Introduction
Lung cancer is currently the leading cause of
cancer-associated mortality worldwide, with an estimated 87,750
mortalities in males and 72,590 in females in 2012 (1). According to its histopathological
classification, lung cancer can be divided into small cell lung
cancer and non-small cell lung cancer (NSCLC), which is the most
common histological type of lung cancer. The incidence of NSCLC has
increased annually; it is a heterogeneous disease that is difficult
to treat, and remains the leading cause of cancer-associated
mortality worldwide (2). In recent
years, despite recent advances in surgery, irradiation,
chemotherapy and targeted therapy, the 5-year survival rate of
patients with advanced stage NSCLC has remained markedly low
(3). Traditional chemotherapy has
been widely used in the treatment of NSCLC, but severe side effects
and drug resistance have limited its use in the clinic. Therefore,
alternative therapeutic drugs that can effectively treat lung
cancer have garnered recent attention (4,5).
Since 1970, polyoxometalates have been widely used
in analytical chemistry, medicine, catalysis and material science
(6), and a large number of them are
potential antitumor, antiviral and antibacterial drugs (7,8). The
biological effects of rare earth elements have attracted much
attention (9–11), since they exhibit inhibitory effects
on tumor cells such as B16 mouse melanoma cells, HeLa human
cervical cancer cells, HepG2 human liver carcinoma cells and PAMC82
human gastric cells (12). Rare earth
elements can inhibit tumor growth; thus, it is plausible that the
introduction of rare earth elements into the structure of
polyoxometalates may enhance their antitumor activities.
K9(C4H4FN2O2)2Nd(PW11O39)2·25H2O
(FNdPW) is a chemically synthesized drug that contains synthesized
rare earth elements, which have a low cytotoxicity (8). However, the physiological and
pathophysiological roles of FNdPW in A549 cells and its underlying
mechanisms of action remain to be determined. In the present study,
it was demonstrated that FNdPW significantly decreased the
viability of human A549 lung cancer cells and induced apoptosis,
with significant activation of caspase 3-dependent and caspase
3-independent signaling pathways.
Materials and methods
Reagents
Antibodies against B-cell lymphoma (Bcl)-2 (D160117)
and Bcl-2-associated X protein (Bax) (D120073) were purchased from
Sangong Biotech Co., Ltd. (Shanghai, China). Antibodies against
Bcl-2-associated death promoter (Bad) (9293), phosphorylated
(p)-Bad (5284), apoptosis-inducing factor (AIF) (4642) and X-linked
inhibitor of apoptosis protein (XIAP) (14334) were obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Other chemicals
were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt,
Germany).
A549 cell culture and drug chemical
synthesis
A549, a human lung cancer cell line, was provided by
Harbin Medical University (Harbin, China). Cells were cultured in
RPMI 1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% (v/v) heat-inactivated
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in an incubator containing humidified air with 5% (v/v)
CO2. FNdPW was purchased from the Department of
Inorganic Chemistry of Harbin Medical University.
Cell viability assay
Cells were seeded in 96-well plates at a density of
8×103 cells/well 24 h prior to treatment. Cells were
treated with FNdPW (3.17×10−7, 6.69×10−7,
3.02×10−6, 5.87×10−6 or 8.52×10−6
mol/l). After 72 h, 15 µl (5 mg/ml) MTT (Sigma-Aldrich; Merck
Millipore) was added to each well and incubated at 37°C for 4 h.
Then, the MTT solution was removed, and 150 µl dimethyl sulfoxide
was added to dissolve the crystals. Then, the mixture was agitated
for 10 min to fully dissolve the crystals. A microplate reader
(Tecan Group Ltd., Männedorf, Switzerland) was used to measure the
absorbance at a wavelength of 570 nm. Cell viability was expressed
as the percentage change in the absorbance values of the treated
group compared with the control group.
Electron microscopy (EM)
A549 lung cancer cells were cultured in 60-mm
plates, collected in PBS and fixed with 2% (v/v) paraformaldehyde
containing 2.5% (w/v) glutaraldehyde (Paesel & Lorei GmbH &
Co. KG, Duisburg, Germany) buffered with Hank's modified salt
solution at 4°C for 4 h. The cells were further fixed in 1% (w/v)
osmium tetroxide solution buffered with 0.1 M cacodylate (pH 7.2)
at 4°C for 2 h. Subsequently, cells were scraped off the plastic
and dehydrated in ethanol. Dehydration was completed in propylene
oxide. The specimens were embedded in Araldite (SERVA
Electrophoresis GmbH, Heidelberg, Germany). Ultrathin sections were
produced on an FCR Reichert Ultracut ultramicrotome (Leica
Microsystems, Inc., Buffalo Grove, IL, USA), mounted on
pioloform-coated copper grids and contrasted with lead citrate.
Specimens were analyzed and documented by EM (10A; Zeiss GmbH,
Jena, Germany).
Acridine orange (AO)/ethidium bromide
(EB)
A549 cells in exponential growth phase were
cultivated on sterile coverslips for 24 h and subsequently treated
with 5.87×10−6 mol/l FNdPW for 72 h. The cells were
washed twice with PBS and then mixed with 1 ml dye mixture
containing 100 mg/ml AO and 100 mg/ml EB in PBS (13). Cellular morphological changes were
examined using fluorescence microscopy (magnification, ×200). The
percentage of apoptotic cells was calculated with the following
formula: Apoptotic rate (%) = number of apoptotic cells/number of
total cells (14,15).
Western blot analysis
Total protein samples were extracted from A549
cells. Cells cultured on 25-mm dishes or 6-well plates were lysed
in a lysis buffer (P0013; Beyotime Institute of Biotechnology,
Haimen, China) containing protease and phosphatase inhibitors.
After centrifugation at 13,500 × g for 15 min at 4°C, cell
lysates were collected. Protein concentration was assessed using
the bicinchoninic acid assay. Aliquots of protein were then mixed
with Laemmli sample buffer and boiled at 100°C for 5 min. Samples
(60 µg protein) were resolved on 10–12% SDS-PAGE, followed by
transfer to nitrocellulose membranes. For visualization, blots were
probed with antibodies against Bcl-2 (1:1,000 dilution), Bax
(1:1,000 dilution), Bad (1:500 dilution), p-Bad (1:500 dilution),
XIAP (1:500 dilution), AIF (1:500 dilution) and GAPDH (1:1,000
dilution) at room temperature (21–23°C) for 1 h. Signals were
detected using horseradish peroxidase-conjugated secondary
antibodies (1:10,000 dilution; Cell Signaling Technology, Inc.) for
1 h at room temperature. The obtained digital images of the western
blots were used for densitometry determinations with Gel-Pro
Analyzer 4.0 (Media Cybernetics, Inc., Rockville, MD, USA). Western
blot bands were quantified using Odyssey version 3.0 software
(LI-COR Biosciences, Lincoln, NE, USA) by measuring the band
intensity (area × absorbance) for each group and normalizing the
value to that of the GAPDH band, which served as an internal
control.
Caspase-3 activity assay
Caspase-3 activity was analyzed using a caspase-3
activity assay kit (Beyotime Institute of Biotechnology) according
to the manufacturer's protocol, using the substrate peptides acetyl
(Ac)-DEVD-p-nitroanilide (pNA), Ac-IETD-pNA and Ac-LEHD-pNA
(Beyotime Institute of Biotechnology). Briefly, the supernatant of
the cell lysates was mixed with buffer containing the above
substrate peptides to allow the binding of caspase-3 to pNA. The
release of pNA was quantified by determining the absorbance with an
ELISA reader at 405 nm. The caspase activities were expressed as a
percentage over the control.
Data analysis
Data are shown as means ± standard deviation of 3–6
independent experiments, and were evaluated using an unpaired
Student's t-test. Statistical analysis was conducted with GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
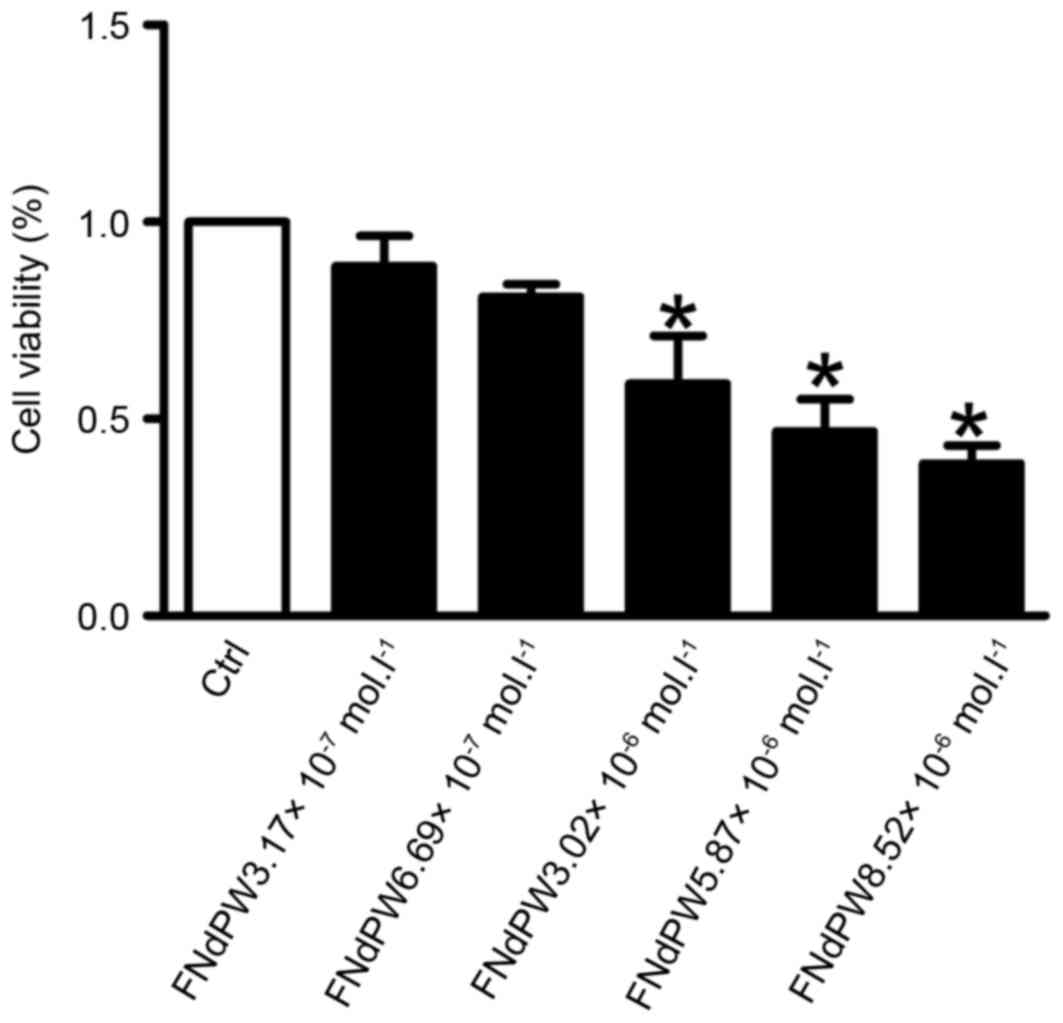
FNdPW suppresses the viability of A549
cells
The antiproliferative effect of FNdPW on A549 cells
was examined by exposing the cells to different concentrations
(3.17×10−7, 6.69×10−7, 3.02×10−6,
5.87×10−6 or 8.52×10−6 mol/l) of FNdPW for 72
h. Cell growth was inhibited in a dose- and time-dependent manner
(Fig. 1). In the presence of
5.87×10−6 mol/l FNdPW, A549 cells exhibited ~50%
inhibition of proliferation after treatment for 72 h. Thus, this
concentration and treatment time were used in subsequent
experiments.
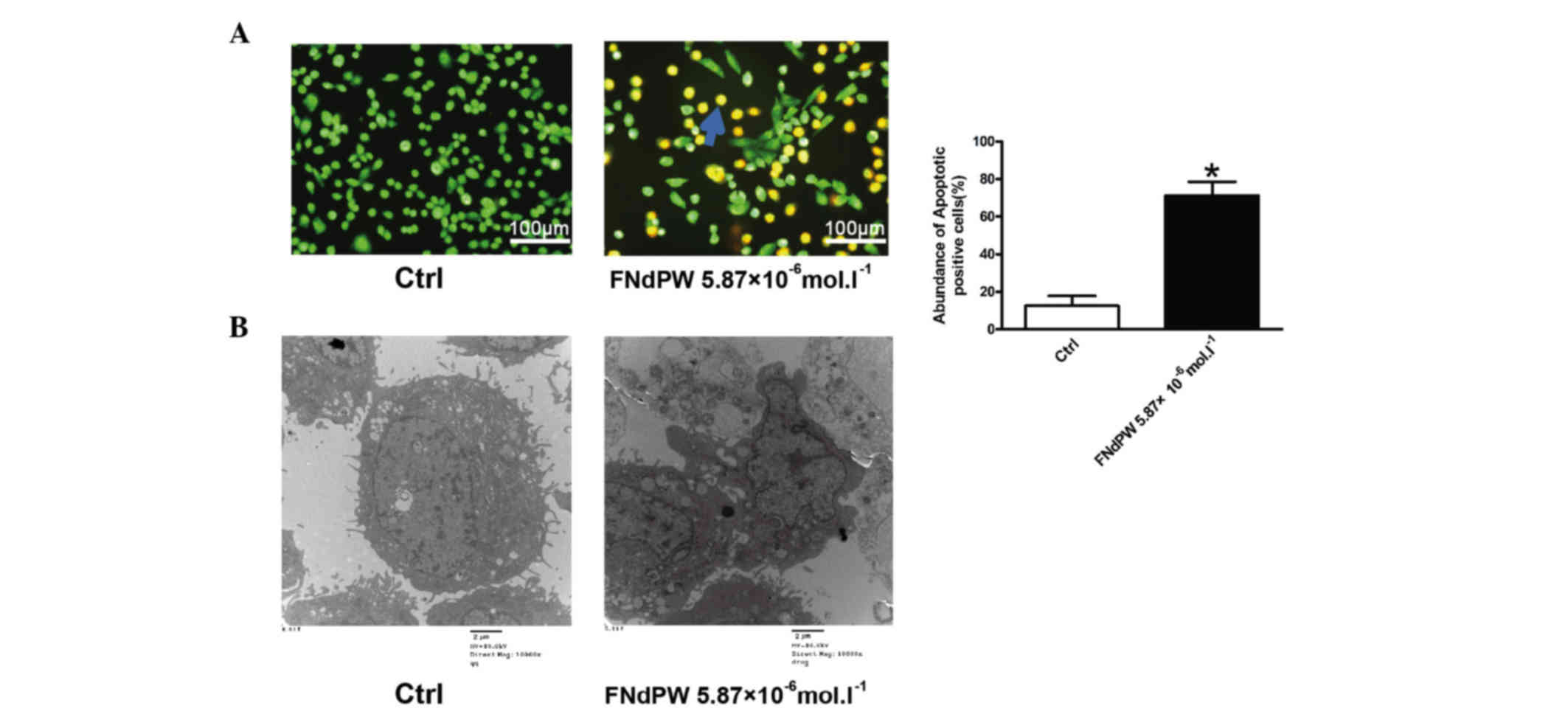
FNdPW induces apoptosis in A549
cells
Cell viability is a balance between proliferation
and apoptosis (16). To investigate
whether FNdPW regulates apoptosis, AO/EB staining and EM were used
to detect the number of apoptotic cells. The results from our
fluorescence microscopy analysis are shown in Fig. 2A. Three types of cells were recognized
under the fluorescence microscope: Living cells (green), apoptotic
cells (yellow) and necrotic cells (red). FNdPW induced the presence
of a substantial number of apoptotic cells (P=0.0365). Under EM,
cells with FNdPW exhibited robust changes in their microstructure,
including cell surface microvilli reduction, nuclear chromatin
condensation, invagination and membrane blistering (Fig. 2B).
FNdPW activates pro-apoptotic
signaling pathways
To explore the mechanisms by which FNdPW induces
apoptosis in A549 cells, the downstream proteins of the FNdPW
apoptotic pathway, including Bax, Bcl-2, Bad, p-Bad and XIAP, were
determined. Fig. 3 demonstrates that
FNdPW upregulated Bad, Bax and AIF, and downregulated p-Bad, Bcl-2
and XIAP expression (Fig. 3A, B and
D). In addition, to determine the potential toxic effects of
FNdPW, the cellular distributions of AIF were detected by western
blotting. As shown in Fig. 3E, FNdPW
increased the protein level of AIF in the nucleus, which is
independent of the caspase-3 (Fig.
3C) pathway.
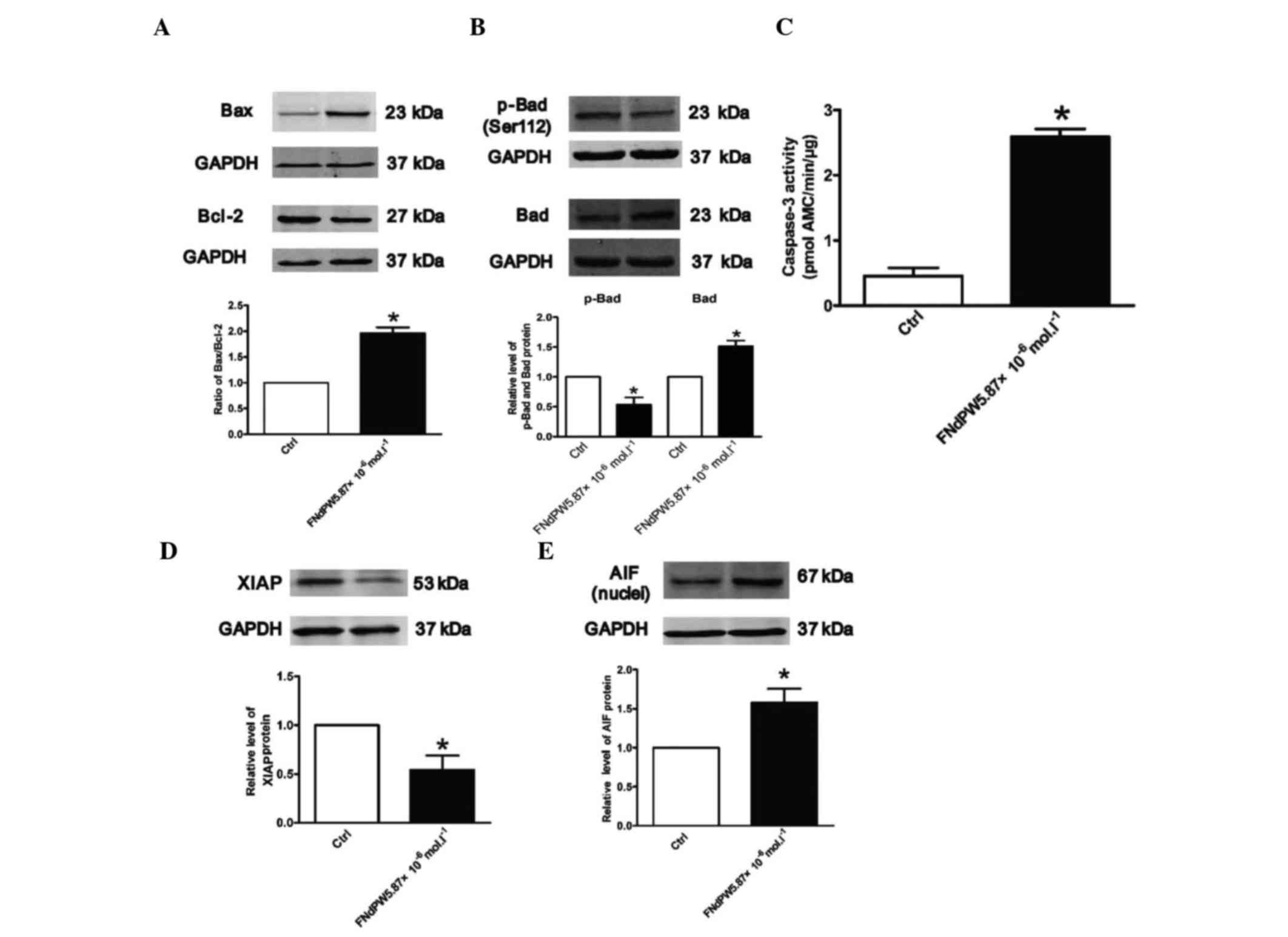 | Figure 3.FNdPW alters p-Bad, Bad, Bax, Bcl-2,
XIAP and AIF expression, and promotes caspase-3 activation. Western
blotting was used to detect (A) Bax, Bcl-2, (B) p-Bad, Bad, (D)
XIAP and (E) AIF expression in A549 cells treated with FNdPW.
Relative expression of Bax, Bcl-2, p-Bad, Bad, XIAP and AIF was
normalized to GAPDH (n=3 independent experiments per each group).
(C) Activation of caspase-3 by FNdPW. Data are averaged from five
independent experiments for each group. *P<0.05 compared with
Ctrl. Bcl, B-cell lymphoma; Bax, Bcl-2-associated X protein; Bad,
Bcl-2-associated death promoter; p, phosphorylated; AIF,
apoptosis-inducing factor; XIAP, X-linked inhibitor of apoptosis
protein; Ctrl, control; FNdPW,
K9(C4H4FN2O2)2Nd(PW11O39)2·25H2O. |
Discussion
Our present understanding of the precise mechanisms
underlying the use of FNdPW for treating human cancer is still
incomplete (17). In our study, it
was demonstrated that FNdPW, a polyoxometalate that includes rare
earth elements, has anti-proliferative effects on A549 cells by
causing apoptosis. In addition, it was identified that activation
of both caspase-3-dependent and caspase-3-independent pathways are
mechanisms underlying FNdPW-induced apoptosis, which led to the
suppression of liver cancer cell growth. Therefore, the current
study provides new insights into the pathophysiological role of
FNdPW in lung cancer diseases.
Lung cancer is the most common malignant cancer
worldwide, and is associated with high fatality rates in humans.
The use of chemotherapy for the clinical management of lung cancer
causes notable side effects (18,19);
therefore, new effective drugs to treat lung cancer are required.
Previous studies have shown that polyoxometalates exert their
antitumor properties by regulating cell invasion, proliferation and
migration in a variety of malignancies such as breast, kidney,
lung, ovary, pancreas and prostate cancer (17). However, FNdPW has not been reported to
induce apoptosis in human lung cancer cells. It appears that the
ability of FNdPW to inhibit cell proliferation may depend upon the
cell type; different cell types have different predominant
apoptotic signaling pathways. Apoptosis (programmed cell death), is
an important homeostatic mechanism balancing cell division and cell
death, while maintaining the appropriate cell number in the body
(20). Therefore, the identification
of novel drugs that trigger the apoptosis of tumor cells has become
an attractive strategy in anticancer drug research (21). The Bcl-2 family members are essential
intracellular players in the apoptotic machinery (22). Several studies have reported that Bax,
Bcl-2 and caspase-3 are key molecules that cause apoptosis in lung
cancer cells (23). Combination
treatment with triptolide and hydroxycamptothecin synergistically
enhances apoptosis by increasing the ratio of Bax/Bcl-2 in A549
cells (24). Formononetin-induced
apoptosis was accompanied by upregulation of Bax and downregulation
of Bcl-2, with the consequent dysfunction of mitochondria and
activation of caspase-3 in A549 cells (25). Cactus pear extracts increased
caspase3 and Bax protein levels, and decreased the Bcl-2 protein
level in the lung cancer cell line A549 (26). Cochinchina momordica seed
induces lung cancer cell apoptosis by activating caspase-3 via
upregulation of Bax and downregulation of Bcl-2 (27). In the present study, it was observed
that the expression of Bcl-2 protein in A549 cells was
significantly lower than that in the control group (P=0.0396),
whereas the expression of Bax protein was significantly higher than
that in the control group (P=0.0236). The ratio of Bcl-2/Bax
significantly decreased upon treatment with FNdPW, indicating that
FNdPW induced apoptosis in A549 cells. In addition, FNdPW treatment
significantly increased the levels of Bax and decreased the levels
of Bcl-2. These data indicated that the caspase-3-dependent
apoptotic signaling pathway serves a critical role in the
anticancer activity of FNdPW.
Bad, a member of the Bcl-2 family, normally binds to
the Bcl-2/Bcl-extra large (xL) complex and triggers apoptosis.
However, p-Bad dissociates from this complex, resulting in the
release of Bcl-2/Bcl-xL and the suppression of apoptosis (28). XIAP interacts with caspase-3 to block
its full activation, substrate cleavage and cell death (29). To fully understand how FNdPW increases
the ratio of Bax over Bcl-2 and activates caspase-3, the present
study examined the levels of Bad, p-Bad and XIAP proteins, and
noticed that FNdPW treatment significantly increased the levels of
Bad and decreased the levels of p-Bad and XIAP. These data
indicated that caspase-3-dependent apoptotic signaling serves a
critical role in the anticancer activity of FNdPW.
The caspase family is important in apoptosis, with
the cysteine aspartic acid protease caspase-3 being a key molecule
that transmits apoptotic signals in multiple signaling pathways
(30). The present study demonstrated
that caspase-3 activity expression in cells increased following
treatment with FNdPW. Although caspases are important mediators of
apoptosis, there is accumulating evidence for the existence of
caspase-independent mechanisms of cell death (28,29). AIF
is a putative caspase-independent effector of cell death that has
recently been cloned and characterized. It is a mitochondrial
intermembrane flavoprotein that is released from mitochondria and
translocates to the nucleus in response to specific death signals
(31,32). The present study observed that FNdPW
treatment resulted in the translocation of AIF from mitochondria to
the nucleus, suggesting that the caspase-3-independent signaling
pathway is involved in the apoptosis induced by FNdPW.
In conclusion, the present study demonstrated for
the first time that FNdPW markedly inhibits cell proliferation by
inducing apoptosis in A549 cells. This event is likely to be
associated with multiple targets involved in A549 cell
proliferation which are regulated by FNdPW. FNdPW increased the
expression of Bad and decreased the level of p-Bad, which
subsequently mediated an increase in the ratio of Bax over Bcl-2,
thus leading to the activation of caspase-3. Our findings suggest
that FNdPW is a potential drug for the clinical treatment of lung
cancer, provided that FNdPW can be applied to the right types of
lung cancer in the adequate cellular context.
Acknowledgements
The present study was supported by the Nature Fund
of Heilongjiang Province of China (Harbin, China; grant no.
D201079).
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ali HA Ahmed, Di J, Mei W, Zhang YC, Li Y,
Du ZW and Zhang GZ: Antitumor activity of lentivirus-mediated
interleukin-12 gene modified dendritic cells in human lung cancer
in vitro. Asian Pac J Cancer Prev. 15:611–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Das D, Preet R, Mohapatra P, Satapathy SR
and Kundu CN: 1,3-Bis(2-chloroethyl)-1-nitrosourea enhances the
inhibitory effect of resveratrol on 5-fluorouracil
sensitive/resistant colon cancer cells. World J Gastroenterol.
19:7374–7388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chermann JC, Raynaud M, Jasmin C and Mathé
G: Powerful new inhibitor of murine leukaemia and sarcoma viruses.
Nature. 227:173–174. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Mei WJ, Xu AW, Tan CP, Shi S and Ji
LN: Synthesis, characterization and antiviral activity against
influenza virus of a series of novel manganese-substituted rare
earth borotungstates heteropolyoxometalates. Antiviral Res.
62:65–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng CG, Xiong YD and Liu X: Synthesis,
spectroscopy and antibacterial activity of supermolecular compounds
of organotitanium substituted heteropolytungstates containing
8-quinolinol. Guang Pu Xue Yu Guang Pu Fen Xi. 31:1153–1160.
2011.PubMed/NCBI
|
|
7
|
Matsumoto Y and Komiyama M: DNA hydrolysis
by rare-earth metal ions. Nucleic Acids Symp Ser. 27:33–34.
1992.
|
|
8
|
Matsumura K and Komiyama M: Enormously
fast RNA hydrolysis by lanthanide (III) ions under physiological
conditions: Eminent candidates for novel tools of biotechnology. J
Biochem. 122:387–394. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palasz A and Czekaj P: Toxicological and
cytophysiological aspects of lanthanides action. Acta Biochim Pol.
47:1107–1114. 2000.PubMed/NCBI
|
|
10
|
Sato T, Hashizume M, Hotta Y and Okahata
Y: Morphology and proliferation of B16 melanoma cells in the
presence of lanthanoid and Al3+ions. Biometals. 11:107–112. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGahon AJ, Martin SJ, Bissonnette RP,
Mahboubi A, Shi Y, Mogil RJ, Nishioka WK and Green DR: The end of
the (cell) line: Methods for the study of apoptosis in vitro.
Methods Cell Biol. 46:153–185. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Takahashi S, Imamura M, Okutani E,
Zhang ZG, Chayama K and Chen BA: Earthworm fibrinolytic enzyme:
Anti-tumor activity on human hepatoma cells in vitro and in vivo.
Chin Med J (Engl). 120:898–904. 2007.PubMed/NCBI
|
|
13
|
Ribble D, Goldstein NB, Norris DA and
Shellman YG: A simple technique for quantifying apoptosis in
96-well plates. BMC Biotechnol. 5:122005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lambert KE, Huang H, Mythreye K and Blobe
GC: The type III transforming growth factor-beta receptor inhibits
proliferation, migration and adhesion in human myeloma cells. Mol
Biol Cell. 22:1463–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Wang LF, Wang JY and Tang N:
Synthesis, characterization, antioxidative and antitumor activities
of solid quercetin rare earth (III) complexes. Mol Biol Cell.
83:41–48. 2001.
|
|
16
|
Martin SJ and Green DR: Apoptosis and
cancer: The failure of controls on cell death and cell survival.
Crit Rev Oncol Hematol. 18:137–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed JC: Apoptosis-targeted therapies for
cancer. Cancer Cell. 3:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delbridge AR and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Sun C, Wang S, He Q and Li D:
microRNA miR-10b inhibition reduces cell proliferation and promotes
apoptosis in non-small cell lung cancer (NSCLC) cells. Mol Biosyst.
11:2051–2059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng G, Wang W, Chai K, Yang S, Li F and
Jiang K: Combination treatment with triptolide and
hydroxycamptothecin synergistically enhances apoptosis in A549 lung
adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt
signaling pathways. Int J Oncol. 46:1007–1017. 2015.PubMed/NCBI
|
|
21
|
Yang Y, Zhao Y, Ai X, Cheng B and Lu S:
Formononetin suppresses the proliferation of human non-small cell
lung cancer through induction of cell cycle arrest and apoptosis.
Int J Clin Exp Pathol. 7:8453–8461. 2014.PubMed/NCBI
|
|
22
|
Zou Y, Qin X, Xiong H, Zhu F, Chen T and
Wu H: Apoptosis of human non-small-cell lung cancer A549 cells
triggered by evodiamine through MTDH-dependent signaling pathway.
Tumour Biol. 36:5187–5193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen Y, Meng L, Sun H, Zhu Y and Liu H:
Cochinchina momordica seed suppresses proliferation and metastasis
in human lung cancer cells by regulating multiple molecular
targets. Am J Chin Med. 43:149–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhivotovsky B: Caspases: The enzymes of
death. Essays Biochem. 39:25–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nair R, Roden DL, Teo WS, McFarland A,
Junankar S, Ye S, Nguyen A, Yang J, Nikolic I, Hui M, et al: c-Myc
and Her2 cooperate to drive a stem-like phenotype with poor
prognosis in breast cancer. Oncogene. 33:3992–4002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hartl M and Bister K: Analyzing Myc in
cell transformation and evolution. Methods Mol Biol. 1012:21–49.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren Y, Han X, Yu K, Sun S, Zhen L, Li Z
and Wang S: microRNA-200c downregulates XIAP expression to suppress
proliferation and promote apoptosis of triple-negative breast
cancer cells. Mol Med Rep. 10:315–321. 2014.PubMed/NCBI
|
|
28
|
Yang E, Zha J, Jockel J, Boise LH,
Thompson CB and Korsmeyer SJ: Bad, a heterodimeric partner for
Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell.
80:285–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu W, Wan OW and Chung KK: S-nitrosylation
of XIAP at Cys 213 of BIR2 domain impairs XIAP's anti-caspase 3
activity and anti-apoptotic function. Apoptosis. 20:491–499. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu X, Wen H, Hu Y, Yu H, Zhang Y, Chen C
and Pan X: STAT1-caspase 3 pathway in the apoptotic process
associated with steroid-induced necrosis of the femoral head. J Mol
Histol. 45:473–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Agarwal E, Chaudhuri A, Leiphrakpam PD,
Haferbier KL, Brattain MG and Chowdhury S: Akt inhibitor MK-2206
promotes anti-tumor activity and cell death by modulation of AIF
and Ezrin in colorectal cancer. BMC Cancer. 14:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ukp Subramanian V, Nicholas AP, Thompson
PR and Ferretti P: Modulation of calcium-induced cell death in
human neural stem cells by the novel peptidylarginine deiminase-AIF
pathway. Biochim Biophys Acta. 1843:1162–1171. 2014. View Article : Google Scholar : PubMed/NCBI
|