Introduction
Esophageal carcinoma is one of the most common
malignant cancers in China. As a result of extensive local cancer
invasion, lymph node involvement and distant metastasis at the time
of diagnosis, patients with esophageal carcinoma typically show
rapid cancer progression and a poor prognosis (1). It has previously been demonstrated that
the development of esophageal carcinoma is a complex, multi-step
process involving a multitude of enzymes (2).
Human matrix metalloproteinases (MMPs) are a group
of endopeptidases that degrade various components of the
extracellular matrix (ECM) (3). MMPs
have been shown to have important roles in tumor metastasis,
invasion and angiogenesis (4). MMP-2,
which is also known as type IV collagenase and gelatinase A, is a
member of the MMP family that is located on the long arm of
chromosome 16 (16q), is comprised of 13 exons and 12 introns, and
has a molecular weight of 72 kDa (5).
MMP-2 degrades type IV collagen within basement membranes, which
are the primary barrier to cancer invasion (6). Previous studies have reported a role for
MMP-2 in the invasion of pancreatic, ovarian and lung cancer
(7–9).
However, the function of MMP-2 in esophageal carcinoma remains
uncertain.
RNA interference (RNAi) using small interfering
(si)RNAs to inhibit the expression of specific genes is a powerful
and promising technology for basic research and therapeutic
intervention (10–13). Our previous study demonstrated that
MMP-2 knockdown using synthesized oligonucleotides inhibited the
invasion and migration of the KYSE150 esophageal carcinoma cell
line in vitro (14). In the
present study, lentiviral vectors targeting the MMP-2 gene were
constructed and transfected into KYSE150 cells, in order to observe
the inhibitory effect of MMP-2 silencing on the growth of
esophageal carcinoma cells in nude mice. The present study aimed to
further clarify the role of MMP-2 in esophageal carcinoma in
vivo and to provide experimental evidence for pre-clinical gene
therapy for esophageal carcinoma.
Materials and methods
Cell culture
The human embryonic kidney 293T packaging cell line
and KYSE150 esophageal carcinoma cell line were obtained from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified incubator containing 5%
CO2.
Lentivirus vector construction and
transfection into KYSE150 cells
The lentiviral vector system is composed of four
plasmids: The expression plasmid and three packaging vectors,
including pMD2.g (VSVG), pRSV-REV and pMDLg/pRRE (Shanghai Telebio
Biomedical, Co., Ltd., Shanghai, China). The human MMP-2 gene (Gen
Bank ID: 4313, NM_001127891.1, NM_004530.4) interference sequence
was obtained using small hairpin (sh)RNA analysis software
(http://www.invitrogen.com/rnai), and a
Basic Local Alignment Search Tool analysis of the NCBI database
(http://blast.ncbi.nlm.nih.gov/Blast.cgi) confirmed
that it had no homology with other genes. Three self-complementary
hairpin DNA oligonucleotides and a negative control targeting MMP-2
mRNA were synthesized. The sequences are shown in Table I. Subsequently, the DNA
oligonucleotides were cloned into the lentiviral vectors and they
were confirmed by DNA sequencing.
 | Table I.Three hairpin DNA oligonucleotide
sequences targeting matrix metalloproteinase-2 mRNA. |
Table I.
Three hairpin DNA oligonucleotide
sequences targeting matrix metalloproteinase-2 mRNA.
| shRNAs | Sequences |
|---|
| shRNA-1 | Sense:
5′-TGCGACAAGAAGTATGGCTTCTTTCAAG |
|
|
AGAAGAAGCCATACTTCTTGTCGCTTTTTTC-3′ |
|
| Antisense:
5′-TCGAGAAAAAAGCGACAAGAAGTATGGCTT |
|
|
CTTCTCTTGAAAGAAGCCATACTTCTTGTCGCA-3′ |
| shRNA-2 | Sense:
5′-TGGAGATACAATGAGGTGAAGATTC |
|
|
AAGAGATCTTCACCTCATTGTATCTCCTTTTTTC-3′ |
|
| Antisense:
5′-TCGAGAAAAAAGGAGATACAATGAGGT |
|
|
GAAGATCTCTTGAATCTTCACCTCATTGTATCTCCA-3′ |
| shRNA-3 | Sense:
5′-TGCAAACAGGACATTGTATTTGTTCAAGA |
|
|
GACAAATACAATGTCCTGTTTGCTTTTTTC-3′ |
|
| Antisense:
5′-TCGAGAAAAAAGCAAACAGGACATTGTATT |
|
|
TGTCTCTTGAACAAATACAATGTCCTGTTTGCA-3′ |
| Non-targeting | Sense:
5′-TGTAGCGACTAAACACATCAATTCAAG |
| control |
AGATTGATGTGTTTAGTCGCATTCTTTTTTC-3′ |
|
| Antisense:
5′-TCGAGAAAAAATAGCGACTAAACACATCAA |
|
|
TCTCTTGAATTGATGTGTTTAGTCGCATGCA-3′ |
Lentiviral vectors and packaging vectors were
transfected into 293T cells. Following transfection, the cells were
cultured for 8 h, after which the culture medium was exchanged with
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.). After 48 h, the supernatant containing the
retroviral particles was collected and then concentrated by
centrifugation at 4,000 × g and 4°C. A total of
2×105 KYSE150 cells/well were transduced with viral
supernatants, and the transfection efficiency was detected directly
by assessing the expression ratio of green fluorescent protein
(GFP) by fluorescence microscopy. Stable cell lines were obtained
after selection by culture in medium containing 100 µg/ml
ampicillin for 18 days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using
the RNA purification kit (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). DNase I was used to remove any contaminating
DNA. Subsequently, RNA was reverse transcribed into cDNA. The
primers for the PCR were designed using Primer Premier 3 software
(Premier Biosoft International, Palo Alto, CA, USA) and were
synthesized by Beijing SBS Genetech, Co., Ltd. (Beijing, China).
PCR was performed using Quantitative RT-PCR ReadyMix
(Sigma-Aldrich; Merck Millipore). The primers for MMP-2 were as
follows: Forward, 5′-TCCAGAGGCAATGCAGTGGGG-3′ and reverse,
5′-CAGCTCTCCTTGGGGCAGCCA-3′. Primers for β-actin were as follows:
Forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′. The PCR conditions involved denaturing
the DNA at 94°C for 3 min, followed by 30 cycles of amplification:
94°C for 30 sec, 55°C for 1 min, 72°C for 1 min and a final
extension step at 72°C for 10 min. The data were analyzed using the
2−ΔΔCq method (15).
Western blotting
The cells were washed with cold PBS and lysed in
pre-cooled radioimmunoprecipitation assay buffer (Pierce; Thermo
Fisher Scientific, Inc.) containing proteinase inhibitors. The
mixture was incubated for 30 min on ice, after which cell lysates
were cleared of cell debris by centrifugation at 140,009 × g
for 5 min at 4°C. Protein concentrations were determined using the
BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The samples were mixed in
59 loading buffer (Pierce; Thermo Fisher Scientific, Inc.),
denatured at 96°C for 10 min and chilled on ice. Subsequently,
equal amounts of protein (60 µg) were separated by 15% SDS-PAGE and
blotted onto nitrocellulose membranes. The membranes were stained
with Ponceau Red (Sigma-Aldrich; Merck Millipore) to verify that
the proteins had been transferred. Subsequently, membranes were
blocked with 5% nonfat milk and incubated with anti-MMP-2 (cat. no.
HPA001939-100UL; 1:10,000 dilution; Sigma-Aldrich; Merck Millipore)
and anti-β-actin (cat. no. CBL171; 1:10,000 dilution;
Sigma-Aldrich; Merck Millipore) primary antibodies overnight at
4°C. After washing with PBS, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat. no., ZDR-5118; Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China) for 60 min at room temperature. Proteins were
detected and quantified using the enhanced chemiluminescence
detection system (ChemiDoc XRS System; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and expression levels were normalized to
β-actin
MTT assays
The viability of KYSE150 cells transfected with
MMP-2-lentivirus and control lentivirus vectors, as well as blank
control cells, were measured using MTT assays. Briefly, KYSE150
cells were infected with lentivirus in 6-well plates and re-seeded
into 96-well plates at a density of 2,000 cells per well. After 1,
2, 3 or 4 days, the cells were treated with 20 µl MTT solution (5
mg/ml) for 4 h, after which the cell supernatants were removed and
150 µl dimethyl sulfoxide was added to each well. After 15 min, the
optical density (OD) of each well was measured using a microplate
reader with an absorbance wavelength of 570 nm. All experiments
were performed in triplicate.
In vivo experiments
A total of 24 female BALB/c nude mice (age, 6–8
weeks; weight, 18–22 g) were supplied by Beijing HFK Bioscience
Co., Ltd. (Beijing, China). The mice were maintained under sterile
conditions at 27°C, exposed to 10 h light/dark cycles and fed a
sterilized mouse diet and water ad libitum. Non-transfected
control KYSE150 cells (blank group), MMP-2-non-targeting shRNA
control (NC group) and MMP-2-shRNA-2-transfected KYSE150 cells
(2×106 cells in 0.1 ml) were injected subcutaneously
into the axilla of each BALB/c nude mouse. Tumor size was measured
every 2 days in two perpendicular dimensions using vernier calipers
and tumor volume was calculated according to the following formula:
Tumor volume (mm3)= 1/2 × (a × b2), where a
and b refer to the longest and shortest dimensions,
respectively.
Statistical analysis
All of the experimental data were analyzed using
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation. Statistical
significance between the different groups was determined using the
Student's t-test. P<0.05 was considered statistically
significant.
Results
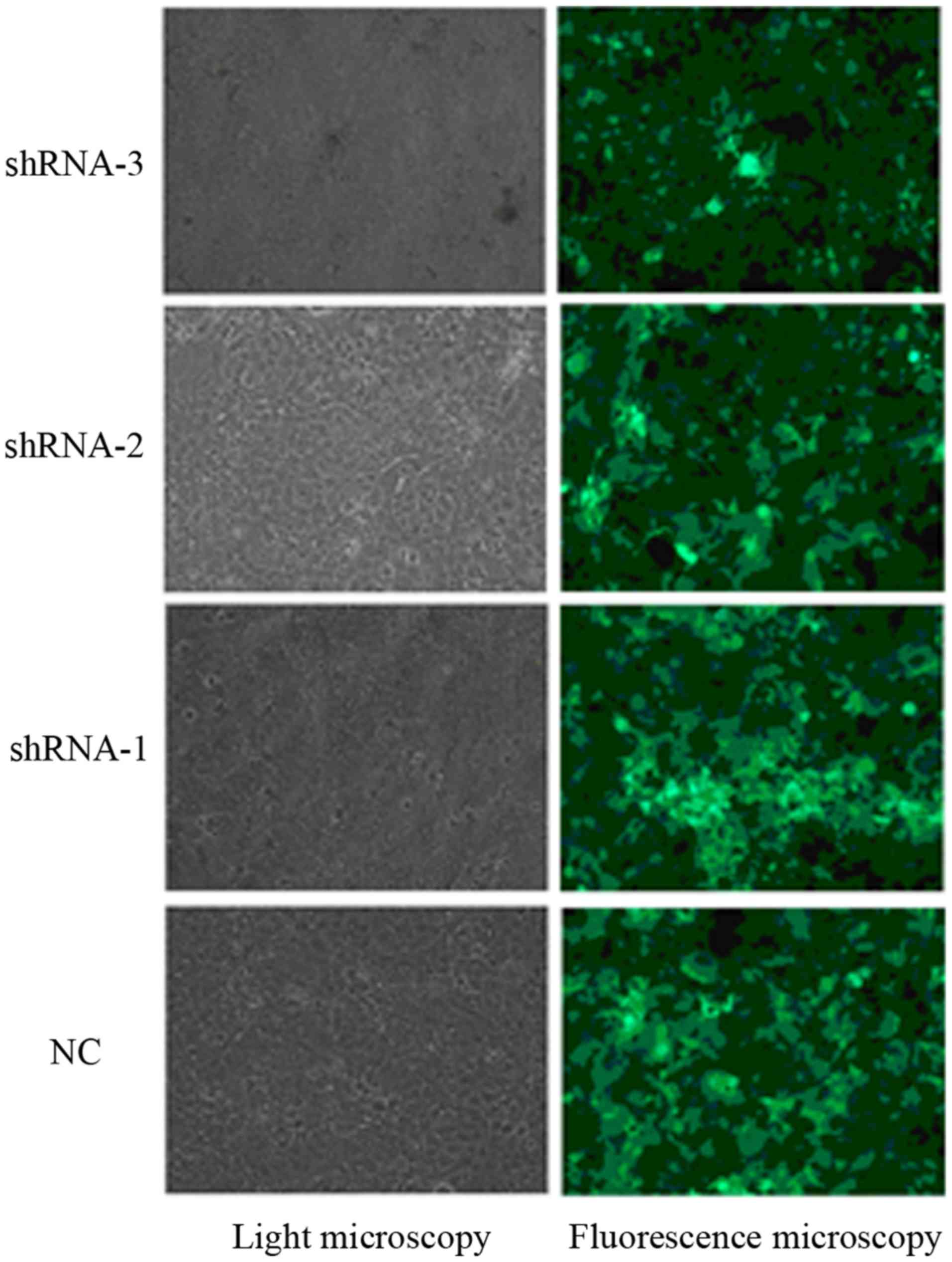
Transfection efficiency of lentiviral
vectors in KYSE150 cells
The recombinant lentivirus vector was successfully
constructed and confirmed by DNA sequencing. After being infected
with the recombinant lentiviruses carrying the reporter GFP gene,
the transfected KYSE150 cells were shown to express GFP, and the
proportion of transfected cells was analyzed under a fluorescence
microscope. The results indicated that 90% of KYSE150 cells were
transfected with the recombinant lentiviruses (Fig. 1).
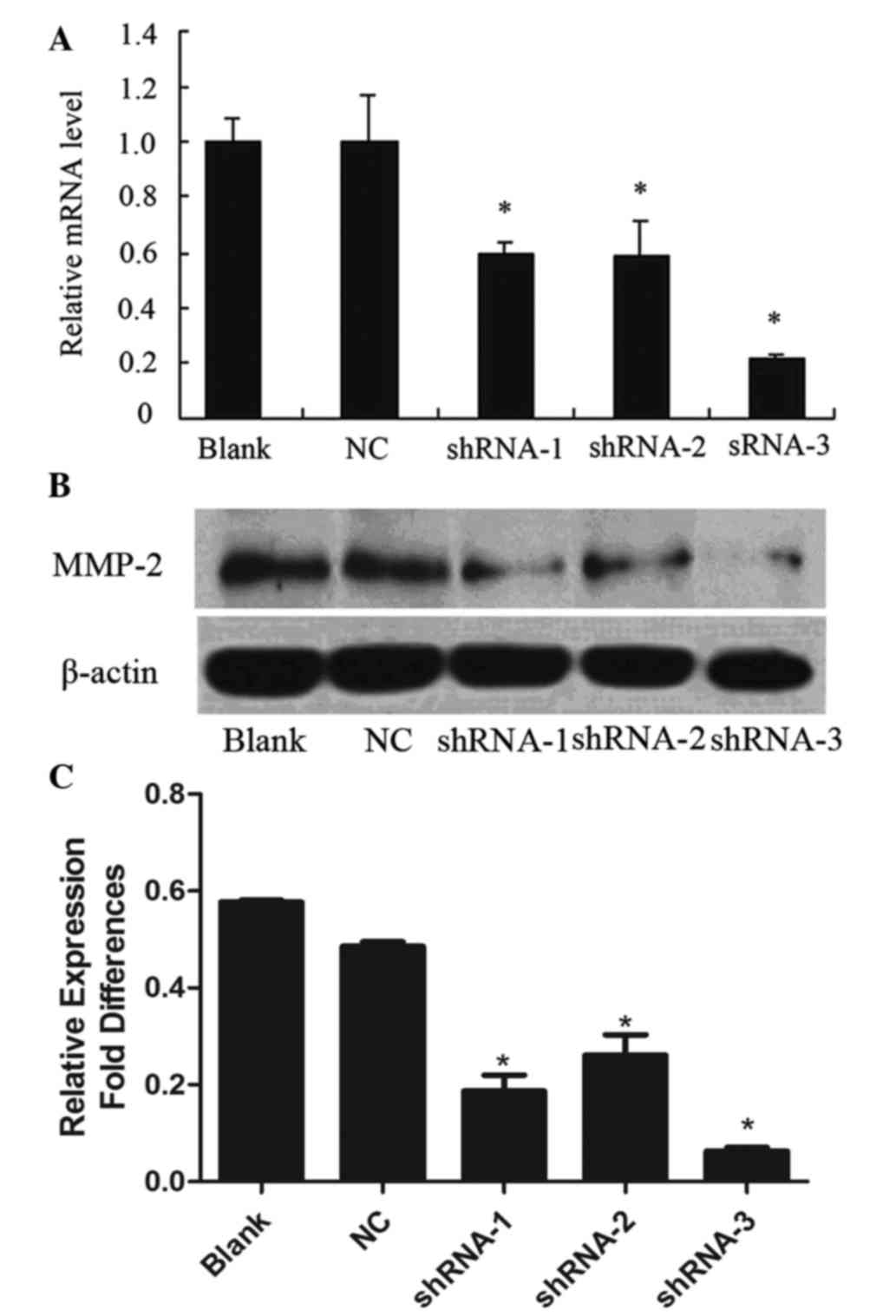
Lentivirus-mediated RNAi inhibits
MMP-2 gene expression in KYSE150 cells
To evaluate the inhibition of MMP-2 mRNA expression,
RT-qPCR was performed 72 h following transfection. The MMP-2 mRNA
expression levels in shRNA-1-, shRNA-2- and shRNA-3-transfected
KESY150 cells were reduced by 40.5, 41.4 and 78.9%, respectively,
as compared with the non-transfected control (blank group)
(P<0.05; Fig. 2A). In addition, no
significant difference was observed between the blank group and the
MMP-2-non-targeting shRNA control (NC group) (Fig. 2A). The results indicated that the
shRNA-3 oligonucleotide was the most effective for silencing of
MMP-2 mRNA expression (Fig. 2A).
Lentivirus-mediated RNAi inhibits
MMP-2 protein expression in KYSE150 cells
Western blotting demonstrated a significant
reduction in MMP-2 protein expression in the KESY150 cells infected
with shRNA-1 (0.187±0.072 of β-actin), shRNA-2 (0.261±0.095 of
β-actin) and shRNA-3 (0.063±0.016 of β-actin), as compared with a
non-targeting control (0.486±0.021 of β-actin) and blank control
(0.577±0.009 of β-actin) (P<0.05; Fig.
2B and C). These results indicated that shRNA-1, shRNA-2 and
shRNA-3 all blocked MMP-2 expression, although shRNA-3 showed the
most effective inhibition of MMP-2 expression. No obvious
inhibition of MMP-2 protein was observed in the non-transfected
control and blank control cells.
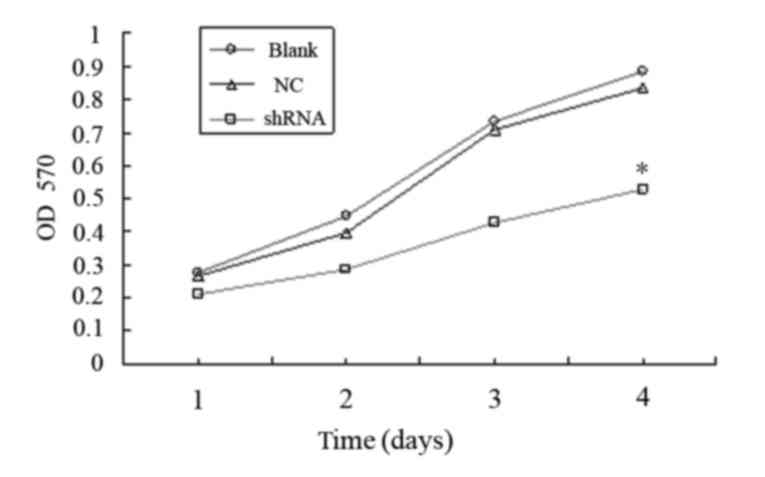
Lentivirus-mediated RNAi against MMP-2
reduces KYSE150 cell viability in vitro
As the shRNA-3 oligonucleotide showed the most
effective inhibition of MMP-2 expression, shRNA-3 was selected for
MTT assays. As shown in Fig. 3, the
viability of KYSE150 cells infected with lentivirus carrying
shRNA-3 was significantly reduced, as compared with the NC and
blank groups (P<0.05). These results suggest that knockdown of
MMP-2 reduces the viability of KYSE150 cells in vitro.
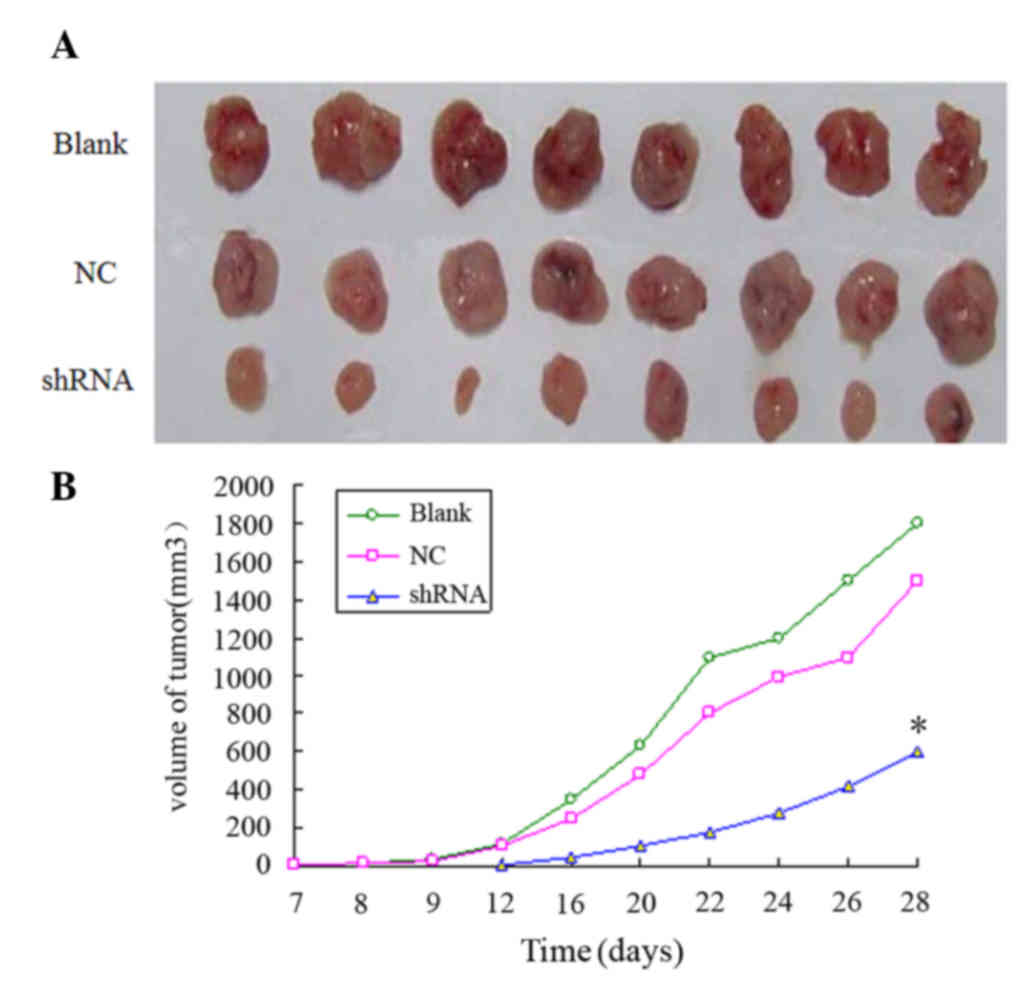
Lentivirus-mediated RNAi against MMP-2
inhibits KYSE150 cell tumorigenicity in vivo
To determine whether lentivirus-mediated RNAi
against MMP-2 was able to inhibit tumor development in vivo,
nude mice were injected with MMP-2-shRNA3-infected KYSE150 cells,
and the volume of the tumor was measured at 2-day intervals. As
compared with the blank and NC groups, the MMP-2-shRNA3-infected
KYSE150 cells resulted in significantly smaller tumors in the nude
mice (P<0.05; Fig. 4), indicating
that MMP-2 knockdown inhibited the tumorigenesis of esophageal
cancer cells.
Discussion
Esophageal cancer was the fourth leading cause of
cancer-associated mortality and the fifth most common diagnosed
cancer in China in 2009, which was increased compared with the data
from 2003–2007 (16). Although the
results of surgery have improved significantly in recent years, the
overall 5-year survival rates remain between 15 and 25% (17). Therefore, novel therapeutic strategies
are urgently required for the treatment of this disease. Cancer
metastasis is a multi-step process, and destruction or penetration
of the basement membrane is thought to be critical for the
successful metastasis of tumor cells (18). Human MMPs are thought to have
important roles in tumor metastasis, invasion and angiogenesis
(18).
Previous studies have reported a role for MMP-2 in
the progression and invasion of numerous types of cancers (19,20). In
our previous study, it was demonstrated that MMP-2-knockdown using
RNAi with synthesized oligonucleotides inhibited the invasion and
migration of the KYSE150 esophageal carcinoma cell line in
vitro (14). Furthermore, MMP-2
has exhibited a tumor-promoting function in many tumors in
vivo, including ovarian (21),
larynx (22) and brain (23) cancers. However, to the best of our
knowledge, few studies have investigated the in vivo
function of MMP-2 in esophageal cancer.
RNAi is a powerful means for post-transcriptional
gene silencing and has been applied to a wide variety of eukaryotic
organisms (24). Currently,
chemically synthetic siRNAs are being evaluated for their use as
highly-specific gene-silencing therapeutics, as well as their
traditional role as an extremely powerful instrument for functional
genomic analyses (25). However,
there are several disadvantages associated with the use of
synthesized siRNAs: i) The transduction of siRNA into cells usually
leads to only transient silencing effects; ii) the transfection
efficiency of siRNA may influence the silencing effects in target
cells; and iii) transfected siRNA is expensive, as they must be
chemically or enzymatically synthesized (26). To overcome these shortcomings, a
stable RNAi DNA vector-based method has been developed (27–29).
Approximately 70% of the vectors used in gene therapy clinical
trials are represented by viral-based delivery systems (30). However, a number of failures with
regard to gene therapy have been observed and thus, further
optimization is required for the safe use of these vectors for
clinical proposes in the future (31).
In the present study, a shRNA-lentiviral expression
vector was used to obtain efficient knockdown of the MMP-2 gene in
KYSE150 cells. The results showed that all shRNAs targeting MMP-2,
including shRNA-1, shRNA-2 and shRNA-3, effectively inhibited the
expression of MMP-2 mRNA and protein in KYSE150 cells, while the
non-transfected and blank control groups showed no difference in
MMP-2 expression. Notably, shRNA-3 was the most effective at
suppressing MMP-2 expression and, therefore, it was selected for
further investigation. Subsequently, it was demonstrated that
MMP-2-shRNA-3 reduced the viability of KYSE150 esophageal carcinoma
cells in vitro and tumorigenesis in vivo, as compared
with the NC and blank control groups.
In conclusion, the results of the present study
suggested that MMP-2 is a feasible RNAi target gene for esophageal
carcinoma and that stable lentivirus-mediated shRNA targeting MMP-2
may be a promising and novel approach to the treatment of
MMP-2-positive esophageal carcinoma. However, to further promote
the application of this technique as a therapeutic approach, an
effective and safe protocol should be developed.
Acknowledgements
The authors of the present study would like to thank
Yong-Min Mao and Li-Li Zhao of the Tianjin Cardiovascular Institute
for their technical assistance.
References
|
1
|
Mukherjee S, Roth MJ, Dawsey SM, Yan W,
RodriguezCanales J, Erickson HS, Hu N, Goldstein AM, Taylor PR,
Richardson AM, et al: Increased matrix metalloproteinase activation
in esophageal squamous cell carcinoma. J Transl Med. 8:912010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Ma J, Guo Q, Duan F, Tang F, Zheng
P, Zhao Z and Lu G: Overexpression of MMP-2 and MMP-9 in esophageal
squamous cell carcinoma. Dis Esophagus. 22:664–667. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poincloux R, Lizárraga F and Chavrier P:
Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to
invadopodia. J Cell Sci. 122:3015–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer-roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Price SJ, Greaves DR and Watkins H:
Identification of novel, functional genetic variants in the human
matrix metalloproteinase-2 gene: Role of Sp1 in allele-specific
transcriptional regulation. J Biol Chem. 276:7549–7558. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vihinen P and Kähäri VM: Matrix
metalloproteinases in cancer: Prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellenrieder V, Alber B, Lacher U, Hendler
SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M, et
al: Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int
J Cancer. 85:14–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Hu C, Dong R, Huang X and Qiu H:
Platelet-derived growth factor-D promotes ovarian cancer invasion
by regulating matrix metalloproteinases 2 and 9. Asian Pac J Cancer
Prev. 12:3367–3370. 2011.PubMed/NCBI
|
|
9
|
Li GH, Cui YS, Wu QY, Zhang XJ and Gao YF:
Clinicopathologic significance of β-catenin and matrix
metalloproteinase-2 expression in non-small cell lung cancer. Med
Oncol. 30:4372013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Izquierdo M: Short interfering RNAs as a
tool for cancer gene therapy. Cancer Gene Ther. 12:217–227. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pushparaj PN, Aarthi JJ, Manikandan J and
Kumar SD: SiRNA, miRNA and shRNA: In vivo applications. J Dent Res.
87:992–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amarzguioui M, Rossi JJ and Kim D:
Approaches for chemically synthesized siRNA and vector-mediated
RNAi. FEBS Lett. 579:5974–5981. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li CX, Parker A, Menocal E, Xiang S,
Borodyansky L and Fruehauf JH: Delivery of RNA interference. Cell
Cycle. 5:2103–2109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen YG, Xu YJ, Shi ZL, Han HL, Sun DQ and
Zhang X: Effects of RNAi-mediated matrix metalloproteinase-2 gene
silencing on the invasiveness and adhesion of esophageal carcinoma
cells, KYSE150. Dig Dis Sci. 57:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, He Y, Zheng R, Zhang S, Zeng H,
Zou X and He J: Esophageal cancer incidence and mortality in China,
2009. J Thorac Dis. 5:19–26. 2013.PubMed/NCBI
|
|
17
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong J, Zhu S, Zhang Y and Wang J:
Interplay of VEGFa and MMP2 regulates invasion of glioblastoma.
Tumour Biol. 34:11879–11885. 2014. View Article : Google Scholar
|
|
20
|
Honkavuori-Toivola M, Santala M, Soini Y,
Turpeenniemi- Hujanen T and Talvensaari-Mattila A: Combination of
strong MMP-2 and weak TIMP-2 immunostainings is a significant
prognostic factor in endometrial carcinoma. Dis Markers.
35:261–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo JM, Park S and Kim JH: Leukotriene B4
receptor-2 promotes invasiveness and metastasis of ovarian cancer
cells through signal transducer and activator of transcription 3
(STAT3)-dependent up-regulation of matrix metalloproteinase 2. J
Biol Chem. 287:13840–13849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Liu M, Yang B, Li B and Lu J: Role
of siRNA silencing of MMP-2 gene on invasion and growth of
laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol.
265:1385–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maddirela DR, Kesanakurti D, Gujrati M and
Rao JS: MMP-2 suppression abrogates irradiation-induced microtubule
formation in endothelial cells by inhibiting αvβ3-mediated
SDF-1/CXCR4 signaling. Int J Oncol. 42:1279–1288. 2013.PubMed/NCBI
|
|
24
|
Zamore PD: RNA interference: Listening to
the sound of silence. Nat Struct Biol. 8:746–750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leng Q, Woodle MC, Lu PY and Mixson AJ:
Advances in systemic siRNA delivery. Drugs Future. 34:7212009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CC, Hsu JT, Huang KL, Tang HK and Lai
YK: Stable RNA interference in Spodoptera frugiperda cells by a DNA
vector-based method. Biotechnol Lett. 28:271–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manjunath N, Wu H, Subramanya S and
Shankar P: Lentiviral delivery of short hairpin RNAs. Adv Drug
Deliv Rev. 61:732–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dykxhoorn DM, Novina CD and Sharp PA:
Killing the messenger: Short RNAs that silence gene expression. Nat
Rev Mol Cell Biol. 4:457–467. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CC, Hsu JT, Huang KL, Tang HK and Lai
YK: Stable RNA interference in Spodoptera frugiperda cells by a DNA
vector-based method. Biotechnol Lett. 28:271–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012 - an
update. J Gene Med. 15:65–77. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chira S, Jackson CS, Oprea I, Ozturk F,
Pepper MS, Diaconu I, Braicu C, Raduly LZ, Calin GA and
Berindan-Neagoe I: Progresses towards safe and efficient gene
therapy vectors. Oncotarget. 6:30675–30703. 2015.PubMed/NCBI
|