Introduction
Bladder cancer (BC) is one of the leading causes of
cancer-associated mortality worldwide, in which 90% of cases
exhibit a transitional cell histology; its incidence is second only
to prostate cancer as a malignancy of the genitourinary tract and
it is the second most common cause of genitourinary cancer-related
mortality (1). Although radical
cystectomy with urinary tract reconstruction is considered the
standard treatment for BC, the mortality rate from invasive
urothelial cell carcinoma is ~50%, and there is a significant
decrease in quality-of-life following radical surgery (2). Every year, >200,000 people succumb to
BC due to cancer metastasis (3).
Therefore, the investigation of the mechanism underlying BC
metastasis, and the search for genes associated with BC metastasis
and therapeutic targets, is necessary.
Numerous genes may be associated with the invasive
capability of BC cells. For example, upregulation of Homo
sapiens longevity assurance homolog 2 of yeast LAG1 (LASS2) was
shown to correlate with an increased invasiveness of BC cells
(4). LASS2, which is also known as
tumor metastasis suppressor gene 1 (TMSG1; GenBank accession no.
AF189062), and is a novel gene isolated from a human liver cDNA
library in the laboratory of Shanghai Medical College, Fudan
University (Shanghai, China), is a human homolog of the yeast
longevity assurance gene LAG1 (Saccharomyces cerevisiae
longevity assurance gene) (5).
Several studies have correlated LASS2 with the extent of invasion
and recurrence in prostate (6–8), liver
(9) and breast (10,11)
carcinomas. Furthermore, previous studies have reported that LASS2
is able to interact with subunit C of vacuolar H+-ATPase
(V-ATPase) (11,12) to regulate V-ATPase activity and the
extracellular H+ concentration, and in turn activate
secreted matrix metalloproteinases (MMPs) 2 and 9, leading to
inhibition of cell proliferation, cell survival, invasion and
metastasis (10,11). However, the mechanism of
LASS2-mediated inhibition of tumor invasion and metastasis in BC
has yet to be investigated. In our previous study, LASS2-negative
BC was associated with a poor clinical prognosis, and the
expression of LASS2 was significantly correlated with clinical
stage, tumor depth and recurrence (13). In addition, different LASS2 expression
levels were observed among the BIU-87, T24, EJ and EJ-M3 human BC
cell lines from patients with poorly-, moderately- or
well-differentiated disease, which indicated that LASS2 expression
may be correlated with the development and progression of human BC
(14).
In the present study, small interfering RNAs
(siRNAs) targeting the LASS2/TMSG1 gene were transfected into the
RT4 human BC cell line, which has a low metastatic potential
(15), in order to further evaluate
the inhibitory effect of LASS2 on the growth, invasion and
metastasis of BC cells. The experiments were designed to elucidate
the potential mechanisms underlying the effects of LASS2 on the
inhibition of cancer metastasis by investigating the activities of
V-ATPase, MMP-2 and MMP-9, as well as the apoptosis of the
transfected cells.
Materials and methods
Cell culture and transfection
The RT4 and T24 human BC cell lines (The Second
Affiliated Hospital of Kunming Medical University, Yunnan Institute
of Urology, Kunming, China) were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2. Invitrogen RPMI-1640
medium, PBS, Opti-MEM I, Lipofectamine 2000 and glutamine were
purchased from Thermo Fisher Scientific, Inc.
Two siRNA sequences targeting LASS2 [National Center
for Biotechnology Information (NCBI) accession nos. NM013384,
NM181747 and NM022075] were synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China): siRNA-1, 5′-GGCUAUUACUUCUUCAAUUTT-3′ and
siRNA-2, 5′-CAGUAUUGGUACUACAUGATT-3′. Unspecific control siRNA
(si-NC) was also obtained from Guangzhou RiboBio Co., Ltd. The
siRNAs were transfected into the RT4 cell line using Lipofectamine
2000, according to the manufacturer's protocol. siRNA was used for
the in vitro experiments, and shRNA was used for cell
selection and then for the Xenograft model.
sh-LASS2 cell line selection
sh-LASS2 cell line selection was performed as
described previously by Xu et al (16). Briefly, two complementary
oligonucleotides
(5′-CACCGAAGAAAGTTTGGGAGGGATATTCAAGACGTA TCC CTC
CCA AAC TTT CTT CTT TTT TG-3′ and
5′-AGCTCAAAAAAGAAGAAAGTTTGGGAGGGATACGTCTTGAATATCCCTCCCAAACTTTCTTC-3′
the 21-nucleotide sense or antisense strand is in bold letters and
the stem loop sequences are in italics) were synthesized (Sangon
Biotech, Co., Ltd., Shanghai, China), annealed to generate dsDNAs
and ligated into the linearized empty vector pSilencer2.1-U6
(Ambion; Thermo Fisher Scientific, Inc.). A random sense sequence
(AAG CTC AGG TCA CAT CTC TGC) was used as a negative control. The
constructed plasmids were verified by direct sequencing (Sangon
Biotech, Co., Ltd.). Stable transfection of RT4 cells was performed
using Lipofectamine 2000 according to the manufacturer's protocol.
Briefly, 1×105 cells was seeded and transfected using 2
µg DNA mixed with 5 µl Lipofectamine in 1 ml Opti-MEM I. The cells
were incubated for 24 h at 37°C, and then 1 ml medium containing
20% RPMI-1640 medium was added into each well. The cells were
cultured and maintained in medium containing 400 mg/ml G418
(Invitrogen; Thermo Fisher Scientific, Inc.) for at least 2 weeks
until the nontransfected RT4 cells cultured in the controlled wells
were all killed. Subsequently, the expression level of LASS2 was
determined by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
RNA extraction and RT-qPCR
Cells were harvested, centrifuged for 10 min at 600
× g at 4°C, and washed with PBS. Total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RNA was quantified at 260
nm in a NanoDrop spectrophotometer, with an optical density 260/280
ratio of 1.7/2.0 for all samples. First-strand cDNA was synthesized
in a volume of 20 µl using 1 µg total RNA and a High Capacity cDNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Target sequences were obtained from the NCBI
GenBank database (https://www.ncbi.nlm.nih.gov/genbank/). qPCR was
performed using a LightCycler® 480 System (Roche
Diagnostics, Basel, Switzerland) and the qPCR Master-Mix (Kapa
Biosystems, Inc., Wilmington, MA, USA), according to the
manufacturers' protocols. To quantify target mRNA levels, Applied
Biosystems LASS2 Gene Expression assays were purchased from Thermo
Fisher Scientific, Inc. The primer sequences were as follows: LASS2
forward, 5′-GCCTTGCTCTTCCTCATCGTTC-3′ and reverse,
5′-TGCTTGCCACTGGTCAGGTAGA-3′ and GAPDH (housekeeping gene) forward,
5′-GGTCTCCTCTGACTTCAACA-3′ and reverse, 5′-GAGGGTCTCTCTCTTCCT-3′.
All PCRs were run in duplicate and were performed for 40 cycles
(95°C for 15 sec and 60°C for 30 sec). Relative expression levels
were determined following normalization to GAPDH using the
2−ΔΔCq method (17). All
tests were performed in triplicate.
Western blot analysis
For the western blot analysis, the cells were
harvested in lysis buffer containing 50 mm Tris (pH 8.0), 2% SDS, 1
mm EDTA and 150 mm NaCl. The homogenate was centrifuged for 10 min
at 14,000 × g at 4°C and the supernatant was used for
protein determination. The protein content was measured using the
Lowry method (Bio-Rad DC Protein Assay; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Cell lysate proteins (30 µg) were separated by
10% SDS-PAGE, transfered onto Trans-Blot Transfer Medium Membranes
(Bio-Rad Laboratories, Inc.) and blocked with 5% skimmed milk for 1
h at room temperature. The membranes were incubated with anti-LASS2
(catalog no. sc-390745; 1:500 dilution), anti-β-actin (sc-47778;
1:1,000 dilution), anti-caspase-3 (catalog no. sc-7272; 1:500
dilution) and anti-poly(ADP-ribose) polymerase (PARP) (catalog no.
sc-7150; 1:500 dilution) antibodies from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) at 4°C overnight. The membranes were then
incubated with goat anti-mouse immunoglobulin G-horseradish
peroxidase-conjugated secondary antibodies (catalog no. sc-2005;
1:2,000 dilution) for 2 h at room temperature. Western blots were
developed using the enhanced chemiluminescence technique (GE
Healthcare Life Sciences, Chalfont, UK) and band intensity was
quantified using Quantity One software (Bio-Rad Laboratories,
Inc.).
Cell viability assay
MTT assays (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) were performed to evaluate cell viability,
according to the manufacturer's protocol. Briefly, the
si-LASS2-transfected RT4 cells and the controls were seeded into
96-well plates at a density of 1.0×103 cells/well in
triplicate. MTT solution (20 µl, 5 mg/ml; Sigma-Aldrich; Merck
Millipore) was added to each well and 150 µl dimethyl sulfoxide was
added 4 h later to dissolve the crystals. Subsequently, the
absorbance of the cells was measured at 490 nm using an ELISA
reader.
Cell migration assay
Cell migration was measured by wound healing
(scratch) assays. For the scratch assay, cells were seeded into
6-well plates in RPMI-1640 medium supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin, at
37°C in a humidified atmosphere containing 5% CO2. Cells
were grown to confluence and a scratch was created using a sterile
pipette tip. The ability of the cells to migrate into the scratch
area was assessed under a Leica DM6000B microscope (Leica
Microsystems GmbH, Wetzlar, Germany) 24 h after the creation of the
wound area.
Cell matrigel invasion assay
The invasive ability of RT4 and T24 cells was
evaluated using a Matrigel invasion assay. BD BioCoat Matrigel
Invasion Chambers with 8-mm pore polyethylene terephthalate
membranes (BD Biosciences, Franklin Lakes, NJ, USA) for 24-well
plates were prepared by hydrating for 2 h at 37°C. A total of
2×105 cells in 0.2 ml were seeded into each insert.
After 12-h cultures, the invasion chamber was removed, the medium
in the top wells was aspirated and the cells on the upper surface
of the membranes were removed using cotton swabs. Invading cells
attached to the lower side of the membrane were removed by flushing
with a pipette, after which migrating cells present in the bottom
chambers were stained with crystal violet and then counted under a
light microscope. Fluorescence intensities were determined and
plotted on a standard histogram and the number of invading cells
was calculated. All determinations were performed in triplicate.
Data are expressed as the number of invaded cells.
Activity of V-ATPase
To measure lysosomal V-ATPase activity, an acridine
orange (Invitrogen; Thermo Fisher Scientific, Inc.) uptake assay
was performed using isolated lysosomes, as previously described
(18). Furthermore, the extracellular
pH (pHe) of the cells was measured using the fluorescent pH
indicator, 2′,7′-bis-(2-carboxyethyl)-5,6-carboxyfluorescein
(BCECF) (Molecular Probes; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Briefly, three groups of
RT4 cells in six-well plates were cultured, then transiently
transfected with si-LASS2. After 24 h, 1×104 cells were
seeded into 96-well plates, cultured at 37°C in 5% CO2
for 24 h, and then the pHe was detected using a 96-well
fluorospectrophotometer following staining with 1 µM BCECF.
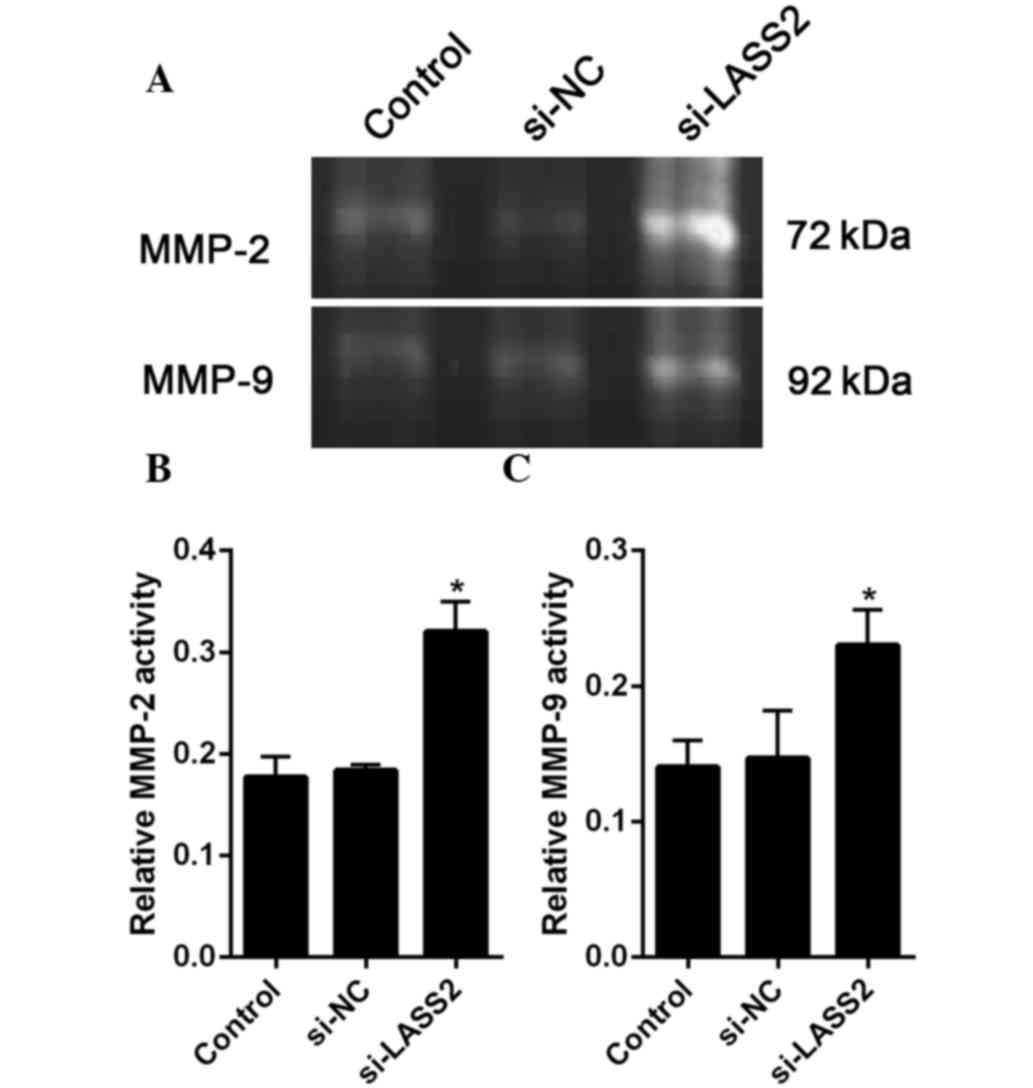
MMP zymography
The zymography assay was performed in 10% SDS
polyacrylamide gels containing 0.1 mg/ml gelatin. Protein sample
(20 µg) was loaded into each lane of the gel. After
electrophoresis, the gel was washed twice with 2.5% Triton X-100
for 1 h at room temperature to remove SDS, and then incubated at
37°C overnight in reaction buffer (Applygen Technologies, Inc.,
Beijing, China). After staining with Coomassie brilliant blue,
MMP-2 and MMP-9 expression was identified as clear zones against
the blue background.
Cell apoptosis analysis
After treating with si-LASS2 or 20 µg/ml doxorubicin
(Dox; Sigma-Aldrich; Merck Millipore) for 48 h, RT4 cells were
harvested and stained with Annexin V-fluorescein isothiocyanate and
propidium iodide (Invitrogen; Thermo Fisher Scientific, Inc.) prior
to fluorescence-activated cell sorting (BD Biosciences) to analyze
apoptosis, according to the manufacturers' protocols.
Xenograft model and treatments
A total of 15 BALB/c male nude mice (4–5 weeks
old; Beijing Vital River Laboratory, Beijing, China) were adapted
to the conditions for 3 days prior to starting the experiment. The
room temperature was 20–25°C, with a relative humidity of 40–70%.
Drinking water, bedding and feeding cages were autoclaved priot to
being provided to then mice. The mice were randomly divided into 3
groups (5 per group) as follows: The control group, the shR-NC
group and the shR-LASS2 group. For the in vivo assays,
1×106 RT4 cells and RT4-sh-LASS2 cells were injected
into the flank of athymic nude mice (5 mice/group) and tumor
dimensions were measured using a caliper once per week. The tumor
volumes were calculated using the following formula: Tumor volume
(mm3) = length (mm) × width (mm) × width (mm) × 0.52. At
week 6, all mice were anesthetized with pentobarbital (50 mg/kg)
and euthanized by cervical dislocation. Tumor tissues were
harvested, fixed in 4% formalin, and subsequently dehydrated and
embedded in paraffin for hematoxylin and eosin staining. All
protocols for treating animals were approved by the Animal Use
Committee of Kunming Medical University (Kunming, China).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). All assays were repeated
three times, data are expressed as the mean ± standard deviation
and P<0.05 was considered to indicate a statistically
significant difference. The significance of the difference was
determined using Student's t-tests and χ2 tests.
All tests were two-tailed for unpaired data.
Results
Downregulation of LASS2 by RNA
interference
RT4 cells, which are BC cells with a low metastatic
potential (15), were transfected
with siRNA targeting LASS2 or with si-NC. After transfection for 48
h, the expression levels of LASS2 in the transfected cells were
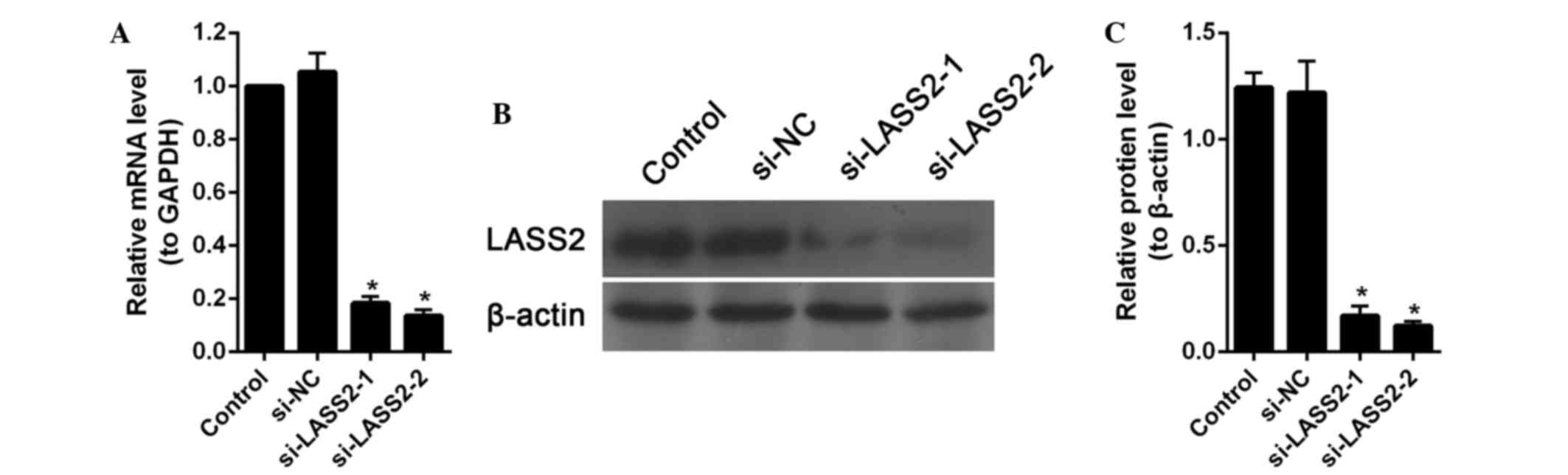
measured using RT-qPCR and western blot analysis. Compared with the
control cells and si-NC-transfected cells, the levels of LASS2 mRNA
and protein were significantly downregulated in the cells
transfected with siRNA targeting LASS2 (P<0.001; Fig. 1). According to these results,
si-LASS2-2, which showed the greatest inhibition on LASS2
expression, was used for further analyses.
siRNA targeting LASS2 promotes cancer
effects in vitro and in vivo
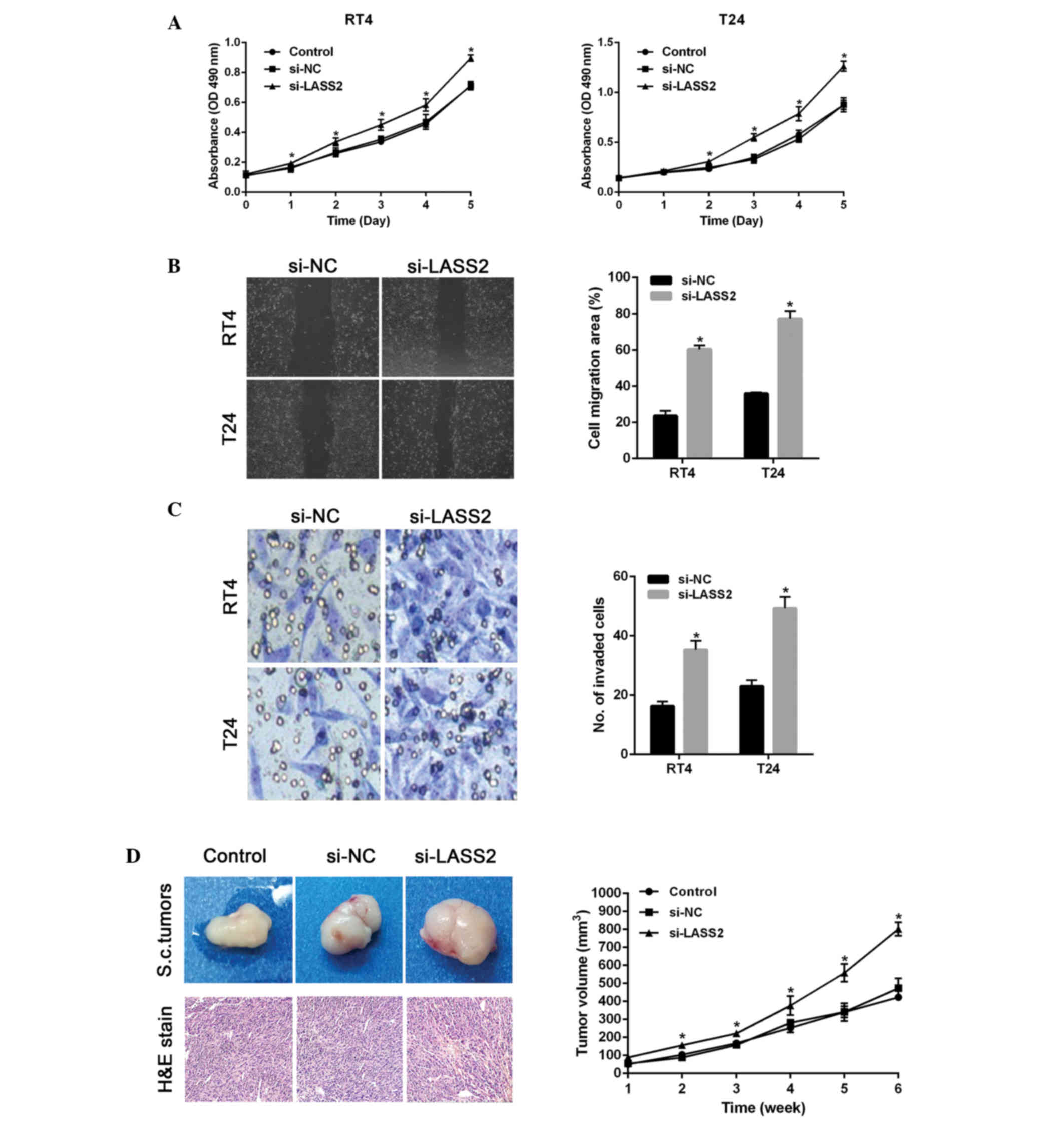
To investigate the cancer promoting potential of
si-LASS2, two BC cell lines, RT4 and T24, were transfected with
si-LASS2. Cell viability was examined using MTT assays. As shown in
Fig. 2A, significant increases in
cell viability were observed following transfection with si-LASS2,
as compared with the si-NC-transfected or untransfected cells.
Subsequently, the impact of LASS2 on the migration and invasion of
BC cells was investigated. The migration of si-LASS2-transfected
cells was significantly increased for both cell lines, as compared
with the si-NC-transfected cells (P<0.001; Fig. 2B). Furthermore, Transwell assays
indicated that the number of invading cells was significantly
increased for si-LASS2-transfected cells compared with
si-NC-transfected cells (P<0.001; Fig.
2C). These results suggest that si-LASS2 exerts cancer
promoting effects in vitro.
Nude mice were subcutaneously injected with
RT4-sh-LASS2 cells, RT4-sh-control cells or untransfected cells.
After 6 weeks, the volumes of tumor xenografts from the
RT4-si-LASS2 group were significantly increased compared with the
control group (P<0.001; Fig.
2D).
LASS2 regulates V-ATPase activity and
the extracellular pH
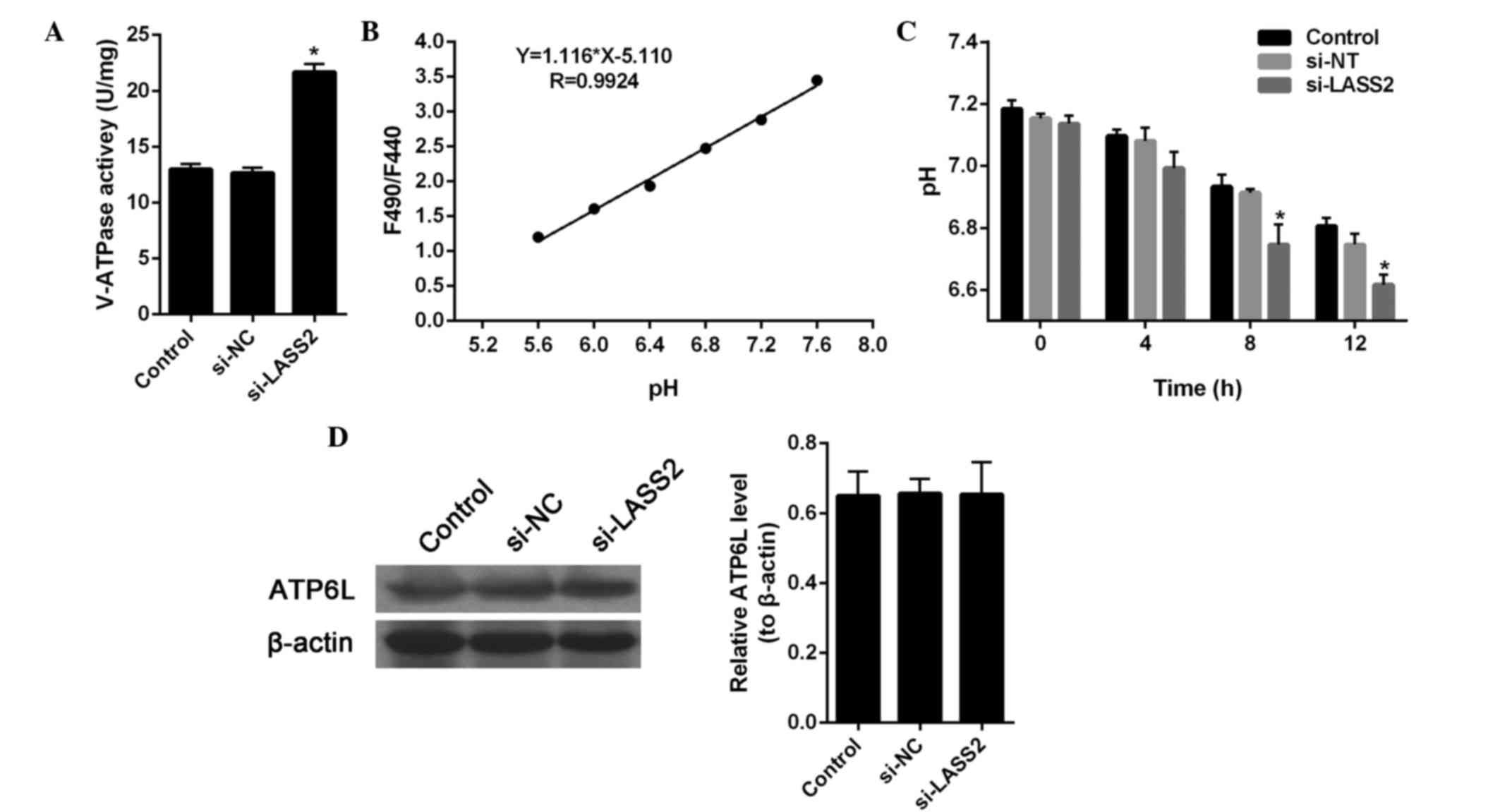
A previous study showed that LASS2 is able to
interact with the C-subunit of V-ATPase and inhibit tumor growth,
invasion and metastasis (9).
Therefore, in the present study, the effect of si-LASS2 on V-ATPase
activity and the H+ potential of RT4 cells was
investigated. The results showed that si-LASS2 significantly
upregulated V-ATPase activity in RT4 cells, as compared with the
untreated cells (P<0.001; Fig.
3A), and the extracellular H+ concentration of the
si-LASS2 cells was markedly increased compared with the
untransfected cells and si-NC-transfected cells (Fig. 3B and C). These results suggest that
si-LASS2 may promote cancer activity by regulating the activity of
the V-ATPase proton pump.
In addition, the expression levels of ATP6L in RT4
cells transfected with si-LASS2 were detected by western blotting
(Fig. 3D). The immunoblot results
showed no change in expression, indicating that LASS2-mediated
regulation of ATP6L does not occur via regulation of its
expression.
Effect of LASS2 on BC cell
apoptosis
It has previously been demonstrated that LASS2 is
able to reduce the ability of V-ATPase to pump H+
out of the cell, resulting in a raise in the intracellular pH (pHi)
and induction of cell apoptosis via the mitochondrial pathway
(9). In order to verify whether this
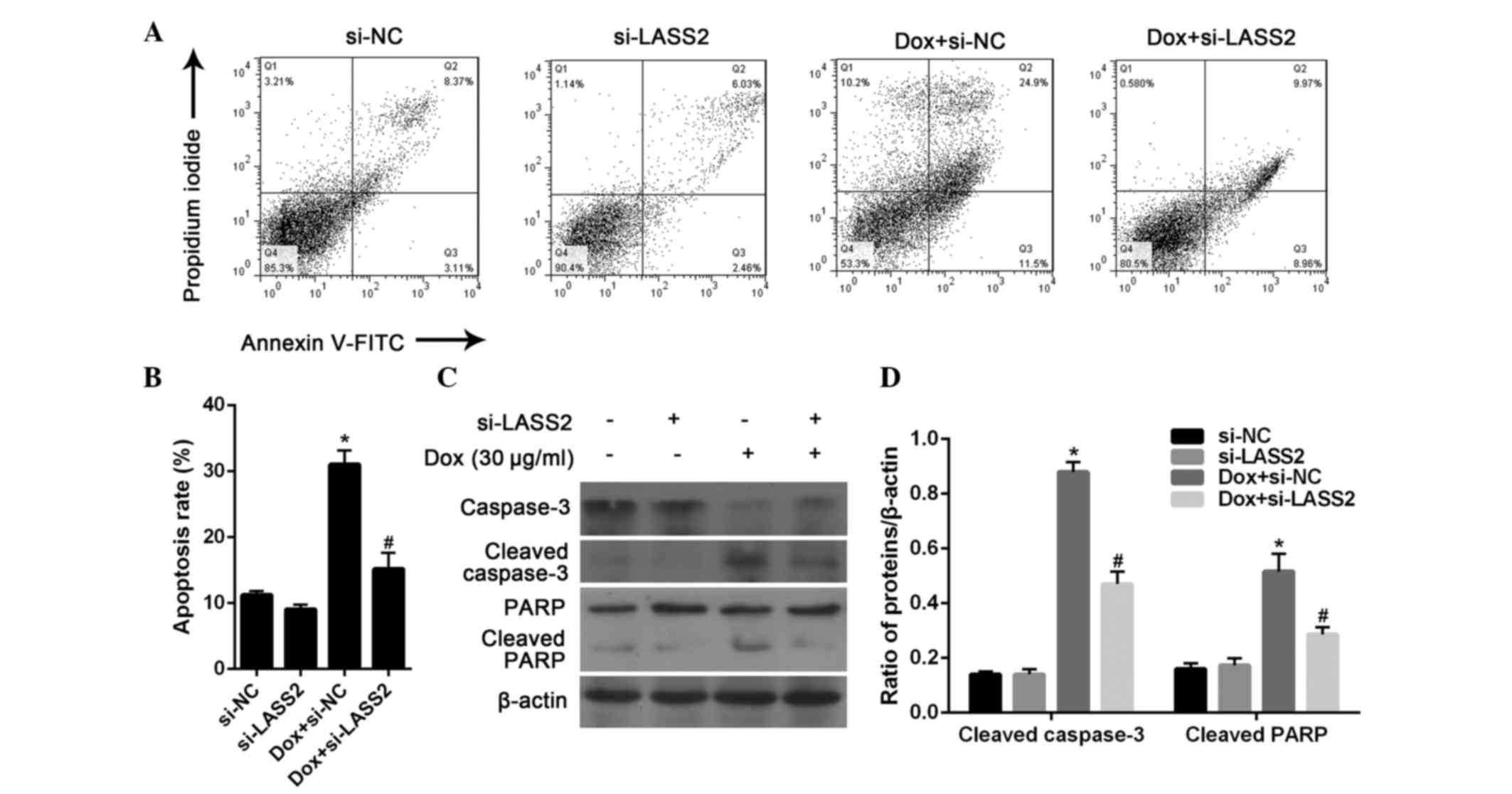
process occurs in BC, the apoptosis of RT4 cells was further
evaluated by flow cytometric analysis. After 24 h, 20 µg/ml Dox
significantly induced the apoptosis of RT4 cells (P<0.001),
which could be significantly inhibited by treatment with si-LASS2
(P=0.0093). There was no significant difference between the RT4
cells transfected with si-NC and si-LASS2 without Dox treatment
(P=0.4357; Fig. 4A and B).
Subsequently, the expression of apoptosis marker
proteins in RT4 cells was analyzed by western blotting. The results
showed that si-LASS2 alone had little effect on the activation of
caspase-3 and PARP cleavage, while Dox treatment was highly
effective. It was also observed that Dox-induced changes in
caspase-3 and PARP activation were significantly suppressed
following treatment with si-LASS2 for 24 h (Fig. 4C and D). These results suggest that
si-LASS2 suppresses the apoptosis of RT4 cells.
LASS2-mediated suppression of V-ATPase
occurs concomitantly with the inhibition of gelatinase
According to previous reports, MMP-2 and MMP-9 are
closely related to cancer metastasis (19). Therefore, the present study assessed
the activities of MMP-2 and MMP-9 using gel gelatin zymography. The
results the gelatinase activity assay showed that the activities of
MMP-2 and MMP-9 were significantly increased in the
si-LASS2-transfected cells compared with the untransfected cells
(P=0.004 and P=0.018, respectively) and si-NC-transfected cells
(P=0.006 and P=0.025, respectively (Fig.
5A and B).
Discussion
LASS2 is a novel gene and has previously been shown
to function as a tumor metastasis suppressor gene, with an
important role in prostate, liver and breast carcinomas (20). Our previous study demonstrated that
LASS2-negative BC was associated with a poor clinical prognosis,
and the expression of LASS2 was significantly correlated with
clinical stage, depth of tumor invasion and recurrence (14). In the present study, the mechanism
underlying LASS2-mediated regulation of tumor cell growth was
investigated. First, BC cell lines were transfected with
siRNA-targeting LASS2, and it was observed that si-LASS2 was able
to promote the growth, invasion and metastasis of BC in vivo
and in vitro.
Xu et al(16)
reported that LASS2 could interact with ATP6L, reduce the ability
of the V-ATPase proton pump to transport H+ out of the
cell, raise the H+ concentration in the cell and induce
cell apoptosis via the mitochondrial pathway, thereby inhibiting
the growth of tumor cells. In addition, the reduction in the
extracellular H+ concentration was shown to reduce the
activation of MMP-2 and MMP-9, thereby inhibiting tumor invasion
and metastasis (16). In the present
study, similar results were observed, although regulation of LASS2
did not alter the expression levels of ATP6L. Furthermore, the
present study indirectly confirmed that LASS2 is able to bind to
ATP6L, which has been reported in previous studies (6,10,16,20). The
authors of the present study hypothesized that, in cells showing a
low metastatic potential, interference with LASS2 may reduce the
number of LASS2 molecules bound to ATP6L protein, rather than
directly regulating the expression level of ATP6L. In turn, the
increased number of unbound ATP6L molecules may play a role through
other molecular mechanisms that promote apoptosis.
Increases in the H+ concentration
resulting in a decrease in pH is a type of external stimuli for
cells, and induce a state of cellular stress (21). It has previously been reported that
LASS2 is able to bind with ATP6L to inhibit H+ from
being pumped out of cells, resulting in a decline in the pHi value
(10). ATP6L is a mediator of
intracellular signaling cascades and can induce the collapse of the
mitochondrial membrane potential, which can trigger a series of
mitochondria-associated events, such as apoptosis (22). The results of the present study
confirmed that si-LASS2 was able to significantly decrease
Dox-induced apoptosis. Dox-induced apoptosis occurs initially via
the c-Jun n-terminal kinase signaling system, which regulates the
expression of nuclear transcription factors to affect cells. In
particular, the P53 protein is phosphorylated, the expression of
B-cell lymphoma-2 (Bcl-2)-associated × protein is increased and
that of Bcl-2 is decreased, which promotes the apoptosis of cells
(23). In the present study, LASS2
was shown to regulate the activity of ATPase and affect the
H+ concentration inside and outside of the cells, but it
did not affect the expression levels of ATP6L. These results
suggested that, in BC, LASS2 may bind to ATP6L to promote BC cell
apoptosis and inhibit the growth of BC cells.
LASS2 genes have a Toll-IL-1-resistance
domain-containing adaptor-inducing IFN-β-related adaptor molecule,
LAG1 and ceroid-lipofuscinosis, neuronal 8 structural domain, which
is necessary for the synthesis of ceramide, and is indispensable in
the process of acetyl-CoA-dependent ceramide synthesis (24). Ceramide is an important second
messenger molecule in cell signal transduction pathways,
participating in the activation of various protein kinases and
phosphatases to regulate cell growth, differentiation and apoptosis
(25). Whether these pathways play
roles in the growth, invasion and metastasis of BC remain to be
elucidated.
In conclusion, the present study confirmed that
LASS2 was able to regulate V-ATPase activity and pHi through by
directly interacting with the C subunit of V-ATPase to influence
the apoptosis and proliferation of BC cells. Furthermore, upon
further study, LASS2 may induce ceramide synthesis and increase
cellular ceramide levels to stimulate cell apoptosis, which may
provide important clues for understanding the role of LASS2 in BC
cells. The results of the present study suggested that LASS2
expression may be correlated with the development and progression
of human BC and may be a potential prognostic indicator for this
cancer.
Acknowledgements
The authors of the present study would like to thank
Mr. Bo Yang (Kunming Institute of Zoology, Chinese Academy of
Sciences, Kunming, Yunnan, China) for drawing the graphs. This
study was supported by the National Natural Science Foundation of
China (grant nos. 81260374 and 81460384), the Yunnan Provincial
Department of Education Fund (grant no. 2014Z072) and the Joint
Project of Science and Technology, Department of Yunnan and Kunming
Medical University (grant nos. 2014FA015 and 2014FZ031).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng L, Davison DD, Adams J, LopezBeltran
A, Wang L, Montironi R and Zhang S: Biomarkers in bladder cancer:
Translational and clinical implications. Crit Rev Oncol Hematol.
89:73–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morgan TM, Keegan KA and Clark PE: Bladder
cancer. Curr Opin Oncol. 23:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Q, Wang H, Yang M, Yang D, Zuo Y and
Wang J: Expression of a tumor-associated gene, LASS2, in the human
bladder carcinoma cell lines BIU-87, T24, EJ and EJ-M3. Exp Ther
Med. 5:942–946. 2013.PubMed/NCBI
|
|
5
|
Pan H, Qin WX, Huo KK, Wan DF, Yu Y, Xu
ZG, Hu QD, Gu KT, Zhou XM, Jiang HQ, et al: Cloning, mapping and
characterization of a human homologue of the yeast longevity
assurance gene LAG1. Genomics. 77:58–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu W, Wang L, Wang Y, Xu X, Zou P, Gong M,
Zheng J, You J, Wang H, Mei F and Pei F: A novel tumor metastasis
suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through
its homeodomain. J Cell Biochem. 114:570–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu XY, You JF, Pei F and Zhang B:
Silencing of tumor metastasis suppressor gene 1 promotes invasion
of prostate cancer cell in vitro and its molecular mechanisms.
Beijing Da Xue Xue Bao. 43:814–819. 2011.(In Chinese). PubMed/NCBI
|
|
8
|
Xu X, You J and Pei F: Silencing of a
novel tumor metastasis suppressor gene LASS2/TMSG1 promotes
invasion of prostate cancer cell in vitro through increase of
vacuolar ATPase activity. J Cell Biochem. 113:2356–2363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang N, Jin J, Deng Y, Ke RH, Shen QJ, Fan
SH and Qin WX: LASS2 interacts with V-ATPase and inhibits cell
growth of hepatocellular carcinoma. Sheng Li Xue Bao. 62:196–202.
2010.(In Chinese). PubMed/NCBI
|
|
10
|
Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H,
Ke R, Song J, Shen Q, Wang W, et al: LASS2 enhances
chemosensitivity of breast cancer by counteracting acidic tumor
microenvironment through inhibiting activity of V-ATPase proton
pump. Oncogene. 32:1682–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schiffmann S, Sandner J, Birod K, Wobst I,
Angioni C, Ruckhäberle E, Kaufmann M, Ackermann H, Lötsch J,
Schmidt H, et al: Ceramide synthases and ceramide levels are
increased in breast cancer tissue. Carcinogenesis. 30:745–752.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartmann D, Lucks J, Fuchs S, Schiffmann
S, Schreiber Y, Ferreirós N, Merkens J, Marschalek R, Geisslinger G
and Grösch S: Long chain ceramides and very long chain ceramides
have opposite effects on human breast and colon cancer cell growth.
Int J Biochem Cell Biol. 44:620–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Wang J, Zuo Y, Ding M, Yan R, Yang
D and Ke C: Expression and prognostic significance of a new tumor
metastasis suppressor gene LASS2 in human bladder carcinoma. Med
Oncol. 29:1921–1927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Tan Q, Geddie WR, Jewett MA,
Phillips N, Ke D, Simmons CA and Sun Y: Biophysical
characterization of bladder cancer cells with different metastatic
potential. Cell Biochem Biophys. 68:241–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu X, Liu B, Zou P, Zhang Y, You J and Pei
F: Silencing of LASS2/TMSG1 enhances invasion and metastasis
capacity of prostate cancer cell. J Cell Biochem. 115:731–743.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu X, Qin W, Li J, Tan N, Pan D, Zhang H,
Xie L, Yao G, Shu H, Yao M, et al: The growth and metastasis of
human hepatocellular carcinoma xenografts are inhibited by small
interfering RNA targeting to the subunit ATP6L of proton pump.
Cancer Res. 65:6843–6849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seiler R, Thalmann GN and Fleischmann A:
MMP-2 and MMP-9 in lymph-node-positive bladder cancer. J Clin
Pathol. 64:1078–1082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei F, You J, Liu B, Zhang M, Liu J, Zhang
B and Pei F: LASS2/TMSG1 inhibits growth and invasion of breast
cancer cell in vitro through regulation of vacuolar ATPase
activity. Tumour Biol. 36:2831–2844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Undem C, Rios EJ, Maylor J and Shimoda LA:
Endothelin-1 augments Na(+)/H(+) exchange
activity in murine pulmonary arterial smooth muscle cells via Rho
kinase. PloS One. 7:e463032012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasazawa Y, Futamura Y, Tashiro E and
Imoto M: Vacuolar H+-ATPase inhibitors overcome
Bcl-xL-mediated chemoresistance through restoration of a
caspase-independent apoptotic pathway. Cancer Sci. 100:1460–1467.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Molecular Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar
|
|
24
|
Mizutani Y, Kihara A and Igarashi Y:
Mammalian Lass6 and its related family members regulate synthesis
of specific ceramides. Biochem J. 390:263–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ponnusamy S, MeyersNeedham M, Senkal CE,
Saddoughi SA, Sentelle D, Selvam SP, Salas A and Ogretmen B:
Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in
the regulation of cell death and drug resistance. Future Oncol.
6:1603–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|