Introduction
Hemangioblastomas of the central nervous system
(CNS) are abundantly vascularized and benign neoplasms that are
most commonly diagnosed in adults. They account for 1.5–2.5% of all
intracranial tumors and for 7–12% of all posterior fossa tumors
(1–3).
Of all hemangioblastomas, 60–75% occur sporadically and 25–40% have
a von Hippel-Lindau disease (VHL) background (4). Since, generally, hemangioblastomas do
not metastasize, the World Health Organization categorizes them as
grade one CNS tumors (4).
Hemangioblastomas are either round or oval and have sharp
boundaries and smooth contours. The majority of hemangioblastomas
(70–75%) have cystic or multi-cystic forms (4). These forms occur predominantly in the
cerebellar hemispheres, while the solid forms of hemangioblastoma
are found more frequently in the brain stem, cerebellar vermis and
spinal cord (4). For the cystic type,
the cavity has to be opened to expose the tumor nodule, which can
be then removed by en bloc resection (3). Solid tumors have characteristics similar
to intracranial arteriovenous malformations (AVMs) (5,6).
Internal decompression and piecemeal resection have
been shown to have potential devastating intraoperative
complications (3). Therefore, the
safe resection of solid tumors involves a sequence of surgical
techniques, including preoperative embolization, wide exposure and
circumferential dissection, which represent a challenge for the
majority of neurosurgeons (3). Early
case reports suggested that the postoperative mortality and
morbidity rates for posterior fossa tumors were ~50%, while the
intraoperative mortality rate was 9% (2,3,5). Generally, the high mortality and
morbidity rates were closely associated with unsuccessful
intraoperative hemostasis (7). Since
the introduction of diagnostic imaging and modern treatment
methods, the mortality and morbidity rates of hemangioblastomas
have decreased significantly (8). The
main risk factor for intraoperative hemorrhage is the tumor size,
such that the surgical treatment of large solid hemangioblastomas
remains relatively difficult (8). In
our previous study, surgical resection was proposed as the optimal
treatment for large tumors of the posterior fossa (8).
This study presents the results of microsurgeries
performed between 2010 and 2014 on 28 patients with posterior fossa
hemangioblastomas, and demonstrated that, with improved techniques
and a better understanding of the vascular pattern of these tumors,
total microsurgical removal can be performed with a relatively low
mortality. Selective preoperative endovascular embolization of the
feeding artery with subsequent en bloc tumor resection was
shown to be the preferred procedure to reduce the risk of
intraoperative hemorrhage and facilitate the removal of large solid
tumors. However, embolization remains controversial, given its
associated risks, including ischemia, bleeding and increased
intracranial pressure (3).
Patients and methods
Patients
This retrospective study included 28 consecutive
patients with confirmed posterior fossa hemangioblastomas, treated
between January 2010 and September 2014 at the Renji Hospital
(Shanghai, China). Only patients with surgical treatment were
included in this study. The therapeutic approach in all cases was
surgical resection with or without preoperative endovascular
embolization. Of the patients, 17 were males and 11 were females,
with an age range of 15–85 years and a mean age of 40 years. In
total, 21 tumors were sporadic and 7 were associated with VHL
disease. The course of the disease ranged from 1 month to 4 years,
with a mean disease duration of 1 year. All patients had varying
degrees of intracranial hypertension symptoms (e.g. a headache); 9
patients had ataxia; 3 had diplopia; 2 had lower cranial nerve
dysfunction; and 11 had preoperative hydrocephalus. The individual
patient data are detailed in Table
I.
 | Table I.Clinical data of 28 patients with
solid hemangioblastomas of the posterior fossa. |
Table I.
Clinical data of 28 patients with
solid hemangioblastomas of the posterior fossa.
| No. | Age (y)/sex | Locationa | Tumor size (cm) | Initial symptoms | Tumor feeding
artery | Preoperative
embolization | VHL | Preoperative
hydrocephalus |
|---|
| 1 | 29/M | I | 2.0 | Headache | SCA, PICA | − | + | − |
| 2 | 44/M | I | 2.0 | Ataxia, nausea | PICA | − | − | − |
| 3 | 44/M | I | 2.5 | Vertigo | AICA, SCA | − | + | + |
| 4 | 85/M | III | 3.5 | Headache,
diplopia | SCA, PICA | + | − | − |
| 5 | 26/M | II | 2.5 | Ataxia | PICA | − | + | + |
| 6 | 66/M | I | 3.5 | Headache | PICA, AICA, MB | − | + | + |
| 7 | 37/F | I | 2.0 | Headache, ataxia | PICA AICA | − | − | − |
| 8 | 43/F | I | 1.0 | Headache | AICA, SCA | − | − | − |
| 9 | 28/M | III | 2.0 | Headache, ataxia | PICA AICA | − | − | − |
| 10 | 41/F | III | 2.5 | Headache | SCA, PICA, MB | + | − | + |
| 11 | 43/F | I | 2.0 | Headache,
diplopia | AICA, SCA | − | − | − |
| 12 | 27/F | I | 1.5 | Headache | PICA, MB | − | − | − |
| 13 | 49/M | I | 1.0 | Vertigo | PICA | − | + | − |
| 14 | 53/M | I | 2.0 | Headache | PICA | − | − | + |
| 15 | 36/F | III | 2.5 | Ataxia, nausea | AICA, SCA | − | − | + |
| 16 | 56/M | I | 3.0 | Headache | SCA, PICA | + | − | − |
| 17 | 49/M | I | 2.0 | Headache | PICA AICA | − | − | − |
| 18 | 33/F | I | 3.5 | Lower CN palsy | SCA, PICA | + | − | − |
| 19 | 26/M | III | 3.0 | Ataxia | PICA, SCA | − | − | + |
| 20 | 21/F | I | 3.0 | Ataxia,
nystagmus | PICA | − | − | − |
| 21 | 34/F | I | 3.5 | Lower CN palsy | PICA AICA | + | + | + |
| 22 | 15/F | I | 1.0 | Headache,
ataxia | PICA | − | − | + |
| 23 | 22/M | I | 2.5 | Headache | AICA, SCA | − | − | − |
| 24 | 42/M | II | 2.0 | Headache | PICA | − | − | − |
| 25 | 25/F | I | 1.5 | Nystagmus | PICA, MB | − | − | − |
| 26 | 59/M | I | 2.0 | Ataxia,
nystagmus | PICA | − | − | − |
| 27 | 36/M | II | 4.0 | Ataxia,
nystagmus | PICA AICA | + | − | + |
| 28 | 60/M | I | 3.5 | Ataxia,
diplopia | PICA | + | + | + |
Imaging and embolization
Brain computed tomography (CT) scans, magnetic
resonance imaging (MRI) scans and digital subtraction angiography
(DSA) were performed routinely for all patients. Tumors were
divided into four types, based on their location: i) Type I was
found in the cerebellar hemispheres and vermis; ii) type II was
found in the cerebellar tonsil and lateral medulla; iii) type III
was found in the fourth ventricle and brain stem; and iv) type IV
was found in the superior vermis and dorsal midbrain. If the
arteriography performed immediately following injection of the
contrast solution (iopamidol) clearly indicated that the tumor had
an abundant blood supply, selective embolization of the feeding
arteries was performed preoperatively. The indication for
preoperative embolization was decided on an individual basis,
depending on the coagulation time of the feeding arteries when
handling the nodule or the location of the nodule. All embolization
procedures were performed by neuroendovascular specialists. The
authors of the present study recommend that endovascular
embolization be performed immediately or within 1 day of tumor
removal, in order to avoid aggravation of edema in the tumor and
the surrounding cerebellar tissue. However, if visualization by DSA
was delayed, embolization of the feeding arteries was avoided. The
feeding arteries could be divided into four types corresponding to
the tumor classification: i) Type I was fed by the superior
cerebellar artery (SCA), posterior inferior cerebellar artery
(PICA) and anterior inferior cerebellar artery (AICA); ii) type II
was fed by the AICA, PICA and meningeal branches; iii) type III was
fed by the PICA; and iv) type IV was fed by the SCA and meningeal
branches.
A general examination was conducted for the 7
patients with VHL disease and revealed three instances of renal
cysts and one of retinal hemangioma.
Surgical procedure
The blood supply of hemangioblastomas is mainly
derived from meningeal branches and intracranial feeding arteries;
tumors in the cerebellar tonsil, lateral medulla, superior vermis
and dorsal midbrain are fed by meningeal branches (4). During the early stages of surgery, the
feeding arteries can be divided based on the various surgical
approaches: The suboccipital ipsilateral approach, modified
far-lateral approach and suboccipital midline approach were applied
to tumor types I, II and III, respectively. Tumors, identified by
microscopy intraoperatively, appeared as superficial racemose
hemangiomas with a bright or dark red coloration. Intraoperative
microvascular Doppler ultrasonography distinguished feeding
arteries from draining veins. The authors of the present study
believe that an early division of the draining veins may induce
local swelling and influence the surgical procedure, and,
therefore, they should be approached after the majority of the
tumor has been dissected from the peripheral tissue.
The surgical principle for hemangioblastoma is
similar to that for AVM (6). Total
removal of the tumor is vital, and, thus, cleavage of the main
draining veins, which are extremely dilated, should be performed at
the very last moment. Surgery was performed under the principle
that feeding arteries should be distinguished first and draining
veins should be ligated last. The blood supply to the tumor was
interrupted prior to resection, and complete resection was
performed after the peripheral tumor tissue had been dissected
(avoiding resection within the tumor). Finally, the draining vein
was ligated. As internal decompression may cause uncontrollable
bleeding (6), en bloc
resection was carried out even for relatively large tumors. At the
end of the operation, any bleeding was carefully handled by careful
coagulation.
Illustrative case
A 36-year-old man (case 27) presented at the Renji
Hospital with headaches, cerebellar ataxia, nystagmus and hearing
difficulty in his right ear. The patient had a 1-month history of
nausea and vertigo prior to operative exploration via the lateral
suboccipital approach at the local hospital. A hypervascular tumor
was identified intraoperatively, and, given its abundant blood
supply, the therapeutic approach was a ventriculoperitoneal shunt,
with recommendation for further treatment.
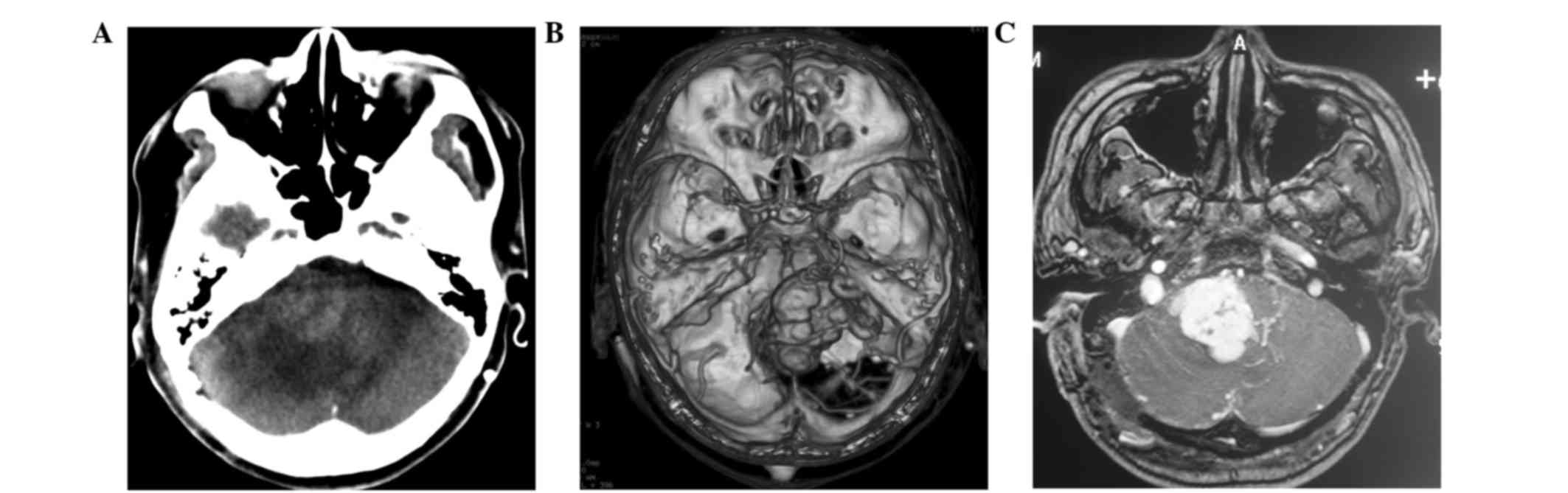
CT and MRI scans at our hospital revealed an intense
mass in the right cerebellopontine angle (CPA), measuring ~40 mm in
diameter (Fig. 1A-C). The cerebral
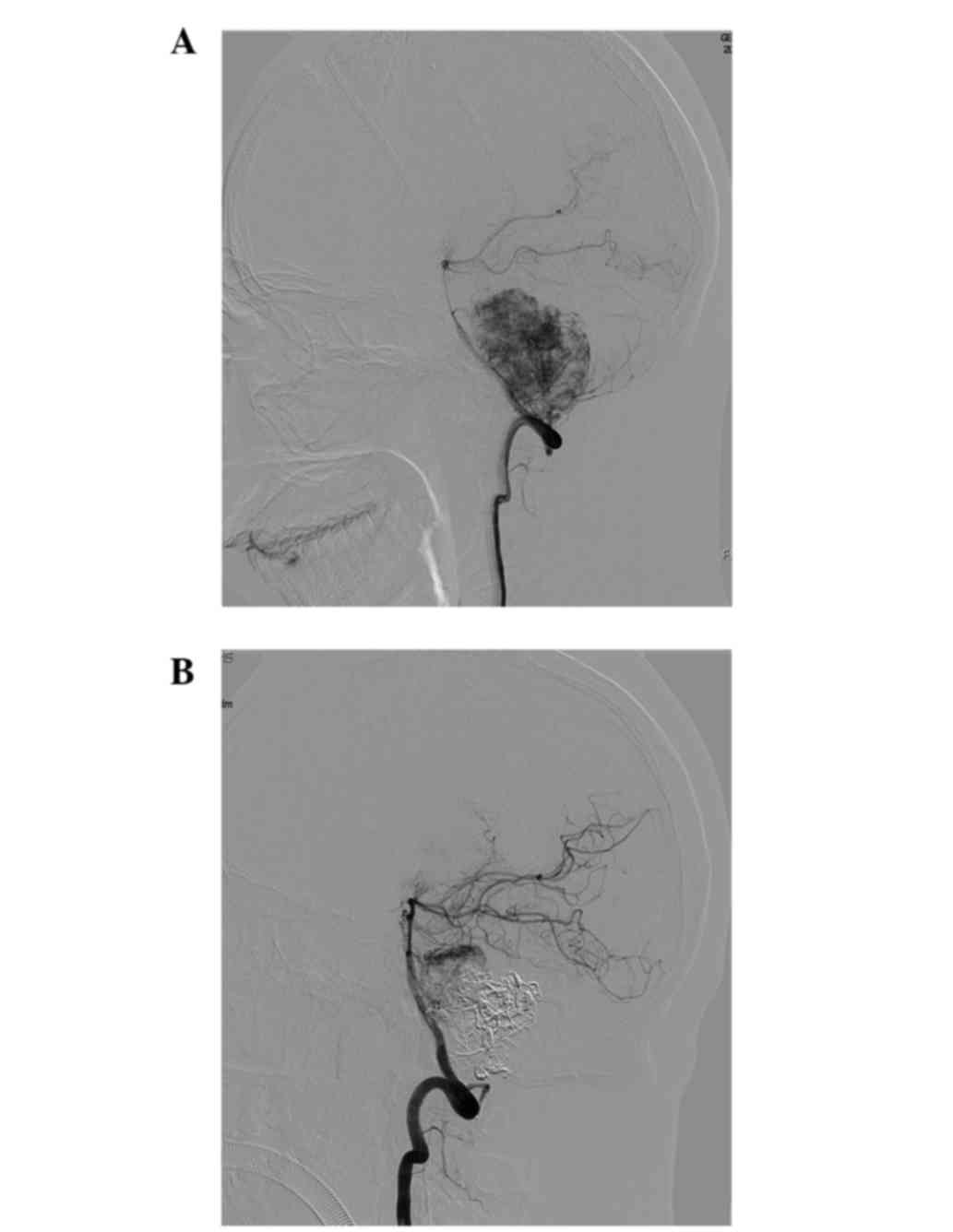
angiogram showed a hypervascular tumor fed by the right AICA and
PICA (Fig. 2A). The patient underwent
preoperative embolization of the feeding artery following the
routine diagnostic angiography. All endovascular procedures were
performed under general anesthesia. A microcatheter was inserted
into the tumor feeders and the glue was delivered under image
acquisition. The angiogram following embolization revealed a 90%
reduction in the tumor vascularization (Fig. 2B). The day after embolization,
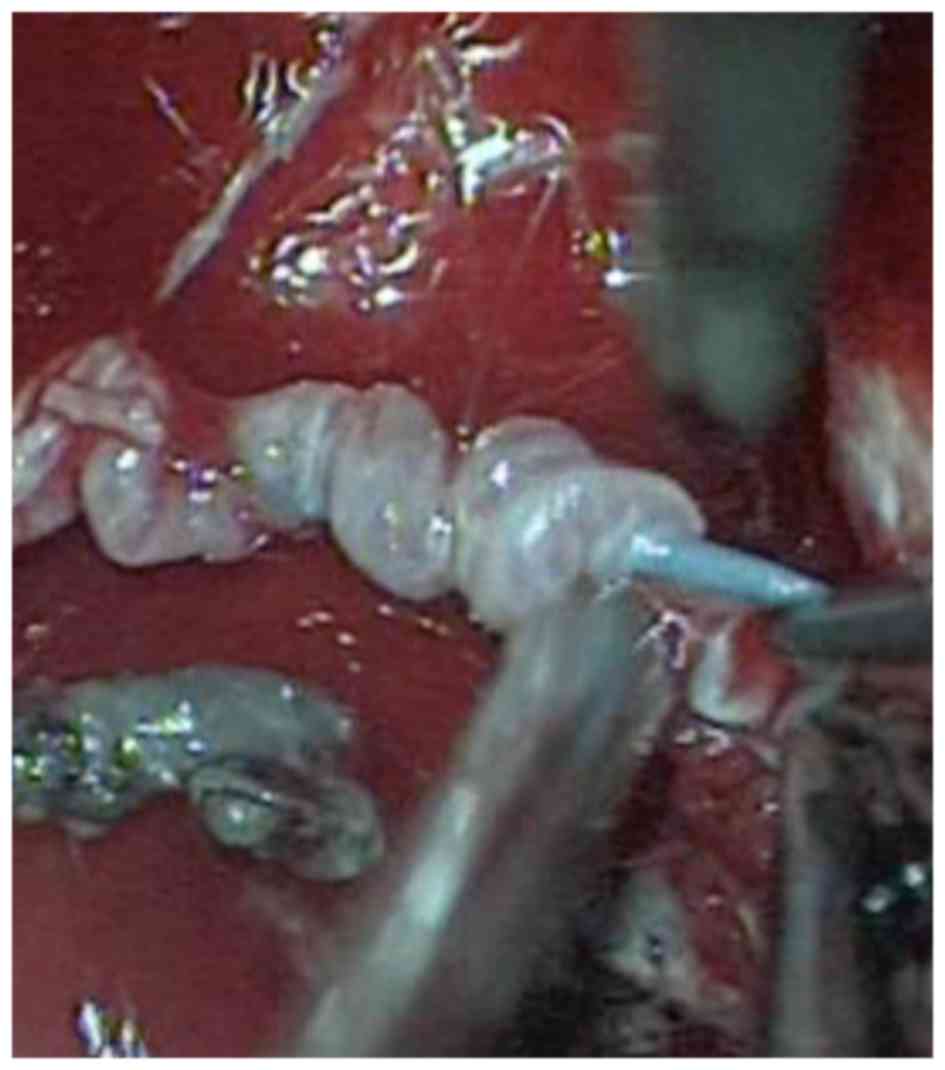
complete surgical tumor resection was performed via a modified
far-lateral approach (6). The
embolized feeding arteries were visible as solid white vessels
directly in the microscopic fields, which was useful for
intraoperative orientation. The microcatheter failed to be
withdrawn following embolization and was removed intraoperatively
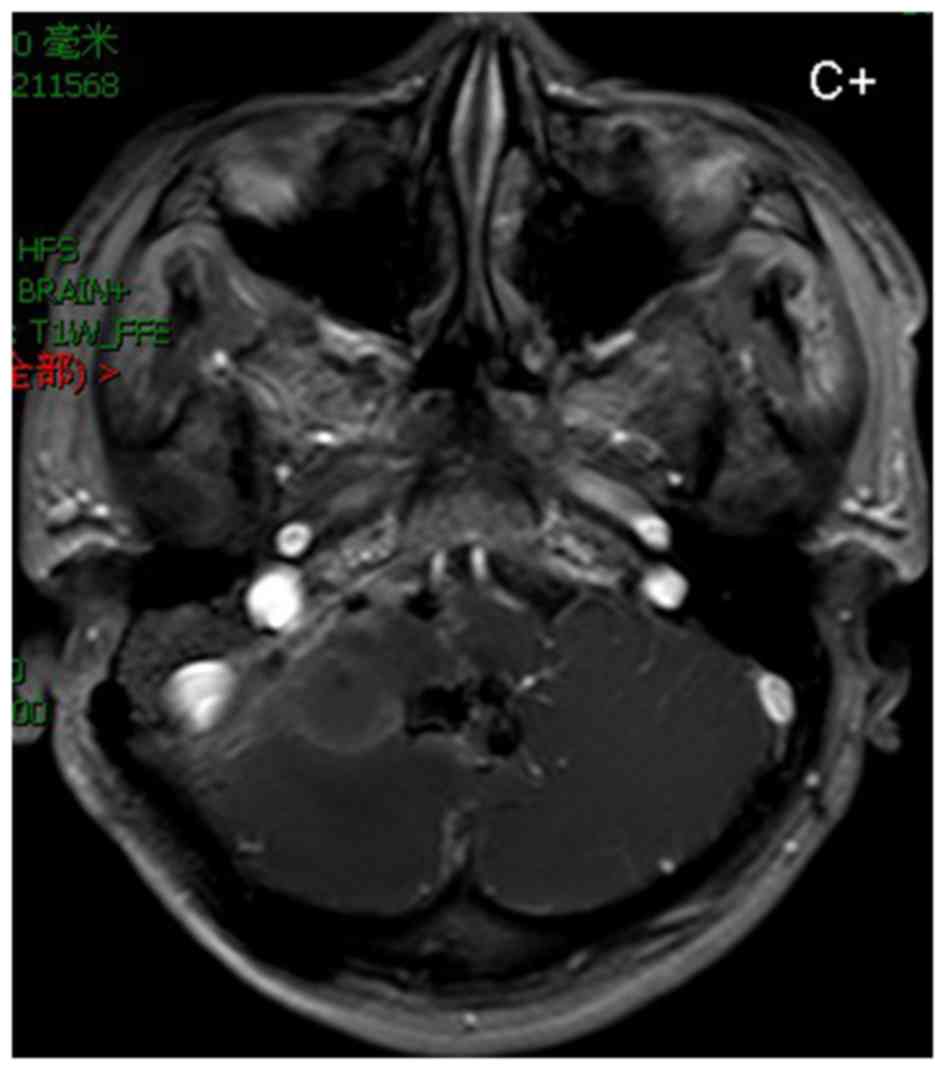
(Fig. 3). MRI showed no residual
tumor at 3 weeks postoperatively (Fig.
4). The postoperative neurological examination did not reveal
any neurological complication, such as facial palsy or auditory
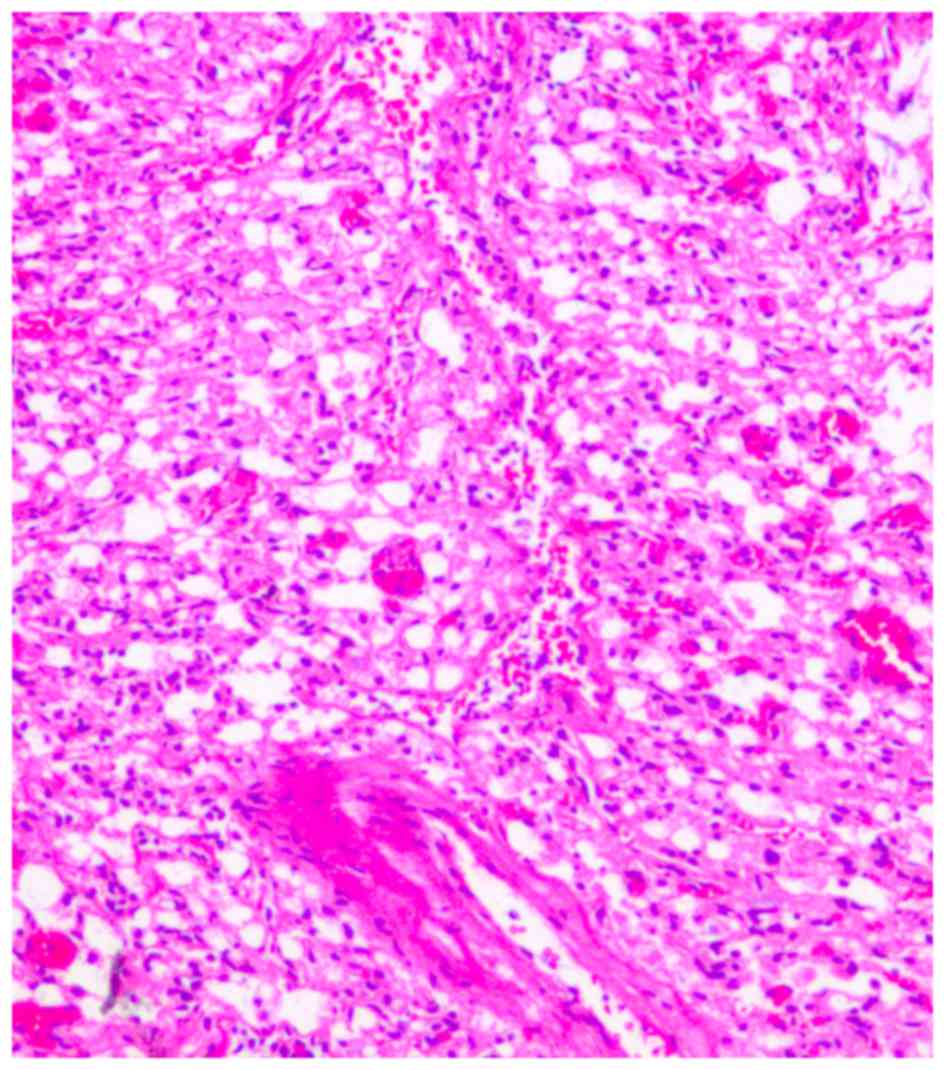
function and lower cranial nerve symptoms. Histological examination
revealed typical hemangioblastomas consisting of numerous vessels,
capillary mesh and stromal cells (Fig.
5). Tumor cells were fixed by formaldehyde and embedded in
paraffin. Slice diameter was 5 µm. Histological analysis of the
tumor specimen stained with hematoxylin and eosin revealed numerous
vessels, a capillary mesh and vacuolated stromal cells, with large
nuclei and an eosinophilic foamy cytoplasm with a light microscope.
At a 6 month follow-up appointment, this patient's symptoms were
markedly improved.
Results
Imaging results
The CT scans of the solid hemangioblastomas showed
lesions that were either isodense or hyperdense, with clear
margins. Enlarged feeding and draining vessels were visualized
following contrast enhancement. The MRI scans showed low or
isointense signals on T1-weighted MRI scans and relatively high
signal intensities on T2-weighted MRI scans. In the majority of
cases, abundant tumor feeding arteries that formed an intense
vascular network around the mass were observed.
Patient outcomes
Preoperative embolization was achieved in 7 cases.
The blood supply of the tumor was reduced significantly after
embolization of the feeding arteries, without any related
neurological deficits. In one case, the microcatheter could not be
withdrawn following embolization and was removed during surgery.
The tumor resection surgery was performed the day following
embolization for all patients, in order to use embolized vessels as
markers for intraoperative orientation.
En bloc resection was performed for all 28
patients. Postoperative pathological examinations of the resected
tumors confirmed that all were hemangioblastomas. Following
surgery, 22 patients (78.6%) showed an improvement of the
neurological deficit. Of the remaining 6 patients (21.4%), 2 showed
neurological improvement or full recovery at the 3-month follow-up.
Of the 7 patients with VHL disease, 6 presented with intracranial
recurrences at other sites. There was no operative mortality.
Discussion
Hemangioblastomas are pathologically benign
neoplasms that originate predominantly from CNS tissues and should
be completely removed. Clinically, about two-thirds appear as
well-circumscribed cystic masses with hypervascular mural nodules
(9). Although there are no
histological differences between the cystic and solid tumor
subtypes (2), solid hemangioblastomas
have an increased risk of excessive intraoperative bleeding and
postoperative complications (10). A
careful strategy and a tailored approach should be considered for
these cases. Based on our previous experiences, large solid
subtypes are associated with a high surgical difficulty, given
their AVM-like characteristics (7,11). Since
most have the risk of uncontrollable intraoperative bleeding, they
are hard to control, and thus, a preoperative intervention may be
particularly important. Therefore, the present study aimed to
reduce vascularization and achieve adequate operation space.
There are many ways to reduce the vascularization of
a tumor. Kamitani et al (5)
reported that radiosurgery at 9 months prior to a craniotomy
significantly reduced the vascularization and subsequently enabled
complete and safe tumor removal (5).
Radiosurgery is also the elected approach for patients with small
tumors, for patients with underlying medical conditions
incompatible with surgery and for patients with multiple tumors
(2). However, in a prospective study,
Wan et al identified 2 patients with large hemangioblastomas
who received γ-knife treatment, followed by surgery (8). They found that these tumors remained
highly vascularized at 6–12 months following γ-knife treatment. In
addition, they reviewed a number of cases that received
radiotherapy as an adjuvant postoperative treatment, and found that
the long-term outcome of tumor control was mostly poor. Since
hemangioblastomas are generally considered to be radio-resistant
tumors, this highlights the importance of preoperative
embolization. In 7 of our cases, preoperative embolization was
performed after a preoperative evaluation of the blood supply by
DSA. The present study found that, following embolization, the
tumors became firm, with clear boundaries, and were easy to orient.
As a result, intraoperative bleeding was significantly decreased,
the surgery time was shortened and the likelihood of total tumor
resection was increased, which is consistent with previous studies
(3,8).
However, preoperative embolization is associated with a high risk
of cerebellar infarction and/or intratumoral hemorrhage, and makes
the texture of the tumor tenacious, which increases surgical
difficulty (12,13). No related complications occurred in
any of the cases in the present study. Generally, embolization is
suitable only for patients with definite tumor-feeding arteries, as
shown by angiography, and only if it does not affect the normal
blood supply (13). The tumor-feeding
arteries are often located deeply on both sides of the tumor, while
the large draining veins typically arise from the tumor surface
(13). With careful observation,
these vessels can be identified accurately during the operation and
embolization can be performed with a high ratio of technical
success, without permanent neurological complications.
Although 6–10% of all intracranial tumors are found
in the CPA, the majority are vestibular schwannomas and meningiomas
(9,11). Because of differences in the surgical
strategies, differential diagnosis is crucial for safe tumor
management. Hemangioblastomas generally have an intra-axial origin
and are rarely found in the CPA (13). To our knowledge, a very small number
of patients with large hemangioblastoma in the CPA have been
reported, and, of those, only 10 cases were presented in English
literature (2,7,9). One large
tumor in the CPA was observed in one of the patients in the present
study. The tumor was completely resected via a modified far-lateral
approach. The embolized feeding arteries were visible in the
microscopic fields during the operation as solid white vessels,
thus becoming useful for intraoperative orientation. Postoperative
MRI showed no residual tumor. Ataxia and nystagmus were not
improved, but no newly developed cranial nerve deteriorations were
observed. There were no operative complications.
Various approaches, including the suboccipital
ipsilateral approach, the modified far-lateral approach and the
suboccipital midline approach, have been used with or without
preoperative embolization for the surgical resection of
hemangioblastomas. About two-thirds of hemangioblastomas appear as
well-circumscribed cystic masses with hypervascular mural nodules.
Although there are no histological differences between the cystic
and solid tumor subtypes, solid subtypes may require a more
extended approach to achieve an adequate operation space (6,11). Dow
et al (11) used the
transcochlear far lateral approach in patients with large (>3
cm) solid hemangioblastoma in the CPA. This approach provided
visual access that was wide enough to permit early control of
proximal feeding vessels and to allow a safe circumferential
dissection of the lesion (11). For
cystic hemangioblastomas in the CPA, Bush et al (9) removed the tumor via a translabyrinthine
approach, as they first mistook it for an atypical cystic
vestibular schwannoma. They suggested that, had an hemangioblastoma
been considered, the suboccipital approach might have provided
adequate exposure and potentially preserved the hearing of the
patient (9). Therefore, novel
microsurgical techniques and a better understanding of the vascular
pattern of this type of tumor have enhanced the surgical strategies
for hemangioblastoma.
In conclusion, given the improvements in
microsurgical techniques and the understanding of the tumor
vascular pattern, en bloc surgical resection is the optimal
treatment for solid hemangioblastomas of the posterior fossa. For
large tumors, selective preoperative embolization of the feeding
artery is critical for tumor removal associated with a low
morbidity and mortality rate.
Acknowledgements
This study was supported by the Science and
Technology Commission Foundation of Shanghai (grant no.
11JC1408602), the Natural Science Foundation of China (grant nos.
81372710, 81000527, 81101801 and 81100547) and the Natural Science
Foundation of Jiangsu Province (grant no. BK2010159). The authors
would like to thank Clarity Manuscript Consultants LLC
(Indianapolis, IN, USA) for technical assistance in editing this
manuscript.
References
|
1
|
Amano T, Tokunaga S, Shono T, Mizoguchi M,
Matsumoto K, Yoshida F and Sasaki T: Cerebellar haemangioblastoma
manifesting as hearing disturbance. Neurol Med Chir (Tokyo).
49:418–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rachinger J, Buslei R, Prell J and Strauss
C and Strauss C: Solid haemangioblastomas of the CNS: A review of
17 consecutive cases. Neurosurg Rev. 32:37–47; discussion 47–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roberti F, Jones RV and Wright DC: Cranial
nerve haemangioblastomas. Report of a rare case and review of
literature. Surg Neurol. 67:640–646; discussion 646. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singounas EG: Haemangioblastomas of the
central nervous system. Acta Neurochir (Wien). 44:107–113. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamitani H, Hirano N, Takigawa H, Yokota
M, Miyata H, Ohama E and Watanabe T: Attenuation of vascularity by
preoperative radiosurgery facilitates total removal of a
hypervascular haemangioblastoma at the cerebello-pontine angle:
Case report. Surg Neurol. 62:238–243; discussion 243–244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsushima T, Kawashima M, Masuoka J,
Mineta T and Inoue T: Transcondylar fossa (supracondylar
transjugular tubercle) approach: Anatomic basis for the approach,
surgical procedures, and surgical experience. Skull Base. 20:83–91.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nair BR, Joseph V, Chacko G and Keshava
SN: Giant solid haemangioblastoma of the cerebellopontine angle: A
technically challenging case. Neurol India. 62:228–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan JQ, Cui H and Wang Y: Surgical
management of large solid haemangioblastomas of the posterior
fossa. J Clin Neurosci. 18:39–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bush ML, Pritchett C, Packer M,
RayChaudhury A and Jacob A: Haemangioblastoma of the
cerebellopontine angle. Arch Otolaryngol Head Neck Surg.
136:734–738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biondi A, Ricciardi GK, Faillot T, Capelle
L, Van Effenterre R and Chiras J: Haemangioblastomas of the lower
spinal region: Report of four cases with preoperative embolization
and review of the literature. AJNR Am J Neuroradiol. 26:936–945.
2005.PubMed/NCBI
|
|
11
|
Dow GR, Sim DW and O'Sullivan MG: Excision
of large solid haemangioblastomas of the cerebellopontine angle by
a skull base approach. Br J Neurosurg. 16:168–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cornelius JF, SaintMaurice JP, Bresson D,
George B and Houdart E: Hemorrhage after particle embolization of
haemangioblastomas: Comparison of outcomes in spinal and cerebellar
lesions. J Neurosurg. 106:994–998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montano N, Doglietto F, Pedicelli A,
Albanese A, Lauretti L, Pallini R, Lauriola L, Fernandez E and
Maira G: Embolization of haemangioblastomas. J Neurosurg.
108:1063–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|