Introduction
Laryngeal cancer is estimated to be the second most
common malignancy of the head and neck, and it has a high mortality
rate and a poor prognosis (1,2). The majority of laryngeal carcinoma
patients are middle-aged males (3,4). The
primary risk factors are endogenic and exogenic factors such as
tobacco smoking and alcohol consumption (4,5). Although
multiple and advanced therapeutic interventions have been
developed, the majority of laryngeal cancer patients present late
to hospital, which leads to reduced therapeutic efficacy and
increased rate of recurrence (2).
Therefore, the development of highly efficacious treatments, as
well as better diagnostic and preventive measures, require a better
understanding of the molecular and pathogenic mechanisms of
laryngeal cancer.
A disintegrins and metalloproteinases (ADAMs) are
primarily located in the cell membrane, and have been shown to be
involved in the proteolytic degradation of cell membrane proteins
for remodelling or processing (6).
Thus, ADAM family members, including ADAM10, serve pivotal roles in
the pathogenesis or progression of cancers, including
proliferation, angiogenesis, migration and invasion (6–9). Analysis
of gene expression profiles revealed that the ADAM10 gene was
significantly overexpressed in a wide variety of human
malignancies, including hepatocellular carcinoma, melanoma, oral
squamous cell carcinoma, lung cancer, pancreatic carcinoma, and
gastric and bladder cancer (10–17).
Accumulating evidence has illustrated that ADAM10 contributed to
the regulation of cancer progression, chemoresistance and
metastasis via the cleavage of growth factors or cell surface
proteins (6,8,10,12,18–21).
Recently, it was reported that ADAM10 is overexpressed in
hepatocellular carcinoma tissues, and that there are significant
associations between ADAM10 expression levels and tumor grade,
tumor differentiation, tumor size and metastasis (10). However, the involvement of ADAM10 in
human laryngeal carcinoma remains unclear.
The present study firstly detected the expression
pattern of ADAM10 in laryngeal carcinoma, and revealed that
upregulated ADAM10 is strongly associated with T classification,
clinical stage, pathology, Ki-67 expression and short survival in
laryngeal carcinoma patients.
Materials and methods
Tissue specimens
A total of 78 laryngeal carcinoma tissue samples and
35 adjacent non-tumor tissues were collected from a cohort of
laryngeal carcinoma patients following surgical excision at the
Department of Otolaryngology, Head and Neck Surgery, Affiliated
Hospital of Nantong University (Nantong, China) between January
2005 and December 2014. Informed consent was obtained from all
patients. The clinicopathological characteristics of the patients
are presented in Table I. All 78
laryngeal carcinoma patients included 72 males and 6 females aged
47–83 years old (mean age, 67.9 years). The follow-up time was 80
months (follow-up period range, 12–80 months), and the median
clinical follow-up time was 65 months. A total of 20 paired fresh
laryngeal carcinoma and adjacent non-tumor tissues were collected
from the Affiliated Hospital of Nantong University, snap frozen in
liquid nitrogen and stored at −80°C until use. None of the patients
had received any therapy prior to surgery. Ethics approval to
perform the current study was obtained from the Affiliated Hospital
of Nantong University.
 | Table I.Distribution of ADAM10 status in 78
human laryngeal carcinoma tissues according to clinicopathological
characteristics. |
Table I.
Distribution of ADAM10 status in 78
human laryngeal carcinoma tissues according to clinicopathological
characteristics.
|
|
| ADAM10 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | N | Low | High | P-value |
|---|
| Gender |
|
|
| 0.665 |
| Male | 72 | 26 | 46 |
|
|
Female | 6 | 3 | 3 |
|
| Age, years |
|
|
| 0.456 |
|
<60 | 25 | 11 | 14 |
|
| ≥60 | 53 | 18 | 35 |
|
| Tobacco smoking |
|
|
| 0.122 |
| No | 23 | 12 | 11 |
|
| Yes | 55 | 17 | 38 |
|
| T status |
|
|
|
<0.010a |
|
T1-T2 | 47 | 23 | 24 |
|
|
T3-T4 | 31 | 6 | 25 |
|
| Lymph node
metastasis |
|
|
| 0.065 |
| No | 43 | 20 | 23 |
|
|
Yes | 35 | 9 | 26 |
|
| TNM clinical
stage |
|
|
|
<0.010a |
|
I–II | 27 | 16 | 11 |
|
|
III–IV | 51 | 13 | 38 |
|
| Pathology
grade |
|
|
| 0.034a |
|
Well/moderate | 47 | 22 | 25 |
|
|
Poor/undifferentiated | 31 | 7 | 24 |
|
| Tumor location |
|
|
|
|
|
Glottic | 47 | 18 | 29 | 0.883 |
|
Supraglottic | 24 | 9 | 15 | 0.879 |
|
Subglottic | 7 | 2 | 5 | 0.649 |
| Ki-67
expression |
|
|
|
<0.010a |
|
Low | 33 | 21 | 12 |
|
|
High | 45 | 8 | 37 |
|
Immunohistochemical staining
Immunohistochemical analysis was performed as
described previously (22). The
sections were incubated with anti-ADAM10 antibody (diluted 1:100;
Sangon Biotech, Co., Ltd., Shanghai, China) and anti-Ki-67 antibody
(diluted 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. Appropriate positive and negative controls were
processed in parallel. VECTASTAIN® Elite ABC kit (Vector
Laboratories, Inc., Burlingame, CA, USA) and 3,3-diaminobenzidine
were used to detect the binding of the primary antibody and to
visualize the reaction, respectively.
Three blinded pathologists evaluated the
immunohistochemistry scoring results independently. Immunostaining
of ADAM10 was scored semiquantitatively in each tumor section based
on the intensity and percentage of staining (17). Cytoplasmic immunostaining in tumor
cells was considered to be positive. The staining intensity was
assessed as strong=2; weak=1; and negative=0. The percentage of
staining was categorized as follows: 0% of cells=0; 1–25% of
cells=1; 26–50% of cells=2; 51–75% of cells=3; and >75% of
cells=4. A final immunoreactivity score of 0–8 was obtained for
each section by multiplying the intensity score by the percentage
score. Tumor samples with a final score <4 were regarded as
negative or weak staining, while tumor samples with a final score
of 4–8 were considered as ADAM10 overexpression. When evaluating
Ki-67 expression, 51–100% of positively stained cell nuclei was
regarded as high-expression group, while 0–50% was considered as
low-expression group.
Western blot analysis
Total proteins were harvested with lysis buffer
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
quantified with the Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). In total, 20 µg protein per lane was separated
by SDS-PAGE and transferred to polyvinylidene difluoride membranes.
The membranes were blocked with 10% dried skim milk for 1 h at room
temperature. Then, primary antibodies were used for immunoblot
analysis against ADAM10 (1:300; D221496-0100; Sangon Biotech, Co.,
Ltd.) and β-actin (1:2,000; sc-47778; Santa Cruz Biotechnology,
Inc.), overnight at 4°C. Then, horseradish peroxidase-linked
immunoglobulin G (1:5,000; sc-2374; Santa Cruz Biotechnology, Inc.)
was used as the secondary antibody at room temperature for 1.5 h.
Immunoreactive bands were visualized by chemiluminescence (NEN;
Life Science Products, Boston, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from frozen tissues was extracted using a
TRIzol extraction kit (Sangon Biotech, Co., Ltd.), and then
subjected to RT to covert it into complementary DNA using a
Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics
GmbH, Mannheim, Germany). The primers were purchased from Sangon
Biotech Co., Ltd., and their sequences were as follows: ADAM10
forward primer, 5′-ATGGATTGTGGCTCATTGGT-3′ and reverse primer,
5′-TGCCTGGAAGTGGTTTAGGA-3′; and GAPDH forward primer,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse primer,
5′-GAAGATGGTGATGGGATTTC-3′. The PCR cycling conditions were as
follows: 42°C for 30 min, followed by 36 cycles of amplification
(94°C for 20 sec, 58°C for 20 sec and 72°C for 30 sec). Melting
curve analysis was performed to assess the specificity and identity
of the RT-qPCR products. The experiment was conducted in
triplicate. GAPDH served as the internal control for messenger RNA
(mRNA) determination of ADAM10. The results were normalized with
the corresponding internal controls. The relative genomic
expression was calculated by the 2−ΔΔCq method (23).
Calculation and statistical
analysis
The data were analyzed with SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). The results were shown as means ±
standard deviation, and the Student's t-test was used to determine
the statistical significance. Survival curves were estimated by the
Kaplan-Meier method and compared by the log-rank test. Univariate
and multivariate analyses were performed using Cox proportional
hazards regression models. All statistical analysis were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Analysis of ADAM10 expression in
laryngeal carcinoma by western blotting and RT-qPCR
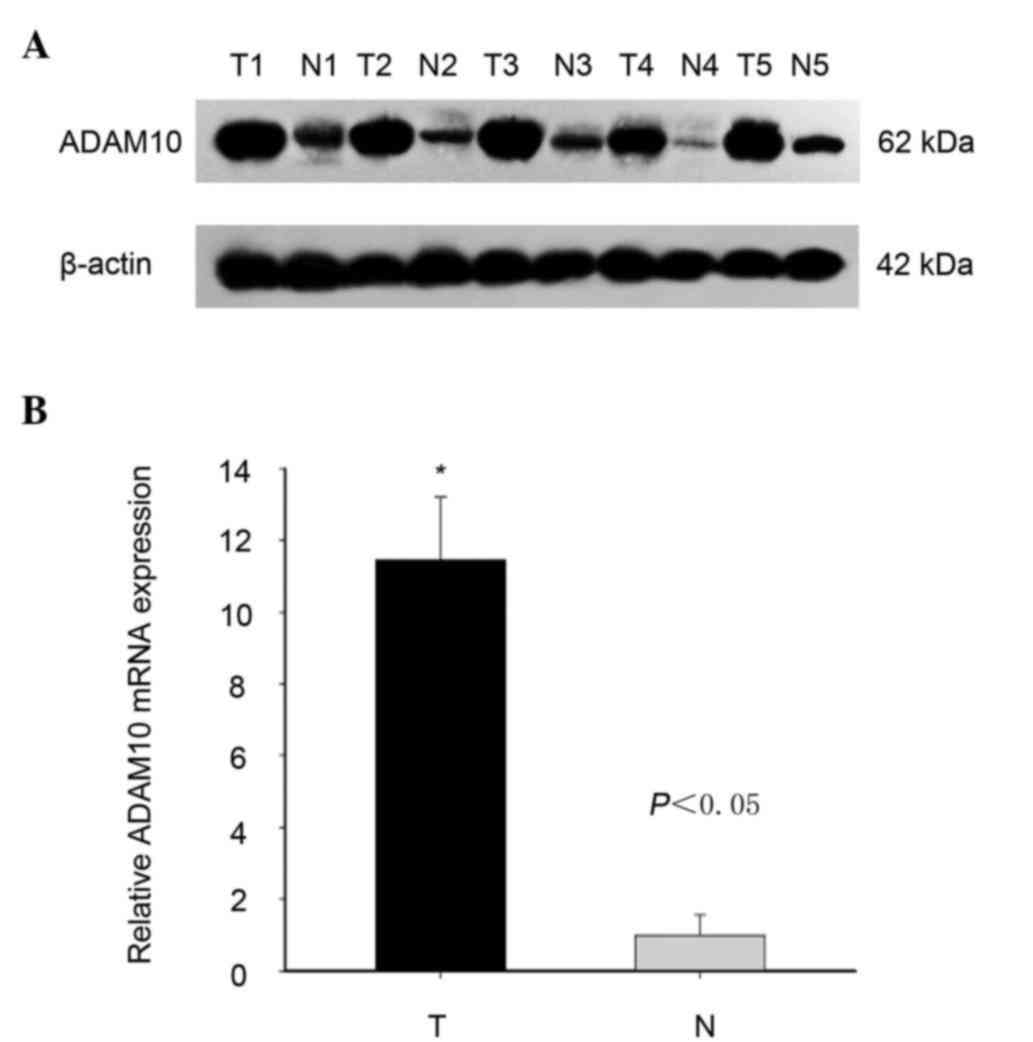
First, the expression level of ADAM10 was
investigated in laryngeal carcinoma tissues compared with that in
adjacent non-tumor tissues using western blotting and RT-qPCR. As
shown in Fig. 1, both the protein and
mRNA expression levels of ADAM10 were effectively higher in
laryngeal carcinoma than in adjacent non-tumor tissues, although
ADAM10 expression was variable among different pairs of laryngeal
tissues (Fig. 1).
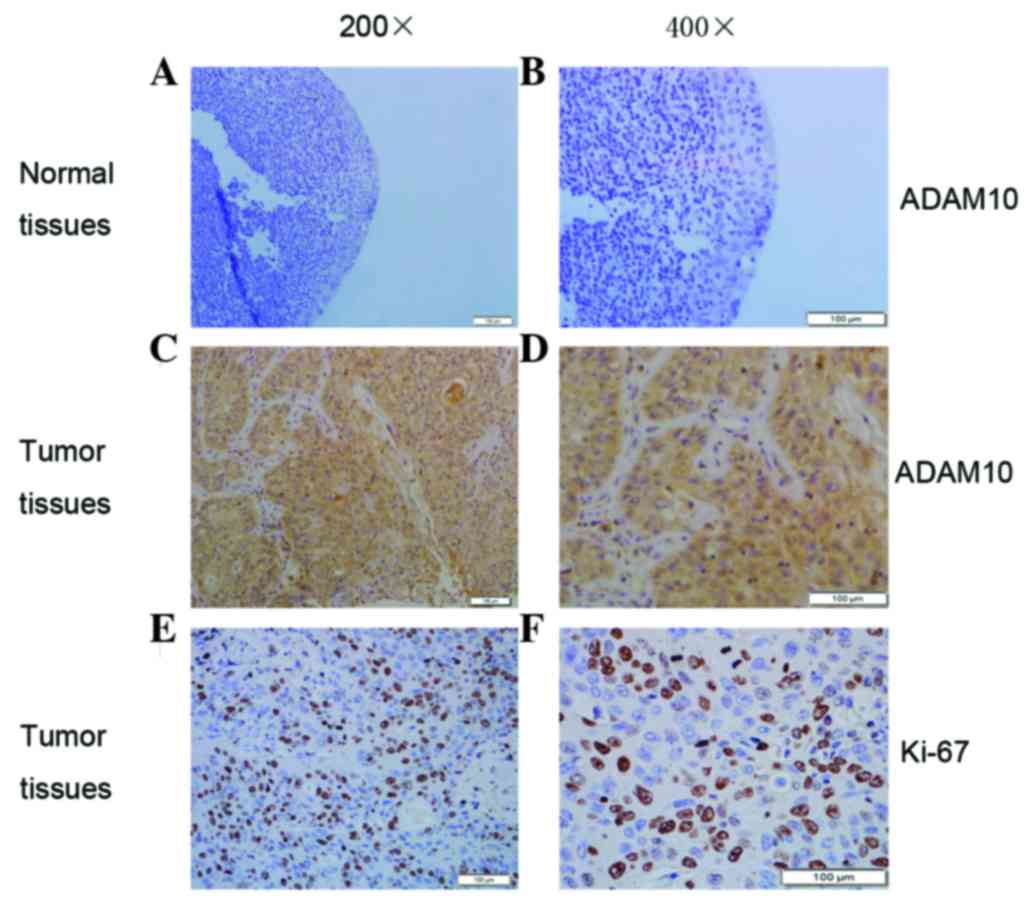
Detection of ADAM10 and Ki-67
expression in laryngeal carcinoma by immunohistochemistry
It was previously reported that overexpressed ADAM10
serves an important role in cancer cell proliferation (7). In the present study,
immunohistochemistry was further performed to explore the
expression of ADAM10 and the proliferation marker Ki-67 in
laryngeal carcinoma (Fig. 2). Both
ADAM10 and Ki-67 were highly expressed in laryngeal carcinoma
compared with the levels detected in the adjacent non-tumor tissues
(Fig. 2). The positive expression
rate of ADAM10 is summarized in Table
II. In brief, high ADAM10 expression was present in 62.82%
(49/78) of laryngeal carcinoma tissues, while it was only present
in 22.86% (8/35) of adjacent non-tumor tissues (Table II). The expression rate of Ki-67 was
57.69% (45/78) in laryngeal carcinoma tissues, while it was 31.43%
(11/35) in normal tissues (Table
II). These results confirmed that both ADAM10 and Ki-67
exhibited higher expression levels in laryngeal carcinoma tissues
than in non-tumor tissues.
 | Table II.Expression of ADAM10 and Ki-67 in 78
laryngeal carcinoma and 35 tumor-adjacent normal tissues. |
Table II.
Expression of ADAM10 and Ki-67 in 78
laryngeal carcinoma and 35 tumor-adjacent normal tissues.
|
|
| ADAM10, n (%) | Ki-67, n (%) | ADAM10/Ki-67, n
(%) |
|---|
|
|
|
|
|
|
|---|
| Tissues | N | +a | P-value | +a | P-value | +/+a | P-value |
|---|
| Laryngeal carcinoma
tissues | 78 | 49 (62.82) | <0.01 | 45 (57.69) | 0.01 | 37 (47.44) | <0.01 |
| Tumor-adjacent
normal tissues | 35 | 8 (22.86) |
| 11 (31.43) |
| 3 (8.57) |
|
Association between ADAM10 expression
and clinicopathological characteristics in laryngeal carcinoma
The association of ADAM10 expression level with
clinicopathological factors of laryngeal carcinoma was evaluated.
As shown in Table I, ADAM10
overexpression was significantly associated with T classification
(P<0.01), clinical stage (P<0.01), pathology (P=0.034) and
Ki-67 expression (P<0.01). However, ADAM10 overexpression
exhibited no significant association with other clinicopathological
characteristics, including gender (P=0.665), age (P=0.456), tobacco
smoking (P=0.122), lymph metastasis location (P=0.065) or tumor
location (all P>0.05). Furthermore, high expression of ADAM10
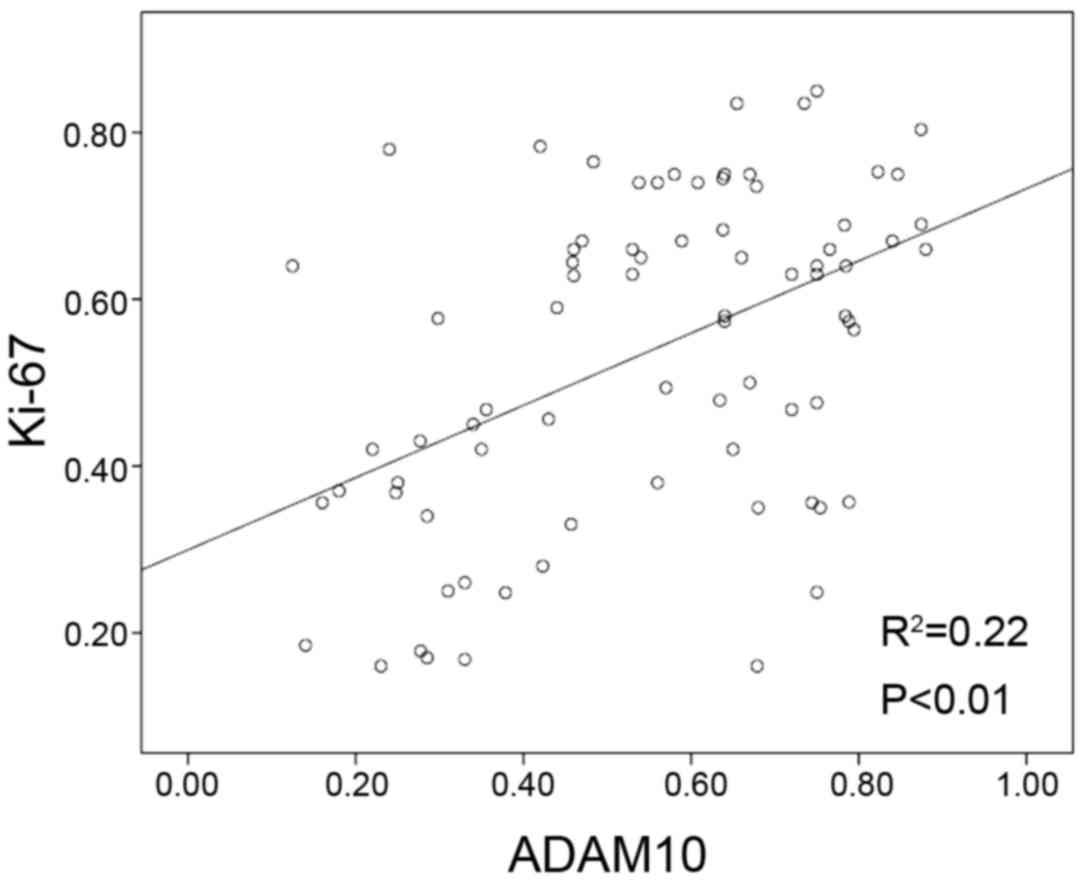
was similar to that of Ki-67 in the majority of specimens (Table I). There was a positive correlation
between ADAM10 expression and Ki-67-based proliferative activity
(P<0.01; Fig. 3).
ADAM10 expression is associated with
survival of laryngeal carcinoma patients
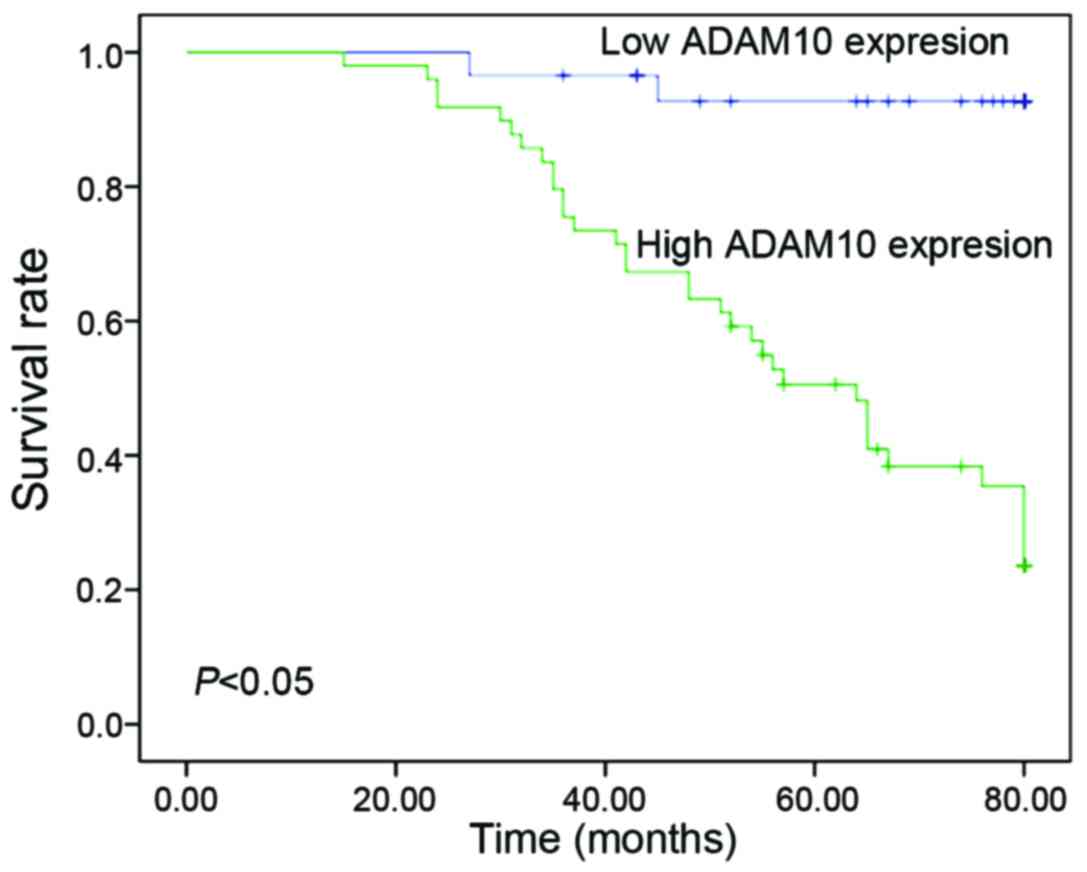
Next, the prognostic value of ADAM10 expression in
laryngeal carcinoma was assessed using Kaplan-Meier analysis with
the log-rank test. It was observed that patients with ADAM10
overexpression exhibited significantly worse survival time than
those with low ADAM10 expression (P<0.05) (Fig. 4). Univariate analyses revealed that
the survival of laryngeal carcinoma patients was significantly
correlated with T classification (P<0.01), lymph node metastasis
(P=0.04), tumor-node-metastasis (TNM) clinical stage (P<0.01),
Ki-67 expression (P<0.01) and ADAM10 expression (P<0.01)
(Table III). Multivariate analysis
revealed that Ki-67 expression (P=0.034) and ADAM10 expression
(P=0.030) were independent prognostic factors in laryngeal
carcinoma patients (Table IV).
 | Table III.Survival status and
clinicopathological parameters in 78 human laryngeal carcinoma
tissues. |
Table III.
Survival status and
clinicopathological parameters in 78 human laryngeal carcinoma
tissues.
|
|
| Survival status,
n |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | N | Alive | Dead | P-value |
|---|
| Gender |
|
|
| 0.406 |
|
Male | 72 | 40 | 32 |
|
|
Female | 6 | 2 | 4 |
|
| Age, years |
|
|
| 0.813 |
|
<60 | 25 | 14 | 11 |
|
|
≥60 | 53 | 28 | 25 |
|
| Tobacco
smoking |
|
|
| 0.807 |
| No | 23 | 13 | 10 |
|
|
Yes | 55 | 29 | 26 |
|
| T
classification |
|
|
|
<0.010a |
|
T1-T2 | 47 | 33 | 14 |
|
|
T3-T4 | 31 | 9 | 22 |
|
| Lymph node
metastasis |
|
|
| 0.040a |
| No | 43 | 28 | 15 |
|
|
Yes | 35 | 14 | 21 |
|
| TNM clinical
stage |
|
|
|
<0.010a |
|
I–II | 27 | 23 | 4 |
|
|
III–IV | 51 | 19 | 32 |
|
| Pathology
grade |
|
|
| 0.107 |
|
Well/moderate | 47 | 29 | 18 |
|
|
Poor/undifferentiated | 31 | 13 | 18 |
|
| Tumor location |
|
|
|
|
|
Glottic | 47 | 25 | 22 | 0.762 |
|
Supraglottic | 24 | 14 | 10 | 0.762 |
|
Subglottic | 7 | 3 | 4 | 0.754 |
| Ki-67 |
|
|
|
<0.010a |
| Low
expression | 33 | 27 | 6 |
|
| High
expression | 45 | 15 | 30 |
|
| ADAM10 |
|
|
|
<0.010a |
| Low
expression | 29 | 27 | 2 |
|
| High
expression | 49 | 15 | 34 |
|
 | Table IV.Contribution of various potential
prognostic factors to survival by Cox regression analysis on 78
human laryngeal carcinoma patients. |
Table IV.
Contribution of various potential
prognostic factors to survival by Cox regression analysis on 78
human laryngeal carcinoma patients.
| Variables | Hazard ratio | P-value | 95% Confidence
interval |
|---|
| T
classification |
| 0.751 | 0.383–1.999 |
| T1-T2
vs. T3-T4 | 0.875 |
|
|
| Lymph node
metastasis |
| 0.694 | 0.388–1.877 |
| No vs.
yes | 0.854 |
|
|
| TNM clinical
stage |
| 0.079 | 0.867–13.858 |
| I–II
vs. III–IV | 3.466 |
|
|
| Ki-67
expression |
|
0.034a | 1.080–7.083 |
| Low vs.
high | 2.766 |
|
|
| ADAM10
expression |
|
0.030a | 0.037–0.842 |
| Low vs.
high | 0.178 |
|
|
Discussion
The ADAMs are a family of proteins that contain an
N-terminal prodomain preceding a metalloproteinase domain, a
disintegrin or integrin-binding domain, a cysteine-rich region, a
transmembrane domain and an intracellular domain (24). ADAMs are involved in modulating the
activity of diverse growth factors, receptors, membrane-bound
cytokines and adhesion molecules by proteolytic cleavage, thus
contributing to tumor developmental processes (8,24). A
number of ADAMs, including ADAM9, ADAM10, ADAM12 and ADAM17, are
critical during the genesis, development and metastasis of cancers
(21,25,26).
Dysregulated expression of ADAM10 has been reported in various
malignancies, and has been suggested to be involved in cancer
progression (10–17). It has been demonstrated that
downregulation of ADAM10 in HepG2 cells via small interfering RNA
significantly suppressed cell proliferation, migration and
invasion, and decreased chemotherapy drug resistance in
vitro and tumor growth in vivo (27,28),
partially via reducing the constitutive phosphorylation of
phosphoinositide 3-kinase and Akt (28). In addition, Maretzky et al
reported that ADAM10 modulated the nuclear translocation of
β-catenin through shedding of cadherin E, resulting in enhanced
expression of cyclin D1 and c-Myc, thus leading to the promotion of
proliferation (29). Furthermore,
ADAM10 contribution to cell proliferation by increasing the
transcriptional activity of β-catenin involves the action of
receptor protein tyrosine phosphatases (30). In the present study, the expression
and clinicopathological significance of ADAM10 in laryngeal
carcinoma was characterized, particularly the prognostic function
of ADAM10.
Our results indicated that ADAM10 expression was
significantly higher in laryngeal carcinoma tissues compared with
that in adjacent non-tumor tissues. This finding was consistent
with that from previous studies on other tumors (10–17). Zhang
et al reported that overexpression of ADAM10 in
hepatocellular carcinoma tissues was significantly associated with
tumor grade, tumor differentiation, tumor size and metastasis
(10). The present study further
analyzed the correlation of ADAM10 with clinical features of
laryngeal carcinoma patients by immunohistochemistry. It was
observed that high ADAM10 expression was associated with T
classification, clinical stage, pathology and Ki-67 expression (all
P<0.05).
Previous reports indicated that ADAM10
overexpression is also involved with the poor prognosis of various
malignancies, including hepatocellular carcinoma and gastric cancer
(10,16). In our study, Kaplan-Meier analysis
also confirmed the prognostic value of ADAM10 in laryngeal
carcinoma. Patients with ADAM10 overexpression exhibited poor
prognosis and short survival time.
Univariate analysis revealed that patient's overall
survival was significantly correlated with T classification
(P<0.01), lymph node metastasis (P=0.04), TNM clinical stage
(P<0.01), Ki-67 expression (P<0.01) and ADAM10 expression
(P<0.01) (Table III). In
addition, our multivariate analyses demonstrated that high
expression of ADAM10 was a useful independent predictor of poor
prognosis for laryngeal carcinoma, regardless of disease status.
These findings suggested that ADAM10 expression may function as a
oncogene and serve an important role in the development and
progression of laryngeal carcinoma. In addition, high ADAM10
expression may serve as an independent prognostic biomarker of
laryngeal carcinoma.
In summary, our data offered convincing evidence
that ADAM10 is overexpressed in laryngeal carcinoma, and that high
ADAM10 expression is involved in the aggressive malignant phenotype
and poor prognosis of laryngeal carcinoma. Therefore, ADAM10 could
serve as a potential independent prognostic factor for laryngeal
carcinoma, and may be a novel therapeutic target for laryngeal
carcinoma therapy using an RNA interference-based approach.
Acknowledgements
The present study was supported by grants from the
Chinese National Natural Science Foundation (grant nos. 81172841,
81202368 and 81471603), the China Postdoctoral Science Foundation
(grant no. 2013M541708), the Natural Science Foundation of Jiangsu
Province (grant no. SBK2015022581), the ‘333 Natural Science
Foundation’ of Jiangsu (grant no. BRA2013286), the ‘Top Six Types
of Talents’ Financial Assistance of Jiangsu Province (grant no. 6),
the Jiangsu Provincial Health Department (grant no. Z201005), the
Innovative Project of Nantong University Postgraduate Students
(grant no. 13025043), the Jiangsu Province's Outstanding Medical
Academic Leader Program (grant no. LJ201136) and the Administration
of Science and Technology of Nantong (grant nos. BK2014007201 and
BK2014003).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vokes EE and Stenson KM: Therapeutic
options for laryngeal cancer. N Engl J Med. 349:2087–2089. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayne ST, Cartmel B, Kirsh V and Goodwin
WJ Jr: Alcohol and tobacco use prediagnosis and postdiagnosis, and
survival in a cohort of patients with early stage cancers of the
oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev.
18:3368–3374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freedman ND, Abnet CC, Leitzmann MF,
Hollenbeck AR and Schatzkin A: Prospective investigation of the
cigarette smoking-head and neck cancer association by sex. Cancer.
110:1593–1601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sapkota A, Hsu CC, Zaridze D, Shangina O,
SzeszeniaDabrowska N, Mates D, Fabiánová E, Rudnai P, Janout V,
Holcatova I, et al: Dietary risk factors for squamous cell
carcinoma of the upper aerodigestive tract in central and eastern
Europe. Cancer Causes Control. 19:1161–1170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klein T and Bischoff R: Active
metalloproteases of the A Disintegrin and Metalloprotease (ADAM)
family: Biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteases: Multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu X, Lu D, Scully M and Kakkar V: ADAM
proteins-therapeutic potential in cancer. Curr Cancer Drug Targets.
8:720–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Liu S, Liu K, Wang Y, Ji B, Zhang
X and Liu Y: A disintegrin and metalloprotease (ADAM)10 is highly
expressed in hepatocellular carcinoma and is associated with tumour
progression. J Int Med Res. 42:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko SY, Lin SC, Wong YK, Liu CJ, Chang KW
and Liu TY: Increase of disintergin metalloprotease 10 (ADAM10)
expression in oral squamous cell carcinoma. Cancer Lett. 245:33–43.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SB, Schramme A, Doberstein K, Dummer
R, AbdelBakky MS, Keller S, Altevogt P, Oh ST, Reichrath J, Oxmann
D, et al: ADAM10 is upregulated in melanoma metastasis compared
with primary melanoma. J Invest Dermatol. 130:763–773. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo J, He L, Yuan P, Wang P, Lu Y, Tong F,
Wang Y, Yin Y, Tian J and Sun J: ADAM10 overexpression in human
non-small cell lung cancer correlates with cell migration and
invasion through the activation of the Notch1 signaling pathway.
Oncol Rep. 28:1709–1718. 2012.PubMed/NCBI
|
|
14
|
Lendeckel U, Kohl J, Arndt M, CarlMcGrath
S, Donat H and Röcken C: Increased expression of ADAM family
members in human breast cancer and breast cancer cell lines. J
Cancer Res Clin Oncol. 131:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaida MM, Haag N, Günther F, Tschaharganeh
DF, Schirmacher P, Friess H, Giese NA, Schmidt J and Wente MN:
Expression of A disintegrin and metalloprotease 10 in pancreatic
carcinoma. Int J Mol Med. 26:281–288. 2010.PubMed/NCBI
|
|
16
|
Wang YY, Ye ZY, Li L, Zhao ZS, Shao QS and
Tao HQ: ADAM 10 is associated with gastric cancer progression and
prognosis of patients. J Surg Oncol. 103:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu L, Liu N, Han Y, Xie C, Li Q and Wang
E: ADAM10 regulates proliferation, invasion, and chemoresistance of
bladder cancer cells. Tumour Biol. 35:9263–9268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue Y, Shao Y, Luo Q, Shi L and Wang Z:
Downregulation of ADAM10 expression inhibits metastasis and
invasiveness of human hepatocellular carcinoma HepG2 cells. Biomed
Res Int. 2013:4345612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao H, Zhu J, Cui K, Xu X, O'Brien M,
Wong KK, Kesari S, Xia W and Wong ST: Bioluminescence imaging
reveals inhibition of tumor cell proliferation by Alzheimer's
amyloid beta protein. Cancer Cell Int. 9:152009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endres K and Fahrenholz F: Upregulation of
the alpha-secretase ADAM10-risk or reason for hope? FEBS J.
277:1585–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin T, Gu J, Zhang L, Davis JJ, Huang X,
Cabbini G, Ji L and Fang B: Enhancing adenovirus-mediated gene
transfer in vitro and in vivo by addition of protamine and
hydrocortisone. J Gene Med. 5:868–875. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You Y, Yao H, You B, Li X, Ni H, Shi S,
Shan Y and Cao X: Clinical significance of HAX-1 expression in
laryngeal carcinoma. Auris Nasus Larynx. 42:299–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu K, Liao M, Liu B and Deng Z: ADAM-17
over-expression in gallbladder carcinoma correlates with poor
prognosis of patients. Med Oncol. 28:475–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zubel A, Flechtenmacher C, Edler L and
Alonso A: Expression of ADAM9 in CIN3 lesions and squamous cell
carcinomas of the cervix. Gynecol Oncol. 114:332–336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Zhang W, Liu K, Ji B and Wang G:
Silencing ADAM10 inhibits the in vitro and in vivo growth of
hepatocellular carcinoma cancer cells. Mol Med Rep. 11:597–602.
2015.PubMed/NCBI
|
|
28
|
Zhang W, Liu S, Liu K, Ji B, Wang Y and
Liu Y: Knockout of ADAM10 enhances sorafenib antitumor activity of
hepatocellular carcinoma in vitro and in vivo. Oncol Rep.
32:1913–1922. 2014.PubMed/NCBI
|
|
29
|
Maretzky T, Reiss K, Ludwig A, Buchholz J,
Scholz F, Proksch E, de Strooper B, Hartmann D and Saftig P: ADAM10
mediates E-cadherin shedding and regulates epithelial cell-cell
adhesion, migration, and beta-catenin translocation. Proc Natl Acad
Sci USA. 102:9182–9187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anders L, Mertins P, Lammich S, Murgia M,
Hartmann D, Saftig P, Haass C and Ullrich A: Furin-, ADAM 10-, and
gamma-secretase-mediated cleavage of a receptor tyrosine
phosphatase and regulation of beta-catenin's transcriptional
activity. Mol Cell Biol. 26:3917–3934. 2006. View Article : Google Scholar : PubMed/NCBI
|