Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide, and its annual incidence approaches
~0.626 million cases worldwide (1).
HCC ranks sixth among the most common malignant tumors, and is the
third most common cause of cancer-associated mortality (1). Invasion and metastasis are important
factors that affect the survival and quality of life of HCC
patients (2). The high rates of
metastasis and recurrence are major obstacles to improve the rate
of survival (3).
Heat shock protein 70 (HSP70) is widely present in
prokaryotic and eukaryotic cells (4).
It can serve as a vehicle, acting as a ‘molecular chaperone’, and
is capable of regulating cellular signals (5). It has been reported that numerous tumor
cells release HSP70 (6). A previous
study demonstrated that HSP70-peptide complexes (HSP70-PCs)
released into the tumor microenvironment could not only regulate
tumor immune response, but were also involved in regulating a
variety of tumor biological behaviors (7). Borges et al (8) observed that extracellular HSP70
inhibited interleukin 10 expression, while Wu et al
(6) noted that, in liver cancer,
extracellular HSP70 enhanced resistance to apoptosis. In view of
these findings, studying the impact of extracellular HSP70-PCs on
tumor invasion and metastasis would be worthwhile. In the present
study, the effect of extracellular HSP70/HSP70-PCs on HCC cell
motility was investigated, and it was observed that extracellular
HSP70/HSP70-PCs regulated HCC cell migration by modulating Ras
homolog family member A (RhoA) expression.
Materials and methods
Cell culture
The human HCC cell line Huh-7 was purchased from the
Cell Bank of the Shanghai Institute for Biological Sciences
(Shanghai, China). Cells were cultured at 37°C in a 5%
CO2 atmosphere, in Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 2–3 days, digested with
0.25% trypsin and passaged. Logarithmic growth-phase cells were
used for the experiments. Cells were divided into the following
groups: Control and treated with extracellular HSP70/HSP70-PCs
(final concentration, 2 µg/ml).
Antibodies
The following antibodies were used for western
blotting analyses: Rabbit monoclonal anti-HSP70 (dilution, 1:1,000;
catalog no., K0414; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), mouse polyclonal anti-RhoA/B/C (dilution, 1:1,000; catalog
no., E2213; Santa Cruz Biotechnology, Inc.) and mouse monoclonal
anti-glyceraldehyde-3-phosphate dehydrogenase (dilution, 1:2,000;
catalog no., SAB2701826; GAPDH; Sigma-Aldrich, St. Louis, MO, USA).
Horseradish peroxidase-conjugated sedondary antibodies were
purchased from Beijing Zhongshan Golden Bridge Biotechnology, Co.,
Ltd. (Beijing, China).
HSP-peptide binding of extracellular
HSP70/HSP70-PCs
In vitro binding of antigen peptide and
glutathione S-transferase (GST)-HSP70 was performed using GST-HSP70
fusion proteins (Sigma-Aldrich) as previously described (9). The reaction system contained 1 mmol/l
adenosine diphosphate, 1 mmol/l MgCl2, 75 µg/ml peptide
and 250 µg/ml GST-HSP70, and was incubated at 37°C in a water bath
for 2 h. The peptides were extracted and purified from
hepatocarcinoma tissue samples, as previously described (10).
Wound healing assay
A lesion was created using a plastic pipette tip 24
h after extracellular HSP70/HSP70-PCs treatment, and cells were
washed twice with phosphate-buffered saline to remove debris. The
monolayer was then maintained in serum-free DMEM and cultured for
24 h. Then, five randomly selected fields at the border of the
lesion were viewed under an inverted microscope (IX71; Olympus
Corporation, Tokyo, Japan).
Transwell invasion and migration
assays
Cell invasion and migration assays were performed
using a Transwell system (Corning Incorporated, Corning, NY, USA)
according to the manufacturer's protocol. To assess invasion
ability, membranes were pre-coated with Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA). Approximately 5×104 cells in
serum-free medium were added to the top chamber; the bottom chamber
was filled with DMEM containing 10% FBS. After 24-h incubation,
cells on the upper surface of the membrane were gently removed with
a cotton swab, and the membrane was then fixed in 4% methanol for
30 min and stained with 0.1% crystal violet for 30 min. Cells that
had migrated to the bottom surface of the membrane were collected,
and the number of cells was counted. The same experimental design
was used for the migration assay, but the membranes were not
pre-coated with Matrigel.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells at 24 and 48 h
after induction with TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Single-strand
complementary DNA was synthesized from 1 µg total RNA by RT,
according to the manufacturer's protocol (Toyobo Co., Ltd., Osaka,
Japan). qPCR was used to measure the RhoA messenger RNA (mRNA)
levels in the cells. Amplification of the cDNA was performed using
the SYBR Premix Ex Taq™ II kit (Takara Bio, Inc., Otsu, Japan) qPCR
was performed using an ABI PRISM® 7000 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Amplification was conducted in a 25-µl volume for 35 cycles,
and the product was detected using SYBR Green I fluorochrome. The
cycling conditions for PCR were: 15 min incubation at 95°C,
followed by 45 cycles of 94°C for 5 sec, 50°C for 15 sec and 72°C
for 10 sec. The primers used were: RhoA forward,
5′-CCGCCATCGCTTACA-3′ and reverse, 5′-GGCACCTGACCCTTGTA-3′; and
GAPDH forward, 5′-GGATTTGGTCGTATTGGG-3′ and reverse,
5′-TCGCTCCTGGAAGATGG-3′. GAPDH was used as a control. The ΔΔCq
method was used for normalization (11).
Western blot analysis
Cells were lysed at the designated time points, and
equal amounts of protein lysate underwent 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. Subsequently, proteins
were transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA) and probed with the aforementioned
primary and secondary antibodies. Bound antibodies were detected
with Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.). Band densities were analyzed using ImageJ
software (version 1.41; National Institutes of Health, Bethesda,
MA, USA).
Transient transfection of RhoA small
interfering RNA (siRNA)
Transfection of RhoA-siRNA was performed using
Lipofectamine™ 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
siRNA duplexes were purchased from Sigma-Aldrich.
Immunofluorescence staining
Cells grown on coverslips were fixed, blocked and
permeabilized, as described previously (12). Cells were then stained with
fluorescein isothiocyanate (FITC)-conjugated phalloidin (5 µg/ml;
Sigma-Aldrich) for 60 min. Actin filaments (F-actin) were
visualized and photographed with a IX71 digital inverted
microscope. In total, >200 cells from three independent
experiments were analyzed for each condition. The average number of
dot or fan-like protrusions around a cell was counted as an index
for lamellipodia formation (12).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was preformed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA) Statistical significance was determined
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
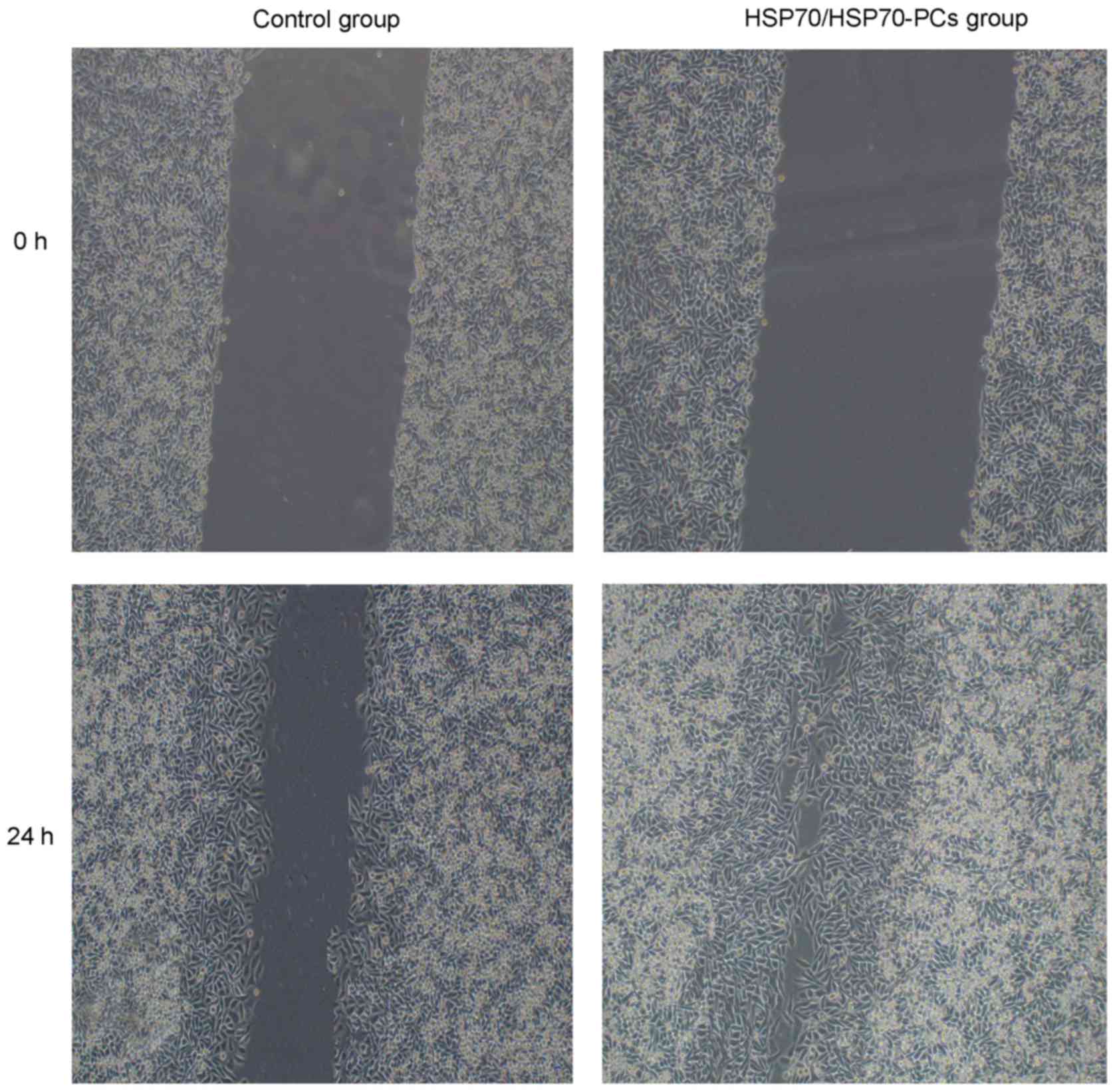
Extracellular HSP70/HSP70-PCs promote
HCC cell migration
To examine the role of extracellular HSP70/HSP70-PCs
in HCC cell migration and invasion, cells were treated with
extracellular HSP70/HSP70-PCs (2 µg/ml). HCC cell motility was then
investigated using a wound healing assay. At 24 h after injury, the
wound in the extracellular HSP70/HSP70-PCs group had healed more
than that in the control group (Fig.
1).
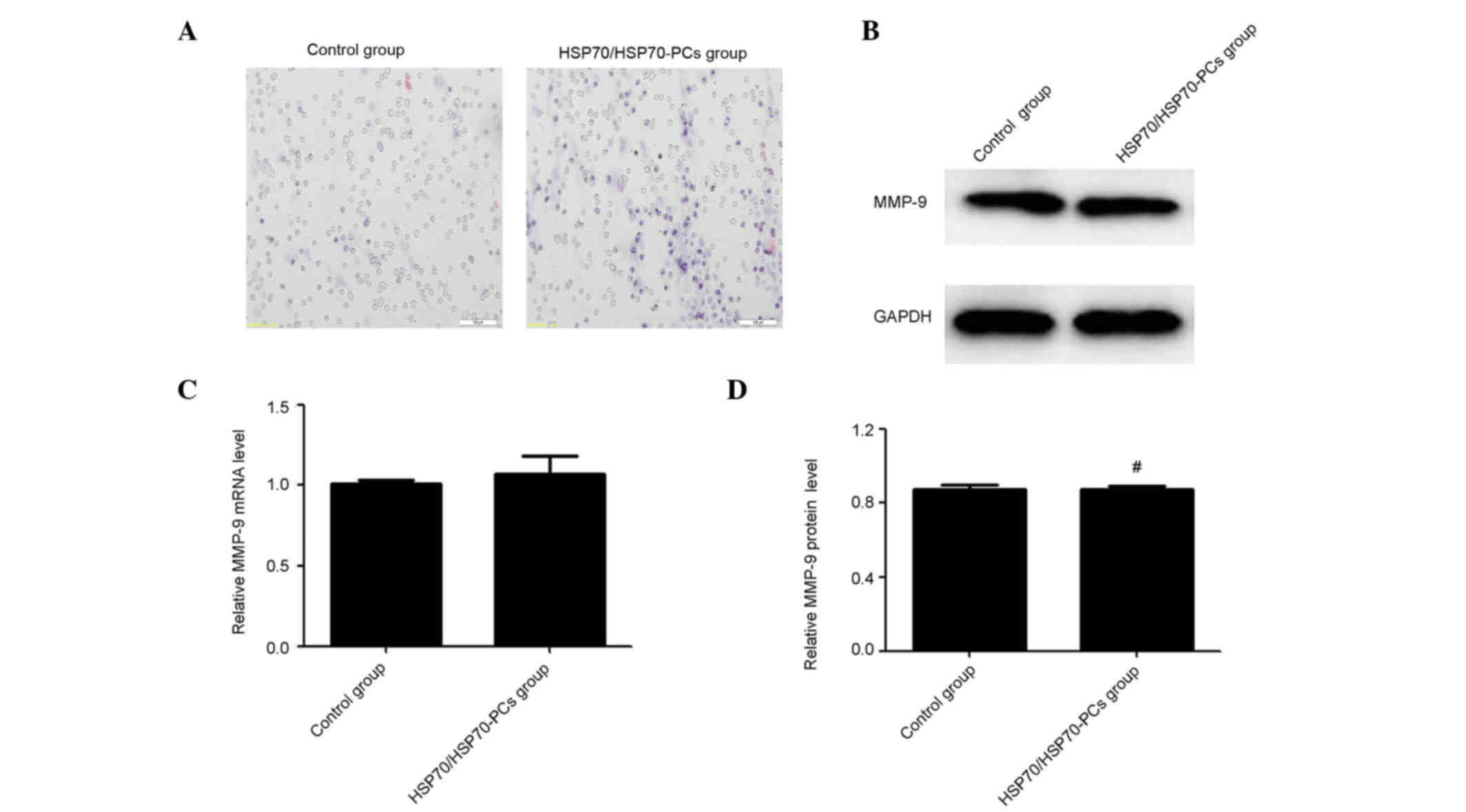
Extracellular HSP70/HSP70-PCs promote
HCC cell invasion
Migration and invasion are widely considered two
closely interrelated processes (13).
As extracellular HSP70/HSP70-PCs promoted HCC cell migration, the
effect of extracellular HSP70/HSP70-PCs on cell invasion was next
tested using Matrigel-precoated Transwell chambers. The number of
invasive cells increased markedly following extracellular
HSP70/HSP70-PCs treatment (Fig.
2A).
Matrix metalloproteinases (MMPs), particularly
MMP-9, are crucial for cell invasion in multiple tumors, including
HCC (14,15). The possibility that extracellular
HSP70/HSP70-PCs affect MMP-9 expression was examined in the present
study. Unexpectedly, RT-qPCR and western blotting demonstrated that
extracellular HSP70/HSP70-PCs altered neither mRNA nor protein
levels of MMP-9 (Fig. 2B-D). Taken
together, these data reveal that regulation of MMP-9 expression
does not mediate the effect of extracellular HSP70/HSP70-PCs on
migration and invasion.
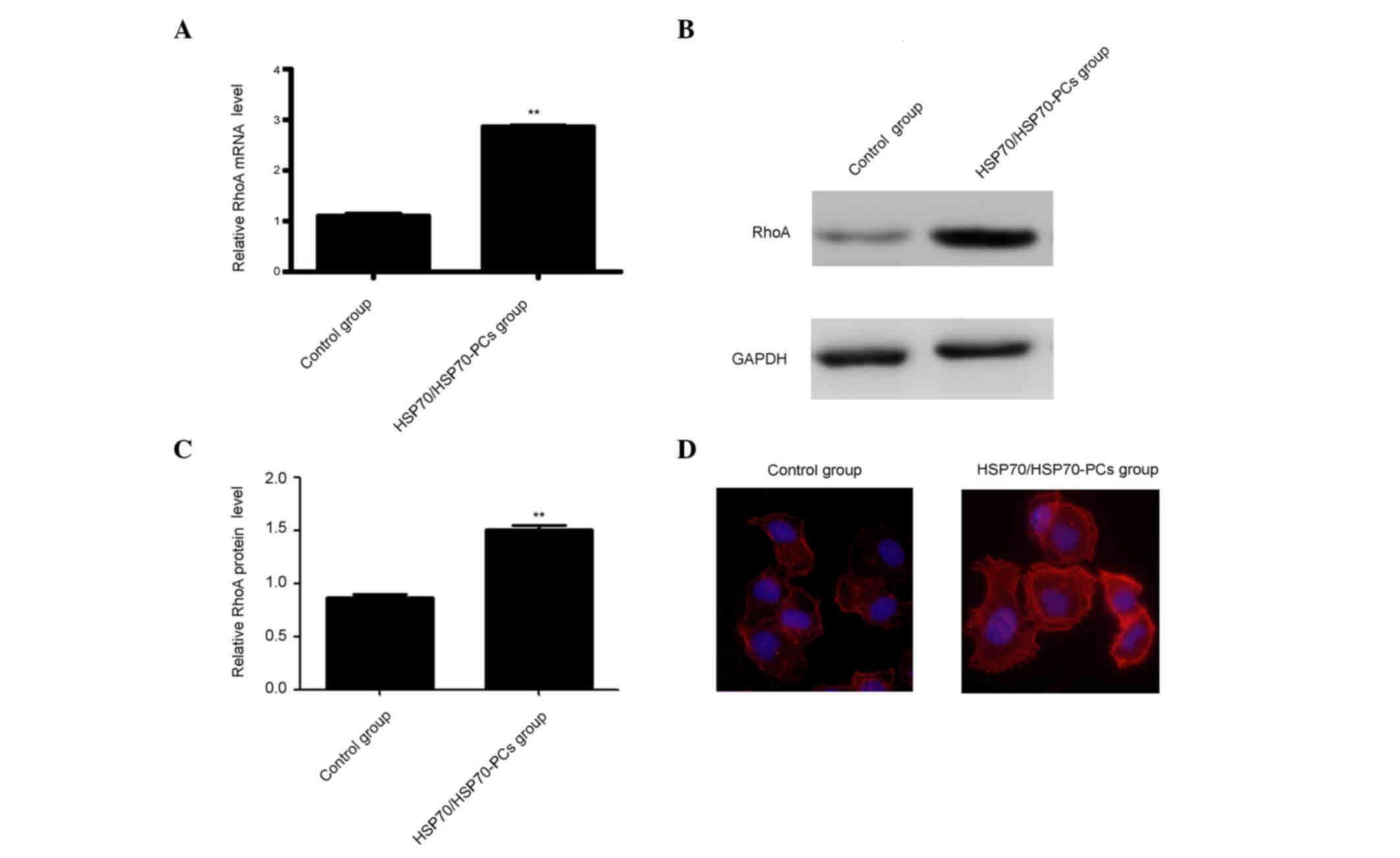
Extracellular HSP70/HSP70-PCs promote
RhoA expression
Previous studies reported that knockdown or
inhibition of the small guanosine triphosphatase RhoA resulted in
the inhibition of cell migration (16). Therefore, it was surmised that
extracellular HSP70/HSP70-PCs may promote RhoA activation or
expression, thereby inducing HCC cell elongation and/or migration.
RhoA expression was measured using western blotting and RT-qPCR.
Both RhoA mRNA and protein levels were significantly increased
following treatment with extracellular HSP70/HSP70-PCs (Fig. 3A-C).
RhoA expression and activity are important for
cytoskeletal regulation in HCC cells, including F-actin stress
fiber and lamellipodia formation (17). Therefore, it was examined whether
extracellular HSP70/HSP70-PCs-mediated regulation affects F-actin
reorganization using FITC-conjugated phalloidin staining. Dot or
fan-like protrusions were detected at the cell periphery in control
cells. Following extracellular HSP70/HSP70-PCs treatment, the
numbers of lamellipodia at the cell margins were markedly increased
(Fig. 3D), and were consistent with
our observation that cellular RhoA expression was increased upon
extracellular HSP70/HSP70-PCs treatment.
RhoA is involved in extracellular
HSP70/HSP70-PCs- induced HCC cell migration
To examine the possibility that RhoA is involved in
the effect of extracellular HSP70/HSP70-PCs on cell migration,
cells were pretreated with RhoA-siRNA, and then stimulated with
extracellular HSP70/HSP70-PCs. RhoA-siRNA attenuated HCC cell
migration (Fig 4). These data
indicate that RhoA is involved in extracellular
HSP70/HSP70-PCs-induced HCC cell migration.
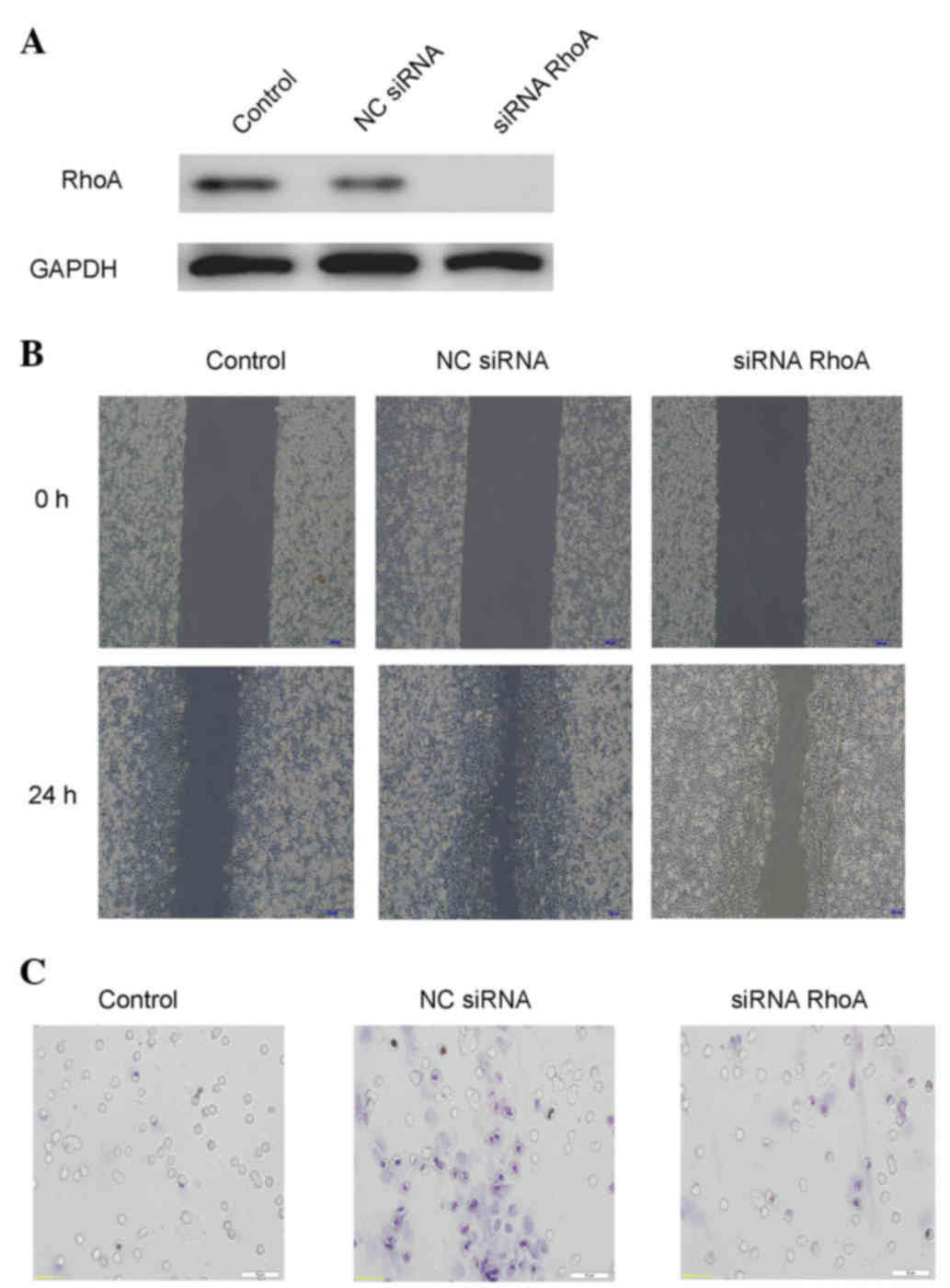 | Figure 4.Extracellular HSP70/HSP70-PCs
mechanism of action through RhoA. (A) Downregulation efficacy of
RhoA-siRNA in Huh-7 cells. Cell lysates from NC-siRNA or
RhoA-siRNA-transfected cells were subjected to western blot
analysis with an anti-RhoA antibody. Glyceraldehyde 3-phosphate
dehydrogenase was used as a loading control. (B) Effect of RhoA
downregulation on Huh-7 cell migration, as examined by a wound
healing assay. Huh-7 cells were transfected with RhoA-siRNA or
NC-siRNA, and then exposed to extracellular HSP70/HSP70-PCs (2
mg/l) for the indicated time periods. Representative digital
pictures were obtained at 0 and 24 h. Magnification, ×10. (C)
Effect of RhoA downregulation on cell migration, as examined by a
Transwell migration assay. Huh-7 cells were transfected with
RhoA-siRNA or NC-siRNA prior to be exposed to extracellular
HSP70/HSP70-PCs (2 mg/l). Magnification, ×20. HSP70, heat shock
protein 70; PCs, peptide complexes; siRNA, small interfering RNA;
NC, negative control; RhoA, Ras homolog family member A; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
HSP70 is generally expressed in living organisms,
and can be released into the extracellular environment in a variety
of ways (18). In the tumor
microenvironment, HSP70 plays a complex and contradictory role at
different stages of tumor progression. Extracellular HSP70 can
inhibit early-stage tumor cell growth by activating the immune
response; however, it promotes late-stage tumor growth by
inhibiting the immune system (6). To
date, no reports in the literature state clearly that extracellular
HSP70/HSP70-PCs affect tumor cell invasion. To this end, the
present study treated the hepatoma cell line Huh-7 with purified
HSP70/HSP70-PCs to observe the effects of extracellular
HSP70/HSP70-PCs on HCC cells. Wound healing and migration assays
revealed that extracellular HSP70/HSP70-PCs enhanced the invasion
and metastasis ability of Huh-7 cells.
The present study attempted to investigate the
mechanism by which extracellular HSP70/HSP70-PCs promote Huh-7 cell
invasion and metastasis. It is common knowledge that MMPs,
particularly MMP-9, are important factors for tumor invasion and
metastasis (19). Thus, the current
study detected MMP-9 mRNA and protein expression with RT-qPCR and
western blotting following extracellular HSP70/HSP70-PCs treatment.
The results revealed that there was no significant change in MMP-9
mRNA or protein expression in extracellular HSP70/HSP70-PCs-treated
cells compared with control cells. This indicates that MMP-9 is not
involved in the HSP70/HSP70-PCs-mediated regulation of Huh-7 cell
invasion and metastasis. Therefore, based on a literature review
(20), the present study focused on
RhoA in an attempt to explain the mechanism behind the
HSP70/HSP70-PCs-mediated regulation of Huh-7 cell invasion and
metastasis. RHOA is an important member of the RHO
gene family, and is a Ras homolog (21). At present, the association between
RhoA and invasion and metastasis of malignant tumors is a popular
research topic (22). RhoA-mediated
regulation of tumor invasion and metastasis occurs through the
activation of cytoskeletal proteins that promote myosin interaction
with F-actin, leading to contractility, which enhances tumor cell
movement and migration ability (23).
To clarify whether RhoA is involved in the extracellular
HSP70/HSP70-PCs-mediated regulation of Huh-7 cells, the effect of
extracellular HSP70/HSP70-PCs on RhoA expression was detected by
RT-qPCR and western blotting. RhoA mRNA and protein expression were
upregulated concomitantly during extracellular
HSP70/HSP70-PCs-mediated promotion of tumor cell invasion and
metastasis. Phalloidin staining revealed a marked increase in the
number of lamellipodia at the cell margins during extracellular
HSP70/HSP70-PCs-mediated promotion of Huh-7 cell invasion and
metastasis. This demonstrates that the role of extracellular
HSP70/HSP70-PCs in the promotion of tumor cell invasion and
metastasis may be associated with RhoA and with the generation of
cytoskeletal proteins. To validate the involvement of RhoA in the
process of extracellular HSP70/HSP70-PCs-mediated promotion of
Huh-7 cell invasion and metastasis, RhoA expression was blocked
with siRNA, and it was observed that the extracellular
HSP70/HSP70-PCs-mediated promotion of Huh-7 cell invasion and
metastasis was significantly decreased. Phalloidin staining
revealed that, once RhoA expression had been blocked, there was no
significant change in the Huh-7 cell cytoskeleton following
extracellular HSP70/HSP70-PCs treatment, compared with the control.
This suggests that increased Huh-7 cell invasion and metastasis
following extracellular HSP70/HSP70-PCs treatment occurs via
upregulated RhoA expression and promotion of cytoskeleton
formation.
The present results suggest that extracellular
HSP70/HSP70-PCs can promote Huh-7 cell invasion and metastasis,
which is achieved by upregulating RhoA expression and promoting
cytoskeleton formation. The current experiments provide a novel
theoretical and experimental basis for preventing and treating
liver cancer in the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81071955) and the Scientific Research from the Education Department
of Liaoning Province (Shenyang, China; grant no. L2010634).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawada K, Hasegawa S, Murakami T, Itatani
Y, Hosogi H, Sonoshita M, Kitamura T, Fujishita T, Iwamoto M,
Matsumoto T, et al: Molecular mechanisms of liver metastasis. Int J
Clin Oncol. 16:464–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH,
Sun J, Yi Y, Shi JY, Shi GM, Ding ZB, et al: CXCR6 upregulation
contributes to a proinflammatory tumor microenvironment that drives
metastasis and poor patient outcomes in hepatocellular carcinoma.
Cancer Res. 72:3546–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mamelak D, Mylvaganam M, Whetstone H,
Hartmann E, Lennarz W, Wyrick PB, Raulston J, Han H, Hoffman P and
Lingwood CA: Hsp70s contain a specific sulfogalactolipid binding
site. Differential aglycone influence on sulfogalactosyl ceramide
binding by recombinant prokaryotic and eukaryotic hsp70 family
members. Biochemistry. 40:3572–3582. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schildkopf P, Frey B, Ott OJ, Rubner Y,
Multhoff G, Sauer R, Fietkau R and Gaipl US: Radiation combined
with hyperthermia induces HSP70-dependent maturation of dendritic
cells and release of pro-inflammatory cytokines by dendritic cells
and macrophages. Radiother Oncol. 101:109–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu FH, Yuan Y, Li D, Liao SJ, Yan B, Wei
JJ, Zhou YH, Zhu JH, Zhang GM and Feng ZH: Extracellular HSPA1A
promotes the growth of hepatocarcinoma by augmenting tumor cell
proliferation and apoptosis-resistance. Cancer Lett. 317:157–164.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitt E, Gehrmann M, Brunet M, Multhoff
G and Garrido C: Intracellular and extracellular functions of heat
shock proteins: Repercussions in cancer therapy. J Leukoc Biol.
81:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borges TJ, Lopes RL, Pinho NG, Machado FD,
Souza AP and Bonorino C: Extracellular Hsp70 inhibits
pro-inflammatory cytokine production by IL-10 driven
down-regulation of C/EBPβ and C/EBPδ. Int J Hyperthermia.
29:455–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blachere NE, Li Z, Chandawarkar RY, Suto
R, Jaikaria NS, Basu S, Udono H and Srivastava PK: Heat shock
protein-peptide complexes, reconstituted in vitro, elicit
peptide-specific cytotoxic T lymphocyte response and tumor
immunity. J Exp Med. 186:1315–1322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Li Y, Liu D, Sun H, Su D, Yang F and
Liu J: Extracellular HSP70/HSP70-PCs promote epithelial-mesenchymal
transition of hepatocarcinoma cells. PLoS One. 8:e847592013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak and Schmittgen, . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo D, Sun W, Zhu L, Zhang H, Hou X, Liang
J, Jiang X and Liu C: Knockdown of BDNF suppressed invasion of
HepG2 and HCCLM3 cells, a mechanism associated with inactivation of
RhoA or Rac1 and actin skeleton disorganization. APMIS.
120:469–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Liu W, Wei D, Hu K, Wu X and Yao
Y: Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor
proliferation, migration and invasion of ovarian cancer cell lines.
Oncol Lett. 7:1581–1585. 2014.PubMed/NCBI
|
|
14
|
Yue P, Gao ZH, Xue X, Cui SX, Zhao CR,
Yuan Y, Yin Z, Inagaki Y, Kokudo N, Tang W and Qu XJ:
Des-γ-carboxyl prothrombin induces matrix metalloproteinase
activity in hepatocellular carcinoma cells by involving the ERK1/2
MAPK signalling pathway. Eur J Cancer. 47:1115–1124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghasemi R, Ghaffari SH, Momeny M,
Pirouzpanah S, Yousefi M, Malehmir M, Alimoghaddam K and
Ghavamzadeh A: Multitargeting and antimetastatic potentials of
silibinin in human HepG-2 and PLC/PRF/5 hepatoma cells. Nutr
Cancer. 65:590–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin Y, Rao J, Zha XL and Xu H:
Angiopoietin-like 3 induces podocyte F-actin rearrangement through
integrin α(V)β3/FAK/PI3K pathway-mediated rac1
activation. Biomed Res Int. 2013:1356082013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Dréan G, Haure-Mirande V, Ferrier L,
Bonnet C, Hulin P, de Coppet P and Segain JP: Visceral adipose
tissue and leptin increase colonic epithelial tight junction
permeability via a RhoA-ROCK-dependent pathway. FASEB J.
28:1059–1070. 2014.[Epub ahead of print]. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mambula SS and Calderwood SK: Heat Shock
Protein 70 is secreted from tumor cells by a nonclassical pathway
involving lysosomal endosomes. J Immunol. 177:7849–7857. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Kempen LC and Coussens LM: MMP9
potentiates pulmonary metastasis formation. Cancer Cell. 2:251–252.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grise F, Bidaud A and Moreau V: Rho
GTPases in hepatocellular carcinoma. Biochim Biophys Acta.
1795:137–151. 2009.PubMed/NCBI
|
|
21
|
Strutt DI, Weber U and Mlodzik M: The role
of RhoA in tissue polarity and Frizzled signalling. Nature.
387:292–295. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cachero TG, Morielli AD and Peralta EG:
The small GTP-binding protein RhoA regulates a delayed rectifier
potassium channel. Cell. 93:1077–1085. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valtcheva N, Primorac A, Jurisic G,
Hollmén M and Detmar M: The orphan adhesion G protein coupled
receptor GPR97 regulates migration of lymphatic endothelial cells
via the small GTPases RhoA and Cdc42. J Biol C. 288:35736–35748.
2013. View Article : Google Scholar
|