pathol.
44:2549–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCafferty DM, Craig AW, Senis YA and
Greer PA: Absence of Fer protein-tyrosine kinase exacerbates
leukocyte recruitment in response to endotoxin. J Immunol.
168:4930–4935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khajah M, Andronegui G, Chan R, Craig AW,
Greer PA and McCafferty DM: Fer kinase limits neutrophil chemotaxis
toward end target chemoattractants. J immunol. 190:2208–2216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salem Y, Shpungin S, Pasder O, Pomp O,
Taler M, Malovani H and Nir U: Fer kinase sustains the activation
level of ERK1/2 and increases the production of VEGF in hypoxic
cells. Cell Signal. 17:341–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Donskov Fa and von der Maase H: Impact of
immune parameters on long-term survival in metastatic renal cell
carcinoma. J Clin Oncol. 24:1997–2005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vandercappellen J, Van Dammet J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mueller SK, Altvater B, Chen C,
Kailayangiri S, Ahlmann M, Dirksen U, Juergens H and Rossig C:
Zoledronic acid negatively affects the expansion of in vitro
activated human NK cells and their cytolytic interactions with
Ewing sarcoma cells. Oncol Rep. 29:2348–2354. 2013.PubMed/NCBI
|
|
40
|
Sangrer W, Shi C, Mullins G, LeBrun D,
Ingalls B and Greer PA: Amplified Ras-MARK signal states correlate
with accelerated EGFR internalization, cytostasis and delayed HER2
tumor onset in Fer-deficient model systems. Oncogene. 34:4109–4117.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hellwig S, Miduturu CV, Kanda S, Zhang J,
Filippakopoulos P, Salah E, Deng X, Choi HG, Zhou W, Hur W, et al:
Small-molecule inhibitors of the c-Fes protein-kinase kinase. Chem
Biol. 19:529–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanda S and Miyata Y: The c-Fes protein
tyrosine kinase as a potential anti-angiogenic target in cancer.
Front Biosci (Landmark Ed). 16:1024–1035. 2011. View Article : Google Scholar : PubMed/NCBI
|