Introduction
Breast conserving surgery (BCS) is a standard
procedure for the treatment of early-stage breast cancer (1). It has been demonstrated in previous
studies that the 10-year overall survival (OS) rate of patients
treated with BCS (65%) is similar to that of those treated with
mastectomy (66%) (1,2); however, there is a higher risk of
ipsilateral breast tumor recurrence (IBTR) following BCS (3). As IBTR is associated with an increased
risk of distant disease and mortality, adjuvant radiation therapy
of the residual breast tissue following BCS is often required to
decrease the risk of IBTR (3).
Furthermore, adjuvant hormone therapy and chemotherapy, which are
used for the majority of patients, may prevent IBTR (3); however, a considerable number of IBTR
cases are diagnosed following BCS administered with an adjuvant
therapy (4,5).
The risk factors for developing IBTR have yet to be
determined, and further studies of the tumor biology of IBTR are
required to identify tumor characteristics that may be used in the
selection of an appropriate treatment strategy. A previous study
revealed a significant difference in distant disease-free survival
following IBTR depending on the subtype of breast cancer tissues
present, as determined by immunohistochemical staining (6). In particular, increased or persistently
high Ki-67 expression levels in cases of IBTR were significantly
associated with a poorer prognosis (7).
A predictive prognostic factor that has gained
significant interest in association with breast cancer is the
presence of cancer stem cells (CSCs). CSCs have self-renewal and
multi-lineage differentiation capacities and are frequently
resistant to conventional anti-cancer drug and radiation therapies
(8). The presence of CSCs may be an
important factor in cases of IBTR occurring subsequent to BCS and
adjuvant therapy. However, to the best of our knowledge, there are
no previous studies that have discussed the presence of CSCs within
IBTR tissues. Aldehyde dehydrogenase 1 (ALDH1) is a marker of CSCs
(9) that may be easily evaluated in
primary breast cancer tissues and is a potentially useful
prognostic factor. In the current study, the expression levels of
ALDH1 in the primary lesion and in IBTR tissues were investigated,
and their association with other clinicopathological factors and
the prognostic impact of ALDH1 in IBTR was determined.
Materials and methods
Patients and samples
A total of 271 consecutive patients with
histologically diagnosed IBTR without distant metastases, who
underwent definitive surgery for IBTR between 1989 and 2008, were
recruited from eight institutions (Okayama University Hospital,
Okayama; The Cancer Institute Hospital of the Japanese Foundation
for Cancer Research, Tokyo; Kumamoto City Hospital, Kumamoto
National Hospital Organization; Osaka National Hospital, Osaka; St.
Luke's International Hospital, Tokyo; Osaka Medical Center for
Cancer and Cardiovascular Diseases, Osaka; Osaka Medical College,
Osaka; Kyoto Prefectural University of Medicine, Kyoto) in Japan.
Each institution's review board approved this retrospective study.
The inclusion criteria for were as follows: i) The patient was
undergoing BCS and axillary surgery (sentinel lymph node biopsy was
only permitted if the nodes had no metastasis); ii) IBTR was
histologically determined; and iii) the patient was undergoing
definitive surgery for IBTR at a time prior to the year 2008. The
exclusion criteria were as follows: i) Presence of synchronous
metastases (defined as occurring within three months); ii)
bilateral breast cancer; iii) history of prior malignancies other
than breast cancer; and iv) presence of tumors located in the skin
or muscle only, without associated parenchymal disease. Of the 271
IBTR cases, 182 met the criteria and were included in the present
study, which investigated the frequency and prognostic impact of
the ALDH1 expression profile in IBTR and primary breast cancer
tissue.
Immunohistological examination
Estrogen receptor (ER) and progesterone receptor
(PgR) status were determined by immunohistochemistry (IHC)
(7), and those tumors with ≥10%
positively stained tumor cells were classified as being positive
for ER. Tissue samples were considered positive for human epidermal
growth factor receptor 2 (HER2) if scored as 3+, or if fluorescence
in situ hybridization identified a HER2/chromosome 17 ratio
of >2.0 (7). The ER and HER2
status in each tissue sample were evaluated independently in each
institution. Proliferation activity was assessed by immunostaining
with the Ki-67 antibody (clone MIB-1, cat. no., M7240; dilution,
1:100; Dako, Glostrup, Denmark) using an autostainer (Benchmark XT;
Ventana Medical Systems, Inc., Tucson, AZ, USA). Ki-67 staining was
centrally evaluated by one pathologist (Kumamoto City Hospital) who
was blinded to the clinical data. The proportion of proliferating
cells was determined by counting ≥500 tumor cells in hot spots,
defined as having a dense concentration of positive cancer nuclei
in each tumor according to a previously described protocol
(10,11) Breast cancer tissues were classified
using the IHC results, according to a previously described protocol
(6), into the following subtypes:
Triple-negative (ER-, PgR- and HER2-negative); HER2 (HER2-positive,
ER- and PgR-negative); luminal A (ER- and/or PgR-positive,
HER2-negative and Ki-67 <15%); and luminal B (ER-positive,
HER2-negative and Ki-67 ≥15%, or ER-positive and
HER2-positive).
IHC was used to evaluate the ALDH1 expression levels
in the surgical tissue specimens of primary cancer and IBTR cases
that were identified to be invasive carcinomas. The antibody used
was anti-ALDH1 (clone 44; #611195; dilution, 1:250; BD Biosciences,
Franklin Lakes, NJ, USA). Imaging analysis of ALDH1 expression in
the breast tumor tissues was performed using fluorescence
microscopy with one selected high-power field (magnification, 400x;
Olympus BX53; Olympus Corporation, Tokyo, Japan) per case, as the
immunoreactivity of ALDH-1 was approximately homogeneous with the
results from a previous study (12–14). The
percentage of ALDH1-positive cells was determined as described in
previous studies (12–14), in which the tumor specimens were
classified into positive and negative groups based on the IHC
staining for ALDH1, with positive defined as >1% of tumor cells
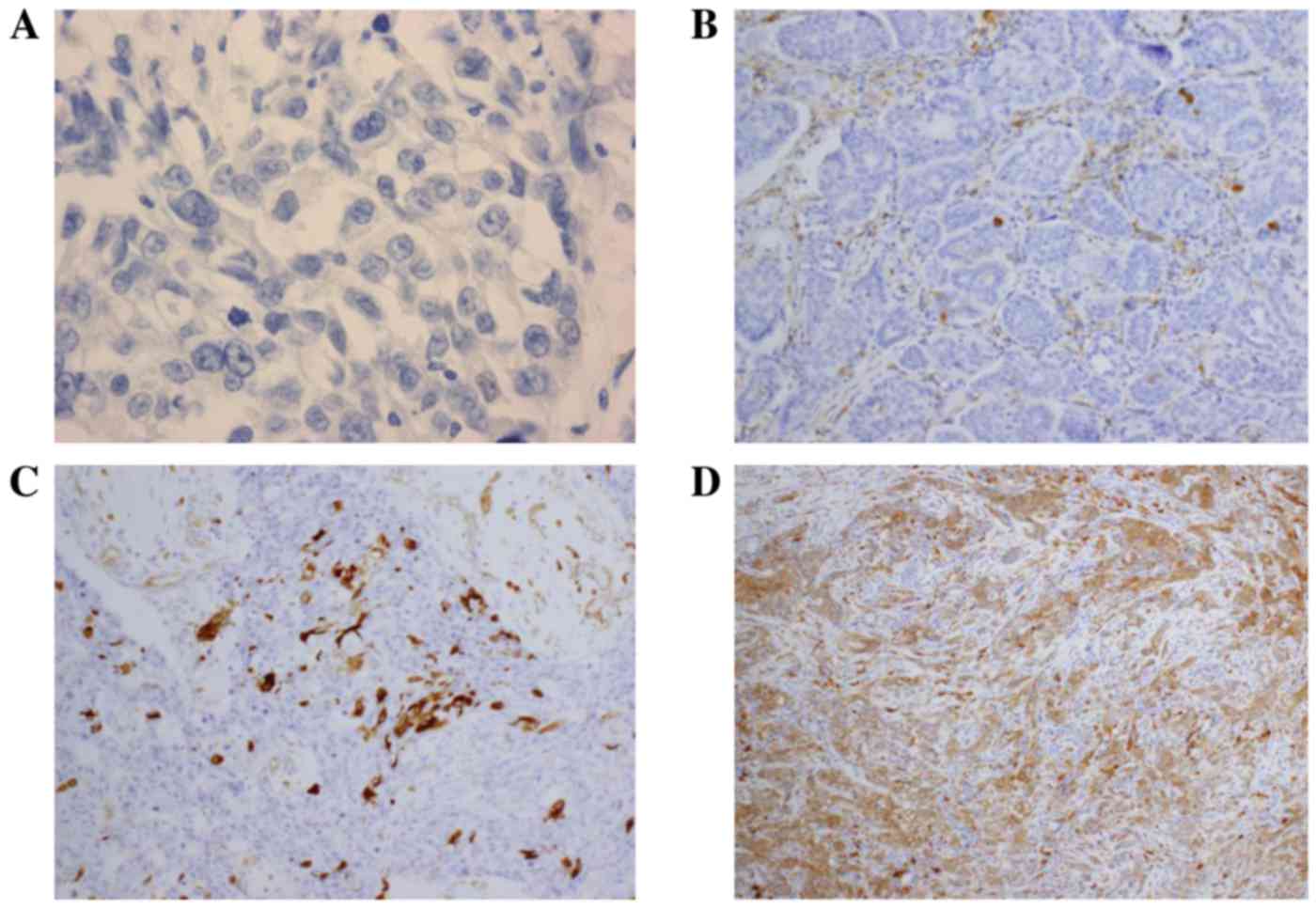
exhibiting positive staining. Representative results of IHC
staining for ALDH1 in breast cancer tissues are presented in
Fig. 1.
Statistical analysis
Disease-free survival (DFS) was calculated as the
duration from the time of initial surgery for IBTR to the diagnosis
of a recurrence. Differences in clinicopathological data were
compared using the χ2 test. For comparison between
patients with recurrent disease and the recurrence-free patients,
the odds ratios for differing variables were assessed by applying a
logistic regression model for univariate and multivariate analyses.
Survival curves were calculated using the Kaplan-Meier method, and
the log-rank test was used to evaluate the statistical significance
of the differences in survival among the patient subgroups.
Varations between overall survival curves were determined using a
log-rank test. For univariate and multivariate analyses, the Cox
regression method was used to evaluate the influence of the
variables on survival. All of the data were analyzed with the use
of JMP version 11.0.0 statistical software (SAS Institute, Cary,
NC, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Patient clinical characteristics are presented in
Table I. A total of 271 patients were
registered for the current study, of which 182 IBTR tissue
specimens were examined for the expression of ALDH1. The median
ages of the patients at the time of initial breast surgery and
surgery for IBTR were 46 years (range, 26–84 years) and 51 years
(range, 29–88 years), respectively. In the primary tumor tissues,
the proportions of cells that were positive for ER, PgR and HER2
were 55, 46 and 11%, respectively; these values were 62, 43 and 22%
in the IBTR tissue samples. Following primary surgery, 10% of
patients exhibited a positive surgical margin. Adjuvant radiation
therapy was administered to 51% of the patients. The median
duration from initial surgery to a diagnosis of IBTR was 46 months
(range, 2–206 months).
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| A, Patient and tumor
characteristics |
|---|
|
|---|
|
| Value |
|---|
|
|
|
|---|
| Characteristic | Primary tumor | IBTR |
|---|
| Age, years; median
(range) | 46 (26–84) | 51 (29–88) |
| Median primary tumor
size, cm (range) | 2 (0–6.8) | – |
| ER, n (%) |
|
|
|
Positive | 101 (55) | 113 (62) |
|
Negative | 64 (35) | 69
(38) |
|
Unknown | 17 (9) | – |
| PgR, n (%) |
|
|
|
Positive | 83 (46) | 78
(43) |
|
Negative | 69 (38) | 104 (57) |
|
Unknown | 30 (16) | – |
| HER2, n (%) |
|
|
|
Positive | 20 (11) | 40
(22) |
|
Negative | 96 (53) | 142 (78) |
|
Unknown | 66 (36) | – |
| Ki-67, n (%) |
|
|
|
≥15% | – | 109 (60) |
|
<15% | – | 73
(40) |
|
| B, Treatment and
survival characteristics |
|
| Characteristic |
| Value |
|
| Adjuvant treatment,
n (%) |
|
|
|
Chemotherapy |
| 61 (34) |
| Hormone
therapy |
| 105 (58) |
|
Trastuzumab |
| 5 (3) |
|
Adjuvant radiation
therapy |
| 90 (51) |
| Disease-free
survival time, months; median (range) |
| 46 (2–206) |
ALDH1 expression in primary tumor and
IBTR
The expression levels of ALDH1 in the primary tumor
and IBTR tissues are presented in Table
II. A total of 37 (20%) primary tumor tissue samples and 43
(23%) IBTR tissue specimens were ALDH1 positive (>1% positively
stained cells). As a minority of tissue samples were classified as
2+ (primary tumor, 2%; IBTR, 4%) or 3+ (primary tumor, 2%; IBTR,
3%), it was determined that tissues with >1% ALDH1-positive
cells would be considered positive cases. The concordance of the
ALDH1 expression status between the primary tumor and IBTR cases is
presented in Table III; the total
concordance rate was 68%.
 | Table II.Expression profile of ALDH1 in breast
cancer. |
Table II.
Expression profile of ALDH1 in breast
cancer.
| ALDH1
expression | Primary tumor, n
(%) | IBTR, n (%) |
|---|
| 0 | 114 (63) | 139 (76) |
| 1+ | 29
(16) | 30
(16) |
| 2+ | 4
(2) | 7
(4) |
| 3+ | 4
(2) | 6
(3) |
| Unknown | 31
(17) | 0
(0) |
 | Table III.Concordance of ALDH1 expression in
primary tumor and IBTR tissues. |
Table III.
Concordance of ALDH1 expression in
primary tumor and IBTR tissues.
|
| ALDH1 expression in
primary tumor, n (%) |
|---|
|
|
|
|---|
| ALDH1 expression in
IBTR | Negative | Positive |
|---|
| Negative | 92 (61) | 22
(15) |
| Positive | 26 (17) | 11 (7) |
Correlation between ALDH1/Ki-67
expression and disease-free survival
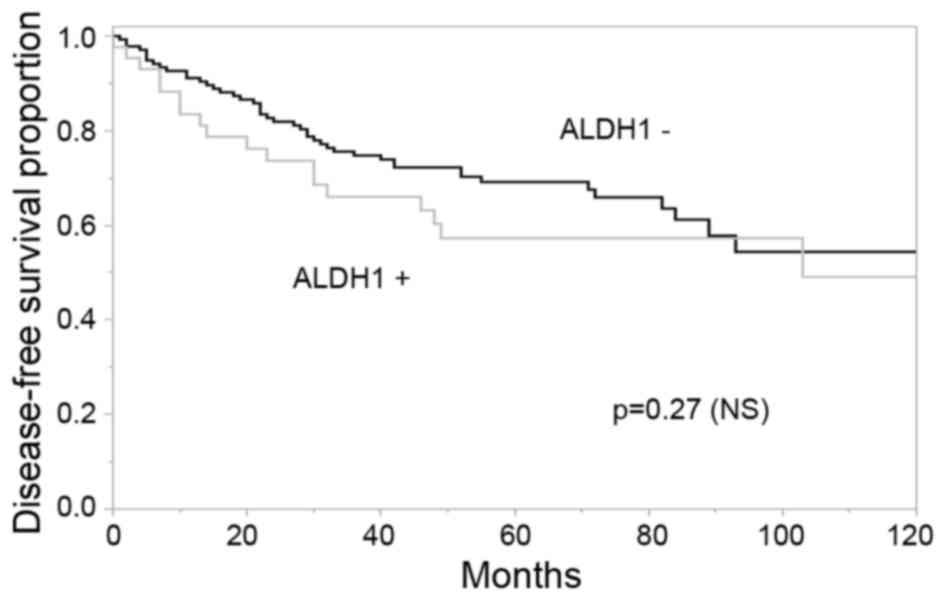
There was no significant association observed
between the rate of DFS following IBTR and ALDH1 expression status
in IBTR tissues (Fig. 2); however,
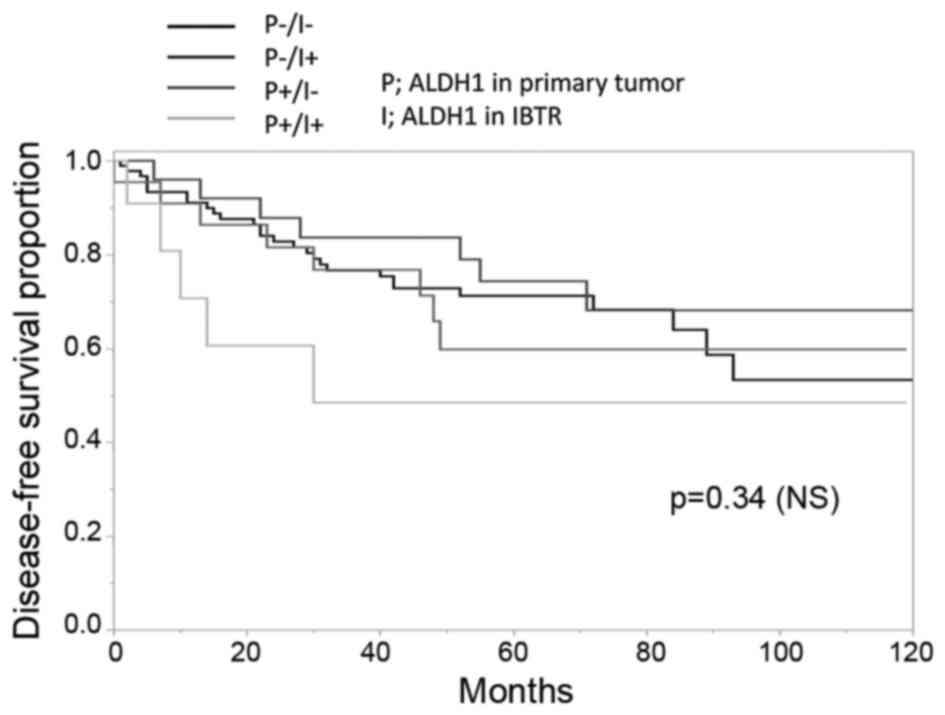
patients who exhibited ALDH1-positive tissues in the primary tumor
and the IBTR had the poorest DFS (Fig.
3). Multivariate analysis revealed that Ki-67 ≥15% (odds ratio,
2.31; P=0.003) and ER-positive status (odds ratio, 0.48; P=0.027)
were statistically significant predictive factors for DFS following
IBTR (Table IV); however, ALDH1 did
not have prognostic value (P=0.513).
 | Table IV.Multivariate analysis using the Cox
proportional hazards model to identify predictors of disease-free
survival. |
Table IV.
Multivariate analysis using the Cox
proportional hazards model to identify predictors of disease-free
survival.
| Variable | Odds ratio | 95% CI | P-value* |
|---|
| ALDH1-positive | 1.21 | 0.676–2.065 | 0.513 |
| Ki-67 ≥15% | 2.31 | 1.320–4.260 | 0.003 |
| ER-positive | 0.48 | 0.233–0.920 | 0.027 |
| PgR-positive | 0.93 | 0.457–1.959 | 0.846 |
| HER2-positive | 0.78 | 0.410–1.430 | 0.436 |
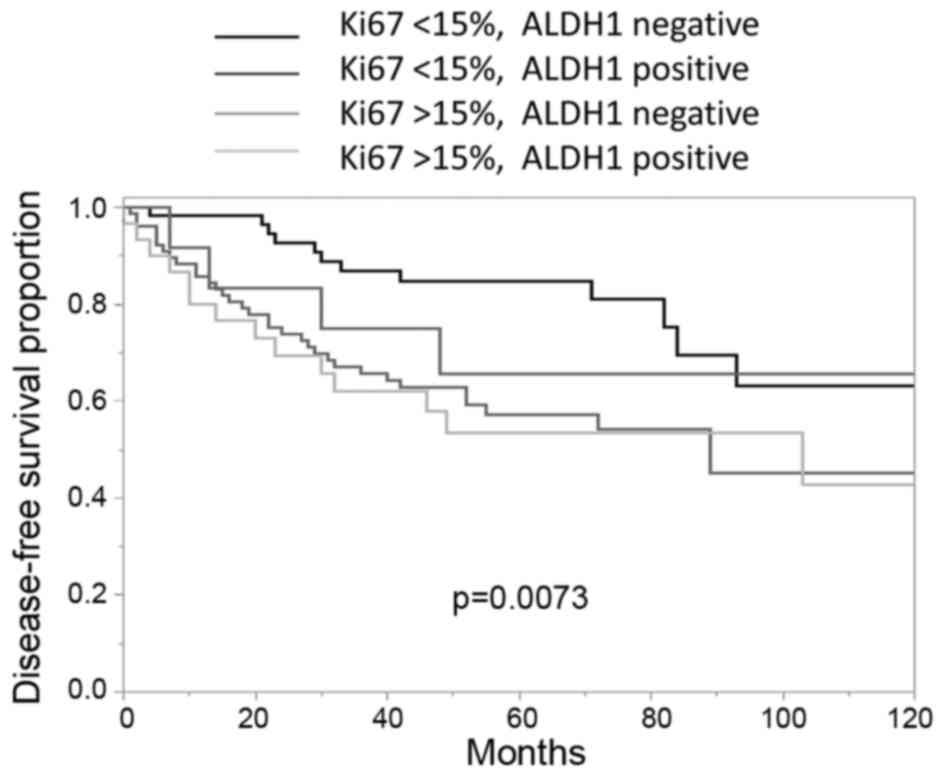
According to ALDH1 and Ki-67 statuses, the tissue
samples were further subdivided (Fig.
4). The ALDH1-negative patients with low Ki-67 expression
(<15%) had typically better outcomes compared with those
patients exhibiting high Ki-67 expression levels (≥15%). However,
there were no significant differences observed between the Ki-67
<15%, ALDH1-positive group and the Ki-67 <15%, ALDH1-negative
group.
ALDH1 expression according to
subtype
When classified according to ER, HER2 and Ki-67
expression profiles, the subtype distribution of IBTR was as
follows: Luminal A, 51 patients (28%); luminal B, 62 patients
(34%); HER2, 26 patients (14%); and triple-negative, 43 patients
(24%). The proportions of each of the subtypes that were positive
for ALDH1 were as follows: Luminal A, 20%; luminal B, 24%; HER2,
35%; and triple-negative, 21% (Table
V). The proportions of patients that received adjuvant drug
therapy following IBTR for luminal A, luminal B, HER2 and
triple-negative types were 93, 92, 58 and 56%, respectively. A
total of 7 patients (27%) with the HER2 subtype received
trastuzumab following IBTR surgery.
 | Table V.Expression of ALDH1 according to ER
and HER2 status in cases of ipsilateral breast tumor
recurrence. |
Table V.
Expression of ALDH1 according to ER
and HER2 status in cases of ipsilateral breast tumor
recurrence.
| Category | Total cases, n | ALDH1-positive
cases, n (%) | P-value* |
|---|
| ER |
|
| 0.54 |
|
Positive | 113 | 25 (22) |
|
|
Negative | 69 | 18 (26) |
|
| PgR |
|
| 0.84 |
|
Positive | 104 | 24 (23) |
|
|
Negative | 78 | 19 (24) |
|
| HER2 |
|
| 0.29 |
|
Positive | 40 | 12 (30) |
|
|
Negative | 142 | 31 (22) |
|
| Ki-67 |
|
| 0.14 |
|
≥15% | 109 | 30 (28) |
|
|
<15% | 73 | 13 (18) |
|
| Subtype |
|
| 0.52 |
| Luminal
Aa | 51 | 10 (20) |
|
| Luminal
Bb | 62 | 15 (24) |
|
| HER2
typec | 26 | 9
(35) |
|
| Triple
negatived | 43 | 9
(21) |
|
There was no significant correlation observed
between ER, PgR, HER2 or Ki-67 expression status and ALDH1
expression status, or between ALDHI and the specific tissue subtype
(Table V). Furthermore, there was no
significant association between the administration of adjuvant
therapy following primary surgery and ALDH1 expression levels in
IBTR tissues (Table VI).
 | Table VI.Association between adjuvant therapy
administered following the primary surgery and ALDH1 expression
levels in IBTR tissues. |
Table VI.
Association between adjuvant therapy
administered following the primary surgery and ALDH1 expression
levels in IBTR tissues.
| Category | ALDH1-positive
cases, n (%) | P-value |
|---|
| RT |
| 0.21 |
| + | 19 (21) |
|
| − | 24 (27) |
|
| CT and/or HT |
| 0.73 |
| + | 34 (25) |
|
| − | 7
(20) |
|
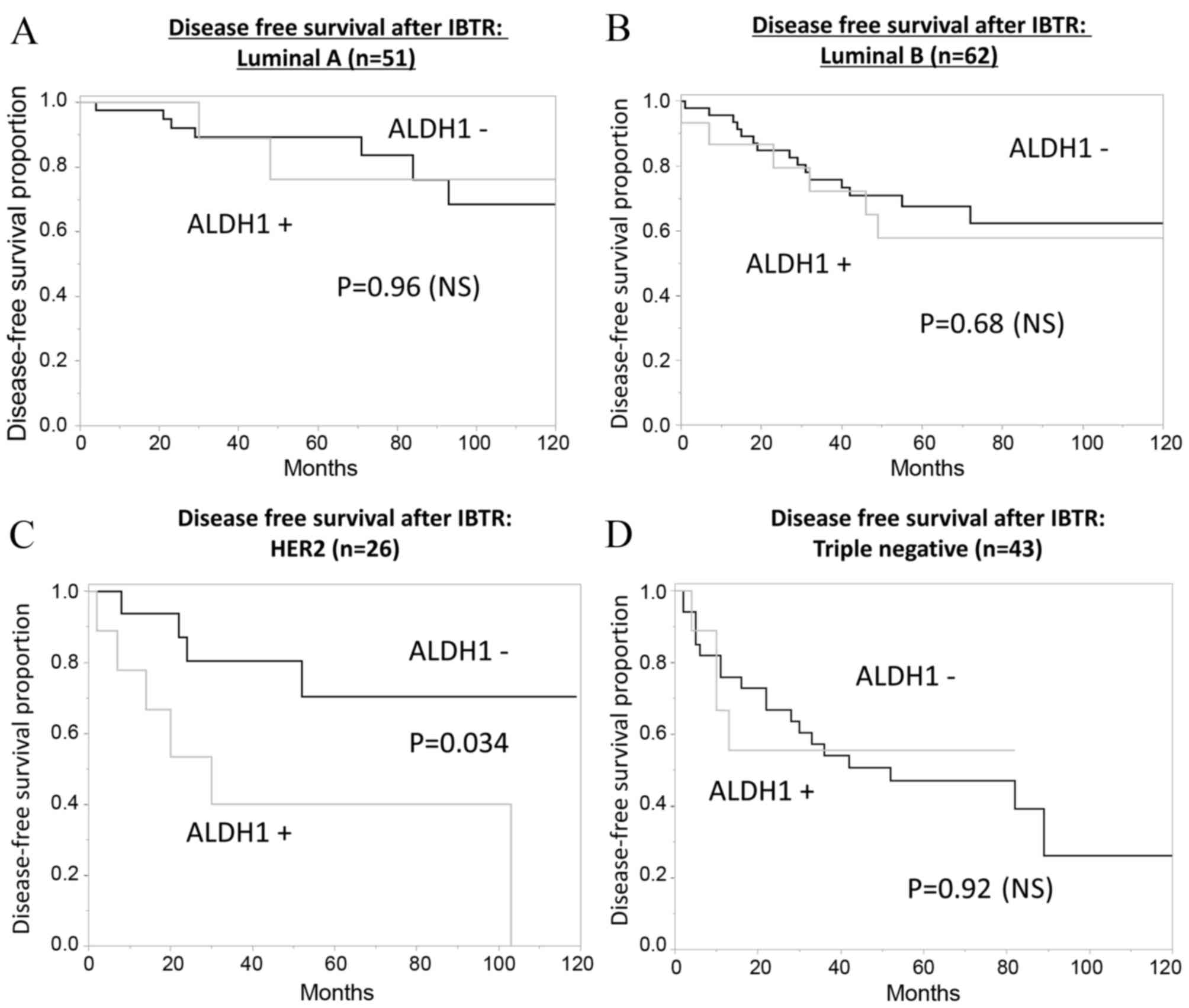
When the prognosis was analyzed with regard to ALDH1
expression and tissue subtype (Fig.
5A-D), the expression status of ALDH1 in IBTR tissues of a HER2
subtype was observed to be a significant prognostic factor for DFS
(P=0.034; Fig. 5C).
There was no significant difference observed in the
rates of adjuvant trastuzumab administration between the
ALDH1-positive and -negative groups (ALDH1-positive, 25%;
ALDH1-negative, 16%).
Discussion
To the best of our knowledge, the present study is
the first to investigate the frequency and prognostic value of
ALDH1 expression in IBTR tissues. The cut-off level for the
classification of positive ALDH1 expression was set at >1% in
the current study, as there is no standardized cut-off level or
method currently available. The ALDH1 positive cells were counted
in hot spots, and positive ALDH1 expression was detected in 20% of
the primary tumor samples and 23% of the IBTR tissue samples. The
frequency of patients with ALDH1-expressing primary breast cancer
(20%) in the current study was similar to that observed in previous
studies (19, 17.5%) (10,11); however, the ALDH1 expression the in
IBTR group (23%) was higher. It was hypothesized that this
variation was due to the dissimilarities between the IBTR and
primary tumors. IBTRs included new primary tumors and true
recurrences following adjuvant therapy. Therefore, ALDH1-positive
cancer stem cells within true recurrence were able to survive
following adjuvant systemic and radiation therapy and may be not
similar to the cancer cells within the primary tumor. A previous
study revealed that the frequency of ALDH1 positive cells in
metastatic tumors is relatively high compared with primary tumors
(14). This discrepancy may be due to
there being no definitive ALDH1 cut-off level; the cut-off level
(>1%) used for the present study was lower than the value used
in previous reports (12–14). The total number of IBTR tissue samples
used in the current study was relatively low, compared with
previous reports that analyzed ALDH1 expression in primary breast
tumors. Therefore, the associations between ALDH1 expression, tumor
subtypes and clinical data could not be analyzed.
A similar expression rate for ALDH1 was observed in
the primary tumor and IBTR tissue specimens. The concordance rate
between ALDH1 expression in primary tumors and associated IBTR was
68%. It was previously reported that variation in the ER, HER2 and
Ki-67 statuses between the primary tumors and IBTR tissues was
observed in 16.2, 13.7 and 36.8% of cases, respectively (7). The discordance rates for ALDH1
expression and Ki-67 status were higher, compared with ER, PgR and
HER2 in our previous study (14). A
number of previous studies have evaluated the variations between
primary tumors and distant metastases and presented similar results
to those observed in the current study (12,15,16). The
discordance rates for the expression of ER, HER2, Ki-67 and ALDH1
between primary breast tumor tissues and lung metastases were 0,
21, 43 and 50%, respectively (14),
and the corresponding rates for primary breast tumor tissue and
axillary lymph node metastases were 12.5, 17.5, 22.5 and 42.5%
(12), respectively. It was
hypothesized that a reason for the variation in the expression of
these specific biomarkers may be the exposure of metastatic tumor
tissues to adjuvant systemic chemotherapy or radiation therapy
(17).
Tanei et al (18) identified a difference in ALDH1
expression levels in breast tumor tissues prior to and following
neoadjuvant chemotherapy (NAC). Although the association between
adjuvant therapy and ALDH1 expression levels in IBTR tissues was
investigated in the current study, no significant correlation was
demonstrated. However, as the current study was retrospective,
further evaluation may be required. The present study also included
a mixture of patient tissue samples consisting of ‘true IBTR’ and
‘new primary tumors’; new primary tumors are not expected to have
any correlation with the first primary tumor (19–21). The
timing of diagnosis of IBTR was distinct from that of the diagnosis
following NAC. The post-NAC surgery was performed immediately
following the end of systemic therapy. However, the duration
between the administration of systemic therapies and time of
surgery for IBTR were not homogeneous. There were many cancer stem
cells present in tissue specimens following NAC administration, as
they are resistant to systemic therapy (18). Therefore, ALDH1 expression levels in
IBTR may be varied according to the timing of surgery following
systemic therapy.
In previous reports, ALDH1 expression in primary
breast tumors was identified to be an independent predictor of
early tumor relapse of invasive ductal carcinoma (22), particularly in patients with
triple-negative breast cancer (23,24). In
the present study, no significant correlation was observed between
ALDH1 expression in primary tumors or IBTR tissues, and the
prognosis of patients with IBTR. However, the results suggested
that low Ki-67 and high ER expression levels in IBTR tissues were
significantly associated with improved prognosis. Furthermore, the
expression levels of Ki-67 and ALDH1 in IBTR tissues were
determined to be significant predictors of poor prognosis and a
short DFS time.
Subgroup analysis identified no significant
correlation between the tumor subtype and ALDH1 expression levels;
however, the expression levels of ALDH1 were significantly
associated with prognosis in HER2-positive IBTR, possibly due to
HER2-positive IBTR tissue including numerous new primary tumors. In
the current study, the rate of HER2 expression in IBTRs (22%) was
higher than that in primary tumors (11%), and 54% of primary tumors
in HER2-type IBTR tissues were diagnosed as HER2-negative or
unknown. Adjuvant trastuzumab therapy was administered to three
patients. Adjuvant trastuzumab following primary surgery is
recommended for patients with HER2-type breast cancer (25); however, no prospective studies have
been conducted to examine the efficacy of adjuvant trastuzumab for
IBTR. A total of 74% of the patients with HER2-type IBTR did not
receive trastuzumab. A number of patients with luminal A or B
subtypes of breast cancer received adjuvant hormonal therapy. Our
previous study reported that the IBTR tumor subtype is more
important in predicting treatment outcomes than the subtype of the
primary tumor (6). There was no
significant difference in the proportions of patients that received
adjuvant trastuzumab treatment between cases of ALDH1-positive and
ALDH1-negative IBTR in the present study. Therefore, trastuzumab
may reduce the variation in disease free survival between patients
with ALDH1-positive and ALDH1-negative IBTR.
In the present study, only tissue specimens from
patients with IBTR were evaluated and no control specimens from
breast cancer without IBTR were included. Therefore, a comparison
between patients with and without IBTR is required in future
studies. Further studies are required to evaluate the value of
ALDH1 expression as a predictor for IBTR. It is possible that ALDH1
expression levels in primary tumors are not a significant predictor
of IBTR, as it was identified that the expression levels of ALDH1
in the primary tumor tissue samples of the patients with IBTR were
not higher compared with previous studies of patients with primary
breast tumors. Further studies are required to investigate
this.
In conclusion, the expression profile of ALDH1 in
IBTR tissue specimens was retrospectively analyzed and ALDH1 was
not demonstrated to be an independent prognostic factor. However,
ALDH1 expression levels may be a useful prognostic factor in
certain subgroups or when examined in combination with other
factors, including Ki67 or breast cancer subtypes. Further studies
regarding the prognostic importance of ALDH1 expression levels in
IBTR are required, with a larger sample number and a prospective
approach.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Scientific Research from the Japanese Breast Cancer Society
(grant no. H25-26), and the Health and Labour Sciences Research
Expenses for Commission, Applied Research for Innovative Treatment
of Cancer (grant no. H26-applied-general-043) from the Ministry of
Health, Labour and Welfare, and Japan Agency for Medical Research
and Development.
References
|
1
|
van Dongen JA, Voogd AC, Fentiman IS,
Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van
Zijl K and Bartelink H: Long-term results of a randomized trial
comparing breast-conserving therapy with mastectomy: European
organization for research and treatment of cancer 10801 trial. J
Natl Cancer Inst. 92:1143–1150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morris AD, Morris RD, Wilson JF, White J,
Steinberg S, Okunieff P, Arriagada R, Lê MG, Blichert-Toft M and
van Dongen JA: Breast-conserving therapy vs mastectomy in
early-stage breast cancer: A meta-analysis of 10-year survival.
Cancer J Sci Am. 3:6–12. 1997.PubMed/NCBI
|
|
3
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty-year
follow-up of a randomized study comparing breast-conserving surgery
with radical mastectomy for early breast cancer. N Engl J Med.
347:1227–1232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishitobi M, Okumura Y, Arima N, Yoshida A,
Nakatsukasa K, Iwase T, Shien T, Masuda N, Tanaka S, Tanabe M, et
al: Breast cancer subtype and distant recurrence after ipsilateral
breast tumor recurrence. Ann Surg Oncol. 20:1886–1892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okumura Y, Nishimura R, Nakatsukasa K,
Yoshida A, Masuda N, Tanabe M, Shien T, Tanaka S, Arima N, Komoike
Y, et al: Change in estrogen receptor, HER2 and Ki-67 status
between primary breast cancer and ipsilateral breast cancer tumor
recurrence. Eur J Surg Oncol. 41:548–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: An old idea-a paradigm shift. Cancer Res. 66:1883–1890,
1895–1896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ginestier C, Hur MH, CharafeJauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arima N, Nishimura R, Osako T, Nishiyama
Y, Fujisue M, Okumura Y, Nakano M, Tashima R and Toyozumi Y: The
importance of tissue handling of surgically removed breast cancer
for an accurate assessment of the Ki-67 index. J Clin Pathol.
69:255–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arima N, Nishimura R, Osako T, Nishiyama
Y, Fujisue M, Okumura Y, Nakano M, Tashima R and Toyozumi Y: A
comparison of the hot spot and the average cancer cell counting
methods and the optimal cutoff point of the Ki-67 index for luminal
type breast cancer. Oncology. 90:43–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nogami T, Shien T, Tanaka T, Nishiyama K,
Mizoo T, Iwamto T, Ikeda H, Taira N, Doihara H and Miyoshi S:
Expression of ALDH1 in axillary lymph node metastases is a
prognostic factor of poor clinical outcome in breast cancer
patients with 1–3 lymph node metastases. Breast Cancer. 21:58–65.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito M, Shien T, Omori M, Mizoo T, Iwamoto
T, Nogami T, Motoki T, Taira N, Doihara H and Miyoshi S: Evaluation
of aldehyde dehydrogenase 1 and transcription factors in both
primary breast cancer and axillary lymph node metastases as a
prognostic factor. Breast Cancer. 23:437–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nogami T, Shien T, Tanaka T, Doihara H,
Taira N, Takabatake D, Nishimura R, Nishiyama K, Mizoo T and Ohsumi
S: The discordance between primary breast cancer lesions and
pulmonary metastatic lesions in expression of aldehyde
dehydrogenase 1-positive cancer cells. Breast Cancer. 21:698–702.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeung C, Hilton J, Clemons M, Mazzarello
S, Hutton B, Haggar F, Addison CL, Kuchuk I, Zhu X, Gelmon K and
Arnaout A: Estrogen, progesterone, and HER2/neu receptor
discordance between primary and metastatic breast tumours-a review.
Cancer Metastasis Rev. 35:427–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossi S, Basso M, Strippoli A, Dadduzio V,
Cerchiaro E, Barile R, D'Argento E, Cassano A, Schinzari G and
Barone C: Hormone receptor status and HER2 expression in primary
breast cancer compared with synchronous Axillary metastases or
recurrent metastatic disease. Clin Breast Cancer. 15:307–312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shien K, Toyooka S, Ichimura K, Soh J,
Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, et al:
Prognostic impact of cancer stem cell-related markers in non-small
cell lung cancer patients treated with induction chemoradiotherapy.
Lung Cancer. 77:162–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanei T, Morimoto K, Shimazu K, Kim SJ,
Tanji Y, Taguchi T, Tamaki Y and Noguchi S: Association of breast
cancer stem cells identified by aldehyde dehydrogenase 1 expression
with resistance to sequential paclitaxel and epirubicin-based
chemotherapy for breast cancers. Clin Cancer Res. 15:4234–4241.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benson JR and Rovere GQ della: Ipsilateral
breast cancer recurrence. Breast. 17:12–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen SL and Martinez SR: The survival
impact of the choice of surgical procedure after ipsilateral breast
cancer recurrence. Am J Surg. 196:495–499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McGrath S, Antonucci J, Goldstein N,
Wallace M, Mitchell C, Grills I, Jolly S, Kestin L and Vicini F:
Long-term patterns of in-breast failure in patients with early
stage breast cancer treated with breast-conserving therapy: A
molecular based clonality evaluation. Am J Clin Oncol. 33:17–22.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong Y, Lin Y, Shen S, Zhou Y, Mao F,
Guan J and Sun Q: Expression of ALDH1 in breast invasive ductal
carcinoma: An independent predictor of early tumor relapse. Cancer
Cell Int. 13:602013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai
Y, Sagara Y, Sagara Y, Sagara Y and Tanimoto A: Aldehyde
dehydrogenase 1 expression predicts poor prognosis in
triple-negative breast cancer. Histopathology. 59:776–780. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Li K, Luo Y, Tian L, Wang M, Li C
and Huang Q: Novel prognostic markers for patients with
triple-negative breast cancer. Hum Pathol. 44:2180–2187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moja L, Tagliabue L, Balduzzi S, Parmelli
E, Pistotti V, Guarneri V and D'Amico R: Trastuzumab containing
regimens for early breast cancer. Cochrane Database Syst Rev.
18:CD0062432012.
|