|
1
|
Boveri T: On multipolar mitosis as a means
of analysis of the cell nucleus. Neu Folge. 35:67–90. 1902.
|
|
2
|
Stehelin D, Varmus HE, Bishop JM and Vogt
PK: DNA related to the transforming gene(s) of avian sarcoma
viruses is present in normal avian DNA. Nature. 260:170–173. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tabin CJ, Bradley SM, Bargmann CI,
Weinberg RA, Papageorge AG, Scolnick EM, Dhar R, Lowy DR and Chang
EH: Mechanism of activation of a human oncogene. Nature.
300:143–149. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dulbecco R: A turning point in cancer
research: Sequencing the human genome. Science. 231:1055–1056.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright CF, Fitzgerald TW, Jones WD,
Clayton S, McRae JF, van Kogelenberg M, King DA, Ambridge K,
Barrett DM, Bayzetinova T, et al: Genetic diagnosis of
developmental disorders in the DDD study: A scalable analysis of
genome-wide research data. Lancet. 385:1305–1314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akbani R, Ng PK, Werner HM, Shahmoradgoli
M, Zhang F, Ju Z, Liu W, Yang JY, Yoshihara K, Li J, et al: A
pan-cancer proteomic perspective on The Cancer Genome Atlas. Nat
Commun. 5:38872014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
The future of cancer genomics. Nat Med.
21:992015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garraway LA and Lander ES: Lessons from
the cancer genome. Cell. 153:17–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wheeler DA and Wang L: From human genome
to cancer genome: The first decade. Genome Res. 23:1054–1062. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Genome Atlas Research Network;
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong J, Cooper LA, Wang F, Gutman DA, Gao
J, Chisolm C, Sharma A, Pan T, Van Meir EG, Kurc TM, et al:
Integrative, multimodal analysis of glioblastoma using TCGA
molecular data, pathology images and clinical outcomes. IEEE Trans
Biomed Eng. 58:3469–3474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
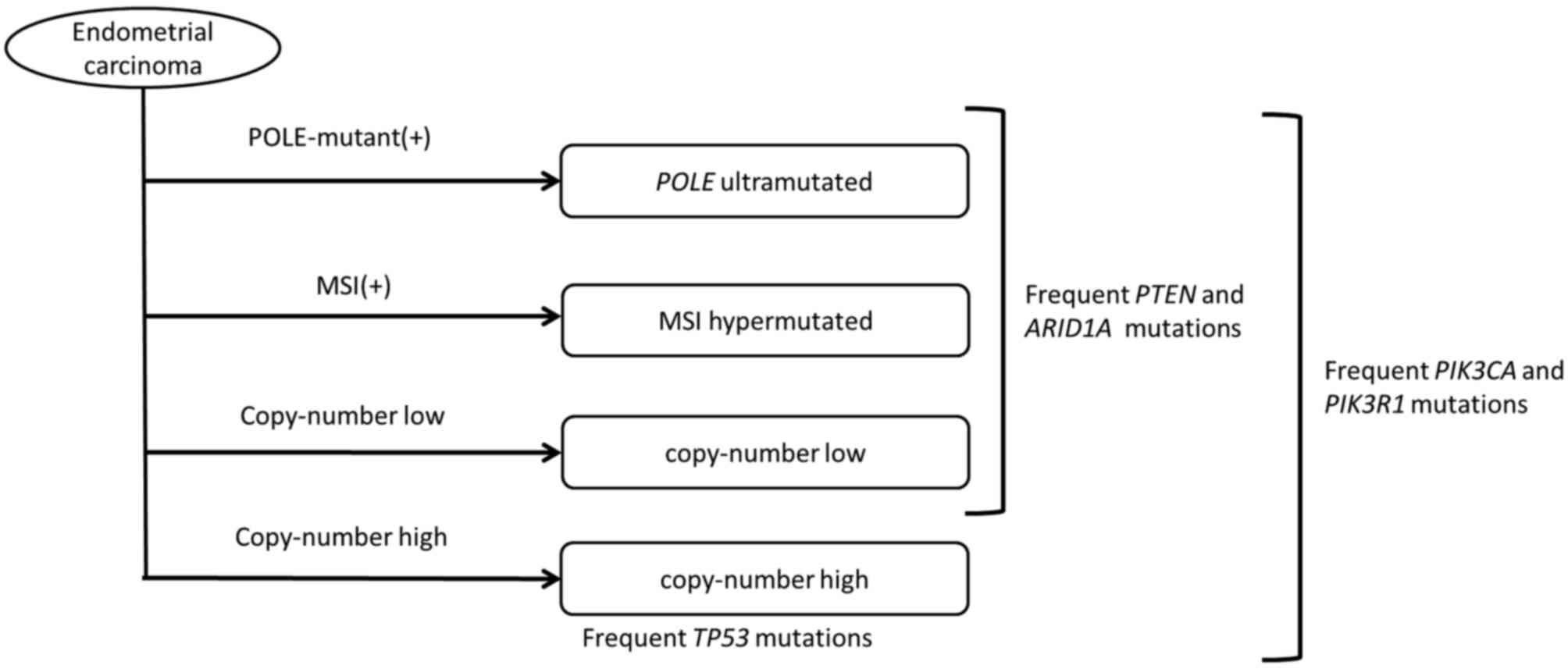
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynaecol Obstet. 95:(Suppl
1). S161–S192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A,
Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ and Shih Ie M:
Frequent activating mutations of PIK3CA in ovarian clear cell
carcinoma. Am J Pathol. 174:1597–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan DS, Iravani M, McCluggage WG, Lambros
MB, Milanezi F, Mackay A, Gourley C, Geyer FC, Vatcheva R, Millar
J, et al: Genomic analysis reveals the molecular heterogeneity of
ovarian clear cell carcinomas. Clin Cancer Res. 17:1521–1534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones S, Wang TL, Shih Ie M, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohrmann L and Verrijzer CP: Composition
and functional specificity of SWI2/SNF2 class chromatin remodeling
complexes. Biochim Biophys Acta. 1681:59–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katagiri A, Nakayama K, Rahman MT, Rahman
M, Katagiri H, Nakayama N, Ishikawa M, Ishibashi T, Iida K,
Kobayashi H, et al: Loss of ARID1A expression is related to shorter
progression-free survival and chemoresistance in ovarian clear cell
carcinoma. Mod Pathol. 25:282–288. 2012.PubMed/NCBI
|
|
28
|
Yamaguchi K, Mandai M, Oura T, Matsumura
N, Hamanishi J, Baba T, Matsui S, Murphy SK and Konishi I:
Identification of an ovarian clear cell carcinoma gene signature
that reflects inherent disease biology and the carcinogenic
processes. Oncogene. 29:1741–1752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuchiya A, Sakamoto M, Yasuda J, Chuma M,
Ohta T, Ohki M, Yasugi T, Taketani Y and Hirohashi S: Expression
profiling in ovarian clear cell carcinoma: Identification of
hepatocyte nuclear factor-1 beta as a molecular marker and a
possible molecular target for therapy of ovarian clear cell
carcinoma. Am J Pathol. 163:2503–2512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaguchi K, Huang Z, Matsumura N, Mandai
M, Okamoto T, Baba T, Konishi I, Berchuck A and Murphy SK:
Epigenetic determinants of ovarian clear cell carcinoma biology.
Int J Cancer. 135:585–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi K, Mandai M, Toyokuni S,
Hamanishi J, Higuchi T, Takakura K and Fujii S: Contents of
endometriotic cysts, especially the high concentration of free
iron, are a possible cause of carcinogenesis in the cysts through
the iron-induced persistent oxidative stress. Clin Cancer Res.
14:32–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barger CJ, Zhang W, Hillman J, Stablewski
AB, Higgins MJ, Vanderhyden BC, Odunsi K and Karpf AR: Genetic
determinants of FOXM1 overexpression in epithelial ovarian cancer
and functional contribution to cell cycle progression. Oncotarget.
6:27613–27627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barsotti AM and Prives C:
Pro-proliferative FOXM1 is a target of p53-mediated repression.
Oncogene. 28:4295–4305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma RY, Tong TH, Cheung AM, Tsang AC, Leung
WY and Yao KM: Raf/MEK/MAPK signaling stimulates the nuclear
translocation and transactivating activity of FOXM1c. J Cell Sci.
118:795–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan Y, Raychaudhuri P and Costa RH: Chk2
mediates stabilization of the FOXM1 transcription factor to
stimulate expression of DNA repair genes. Mol Cell Biol.
27:1007–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brachova P, Mueting SR, Carlson MJ,
Goodheart MJ, Button AM, Mott SL, Dai D, Thiel KW, Devor EJ and
Leslie KK: TP53 oncomorphic mutations predict resistance to
platinum- and taxane-based standard chemotherapy in patients
diagnosed with advanced serous ovarian carcinoma. Int J Oncol.
46:607–618. 2015.PubMed/NCBI
|
|
38
|
Brachova P, Mueting SR, Devor EJ and
Leslie KK: Oncomorphic TP53 mutations in gynecologic cancers lose
the normal protein: Protein interactions with the microRNA
microprocessing complex. J Cancer Ther. 5:506–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu G, Yang D, Sun Y, Shmulevich I, Xue F,
Sood AK and Zhang W: Differing clinical impact of BRCA1 and BRCA2
mutations in serous ovarian cancer. Pharmacogenomics. 13:1523–1535.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Scully R, Puget N and Vlasakova K: DNA
polymerase stalling, sister chromatid recombination and the BRCA
genes. Oncogene. 19:6176–6183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kurman RJ and Shih Ie M: Molecular
pathogenesis and extraovarian origin of epithelial ovarian cancer:
Shifting the paradigm. Hum Pathol. 42:918–931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shah RH, Scott SN, Brannon AR, Levine DA,
Lin O and Berger MF: Comprehensive mutation profiling by
next-generation sequencing of effusion fluids from patients with
high-grade serous ovarian carcinoma. Cancer Cytopathol.
123:289–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kurman RJ and Shih Ie M: Molecular
pathogenesis and extraovarian origin of epithelial ovarian
cancer-shifting the paradigm. Hum Pathol. 42:918–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wiedemeyer WR, Beach JA and Karlan BY:
Reversing platinum resistance in high-grade serous ovarian
carcinoma: Targeting BRCA and the homologous recombination system.
Front Oncol. 4:342014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rao SS, O'Neil J, Liberator CD, Hardwick
JS, Dai X, Zhang T, Tyminski E, Yuan J, Kohl NE, Richon VM, et al:
Inhibition of NOTCH signaling by gamma secretase inhibitor engages
the RB pathway and elicits cell cycle exit in T-cell acute
lymphoblastic leukemia cells. Cancer Res. 69:3060–3068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jones S, Wang TL, Kurman RJ, Nakayama K,
Velculescu VE, Vogelstein B, Kinzler KW, Papadopoulos N and Shih Ie
M: Low-grade serous carcinomas of the ovary contain very few point
mutations. J Pathol. 226:413–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tothill RW, Tinker AV, George J, Brown R,
Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro
B, et al: Novel molecular subtypes of serous and endometrioid
ovarian cancer linked to clinical outcome. Clin Cancer Res.
14:5198–5208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rajagopalan H, Bardelli A, Lengauer C,
Kinzler KW, Vogelstein B and Velculescu VE: Tumorigenesis: RAF/RAS
oncogenes and mismatch-repair status. Nature. 418:9342002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Singer G, Oldt R III, Cohen Y, Wang BG,
Sidransky D, Kurman RJ and Shih Ie M: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
J Natl Cancer Inst. 95:484–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kannan K, Coarfa C, Chao PW, Luo L, Wang
Y, Brinegar AE, Hawkins SM, Milosavljevic A, Matzuk MM and Yen L:
Recurrent BCAM-AKT2 fusion gene leads to a constitutively activated
AKT2 fusion kinase in high-grade serous ovarian carcinoma. Proc
Natl Acad Sci USA. 112:E1272–E1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Buchanan EM, Weinstein LC and Hillson C:
Endometrial cancer. Am Fam Physician. 80:1075–1080. 2009.PubMed/NCBI
|
|
53
|
Setiawan VW, Yang HP, Pike MC, McCann SE,
Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, et al:
Type I and II endometrial cancers: Have they different risk
factors? J Clin Oncol. 31:2607–2618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsumura N, Huang Z, Mori S, Baba T,
Fujii S, Konishi I, Iversen ES, Berchuck A and Murphy SK:
Epigenetic suppression of the TGF-beta pathway revealed by
transcriptome profiling in ovarian cancer. Genome Res. 21:74–82.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Talhouk A, McConechy MK, Leung S, Li-Chang
HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, et al:
A clinically applicable molecular-based classification for
endometrial cancers. Br J Cancer. 113:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Le Gallo M and Bell DW: The emerging
genomic landscape of endometrial cancer. Clin Chem. 60:98–110.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garcia-Dios DA, Lambrechts D, Coenegrachts
L, Vandenput I, Capoen A, Webb PM, Ferguson K, Akslen LA, Claes B,
Vergote I, et al: Australian National Endometrial Cancer Study
Group: High-throughput interrogation of PIK3CA, PTEN, KRAS, FBXW7
and TP53 mutations in primary endometrial carcinoma. Gynecol Oncol.
128:327–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hoang LN, McConechy MK, Köbel M, Han G,
Rouzbahman M, Davidson B, Irving J, Ali RH, Leung S, McAlpine JN,
et al: Histotype-genotype correlation in 36 high-grade endometrial
carcinomas. Am J Surg Pathol. 37:1421–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Athanassiadou P, Athanassiades P, Grapsa
D, Gonidi M, Athanassiadou AM, Stamati PN and Patsouris E: The
prognostic value of PTEN, p53, and beta-catenin in endometrial
carcinoma: A prospective immunocytochemical study. Int J Gynecol
Cancer. 17:697–704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kuhn E, Wu RC, Guan B, Wu G, Zhang J, Wang
Y, Song L, Yuan X, Wei L, Roden RB, et al: Identification of
molecular pathway aberrations in uterine serous carcinoma by
genome-wide analyses. J Natl Cancer Inst. 104:1503–1513. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao S, Choi M, Overton JD, Bellone S,
Roque DM, Cocco E, Guzzo F, English DP, Varughese J, Gasparrini S,
et al: Landscape of somatic single-nucleotide and copy-number
mutations in uterine serous carcinoma. Proc Natl Acad Sci USA.
110:2916–2921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Le Gallo M, O'Hara AJ, Rudd ML, Urick ME,
Hansen NF, O'Neil NJ, Price JC, Zhang S, England BM, Godwin AK, et
al: Exome sequencing of serous endometrial tumors identifies
recurrent somatic mutations in chromatin-remodeling and ubiquitin
ligase complex genes. Nat Genet. 44:1310–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Giannakis M, Hodis E, Mu X Jasmine,
Yamauchi M, Rosenbluh J, Cibulskis K, Saksena G, Lawrence MS, Qian
ZR, Nishihara R, et al: RNF43 is frequently mutated in colorectal
and endometrial cancers. Nat Genet. 46:1264–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Markowska A, Pawałowska M, Lubin J and
Markowska J: Signalling pathways in endometrial cancer. Contemp
Oncol (Pozn). 18:143–148. 2014.PubMed/NCBI
|
|
65
|
Jo YS, Kim MS, Lee JH, Lee SH, An CH and
Yoo NJ: Frequent frameshift mutations in 2 mononucleotide repeats
of RNF43 gene and its regional heterogeneity in gastric and
colorectal cancers. Hum Pathol. 46:1640–1646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu Y, Patel L, Mills GB, Lu KH, Sood AK,
Ding L, Kucherlapati R, Mardis ER, Levine DA, Shmulevich I, et al:
Clinical significance of CTNNB1 mutation and Wnt pathway activation
in endometrioid endometrial carcinoma. J Natl Cancer Inst.
106:dju2452014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Carvajal-Carmona LG, O'Mara TA, Painter
JN, Lose FA, Dennis J, Michailidou K, Tyrer JP, Ahmed S, Ferguson
K, Healey CS, et al: Candidate locus analysis of the TERT-CLPTM1L
cancer risk region on chromosome 5p15 identifies multiple
independent variants associated with endometrial cancer risk. Hum
Genet. 134:231–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fredriksson NJ, Ny L, Nilsson JA and
Larsson E: Systematic analysis of noncoding somatic mutations and
gene expression alterations across 14 tumor types. Nat Genet.
46:1258–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Oshita T, Nagai N and Ohama K: Expression
of telomerase reverse transcriptase mRNA and its quantitative
analysis in human endometrial cancer. Int J Oncol. 17:1225–1230.
2000.PubMed/NCBI
|
|
70
|
Merritt MA and Cramer DW: Molecular
pathogenesis of endometrial and ovarian cancer. Cancer Biomark.
9:287–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Erickson BK, Kinde I, Dobbin ZC, Wang Y,
Martin JY, Alvarez RD, Conner MG, Huh WK, Roden RB, Kinzler KW, et
al: Detection of somatic TP53 mutations in tampons of patients with
high-grade serous ovarian cancer. Obstet Gynecol. 124:881–885.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
McConechy MK, Ding J, Cheang MC, Wiegand
KC, Senz J, Tone AA, Yang W, Prentice LM, Tse K, Zeng T, et al: Use
of mutation profiles to refine the classification of endometrial
carcinomas. J Pathol. 228:20–30. 2012.PubMed/NCBI
|
|
73
|
Parkinson DR, Johnson BE and Sledge GW:
Making personalized cancer medicine a reality: Challenges and
opportunities in the development of biomarkers and companion
diagnostics. Clin Cancer Res. 18:619–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shih Ie M and Kurman RJ: Ovarian
tumorigenesis: A proposed model based on morphological and
molecular genetic analysis. Am J Pathol. 164:1511–1518. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Piek JM, van Diest PJ, Zweemer RP, et al:
Dysplastic changes in prophylactically removed Fallopian tubes of
women predisposed to developing ovarian cancer. J Pathol.
195:451–456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Curtin N: PARP inhibitors for anticancer
therapy. Biochem Soc Trans. 42:82–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cass I, Baldwin RL, Varkey T, Moslehi R,
Narod SA and Karlan BY: Improved survival in women with
BRCA-associated ovarian carcinoma. Cancer. 97:2187–2195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ashworth A: A synthetic lethal therapeutic
approach: Poly (ADP) ribose polymerase inhibitors for the treatment
of cancers deficient in DNA double-strand break repair. J Clin
Oncol. 126:3785–3790. 2008. View Article : Google Scholar
|
|
79
|
McCabe N, Turner NC, Lord CJ, Kluzek K,
Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka
MZ, et al: Deficiency in the repair of DNA damage by homologous
recombination and sensitivity to poly(ADP-ribose) polymerase
inhibition. Cancer Res. 66:8109–8115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gelmon KA, Tischkowitz M, Mackay H,
Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M,
Gilks B, et al: Olaparib in patients with recurrent high-grade
serous or poorly differentiated ovarian carcinoma or
triple-negative breast cancer: A phase 2, multicentre, open-label,
non-randomised study. Lancet Oncol. 12:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oza AM, Cibula D, Benzaquen AO, Poole C,
Mathijssen RH, Sonke GS, Colombo N, Špaček J, Vuylsteke P, Hirte H,
et al: Olaparib combined with chemotherapy for recurrent
platinum-sensitive ovarian cancer: A randomised phase 2 trial.
Lancet Oncol. 16:87–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lee JM, Hays JL, Annunziata CM, Noonan AM,
Minasian L, Zujewski JA, Yu M, Gordon N, Ji J, Sissung TM, et al:
Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2
mutation-associated breast or ovarian cancer with biomarker
analyses. J Natl Cancer Inst. 106:dju0892014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fingar DC, Richardson CJ, Tee AR, Cheatham
L, Tsou C and Blenis J: mTOR controls cell cycle progression
through its cell growth effectors S6K1 and 4E-BP1/eukaryotic
translation initiation factor 4E. Mol Cell Biol. 24:200–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Phase 3 trial of everolimus for metastatic
renal cell carcinoma: Final results and analysis of prognostic
factors. Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Husseinzadeh N and Husseinzadeh HD: mTOR
inhibitors and their clinical application in cervical, endometrial
and ovarian cancers: A critical review. Gynecol Oncol. 133:375–381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hirasawa T, Miyazawa M, Yasuda M, Shida M,
Ikeda M, Kajiwara H, Matsui N, Fujita M, Muramatsu T and Mikami M:
Alterations of hypoxia-induced factor signaling pathway due to
mammalian target of rapamycin (mTOR) suppression in ovarian clear
cell adenocarcinoma: In vivo and in vitro explorations for clinical
trial. Int J Gynecol Cancer. 23:1210–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Köbel M, Huntsman D and Gilks CB: Critical
molecular abnormalities in high-grade serous carcinoma of the
ovary. Expert Rev Mol Med. 10:e222008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zoratto F, Rossi L, Giordani E, Strudel M,
Papa A and Tomao S: From conventional chemotherapy to targeted
therapy: Use of monoclonal antibodies (moAbs) in gastrointestinal
(GI) tumors. Tumour Biol. 35:8471–8482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kashiyama T, Oda K, Ikeda Y, Shiose Y,
Hirota Y, Inaba K, Makii C, Kurikawa R, Miyasaka A, Koso T, et al:
Antitumor activity and induction of TP53-dependent apoptosis toward
ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor
DS-7423. PLoS One. 9:e872202014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shoji K, Oda K, Kashiyama T, Ikeda Y,
Nakagawa S, Sone K, Miyamoto Y, Hiraike H, Tanikawa M, Miyasaka A,
et al: Genotype-dependent efficacy of a dual PI3K/mTOR inhibitor,
NVP-BEZ235 and an mTOR inhibitor, RAD001, in endometrial
carcinomas. PLoS One. 7:e374312012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gershenson DM, Sun CC, Bodurka D, Coleman
RL, Lu KH, Sood AK, Deavers M, Malpica AL and Kavanagh JJ:
Recurrent low-grade serous ovarian carcinoma is relatively
chemoresistant. Gynecol Oncol. 114:48–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gershenson DM, Sun CC, Lu KH, Coleman RL,
Sood AK, Malpica A, Deavers MT, Silva EG and Bodurka DC: Clinical
behavior of stage II–IV low-grade serous carcinoma of the ovary.
Obstet Gynecol. 108:361–368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Miller CR, Oliver KE and Farley JH: MEK1/2
inhibitors in the treatment of gynecologic malignancies. Gynecol
Oncol. 133:128–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Farley J, Brady WE, Vathipadiekal V,
Lankes HA, Coleman R, Morgan MA, Mannel R, Yamada SD, Mutch D,
Rodgers WH, et al: Selumetinib in women with recurrent low-grade
serous carcinoma of the ovary or peritoneum: An open-label,
single-arm, phase 2 study. Lancet Oncol. 14:134–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kuo KT, Guan B, Feng Y, Mao TL, Chen X,
Jinawath N, Wang Y, Kurman RJ, Shih Ie M and Wang TL: Analysis of
DNA copy number alterations in ovarian serous tumors identifies new
molecular genetic changes inlow-grade and high-grade carcinomas.
Cancer Res. 69:4036–4042. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hamilton MP, Rajapakshe K, Hartig SM, Reva
B, McLellan MD, Kandoth C, Ding L, Zack TI, Gunaratne PH, Wheeler
DA, et al: Identification of a pan-cancer oncogenic microRNA
superfamily anchored by a central core seed motif. Nat Commun.
4:27302013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jacobsen A, Silber J, Harinath G, Huse JT,
Schultz N and Sander C: Analysis of microRNA-target interactions
across diverse cancer types. Nat Struct Mol Biol. 20:1325–1332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zovoilis A, Mungall AJ, Moore R, Varhol R,
Chu A, Wong T, Marra M and Jones SJ: The expression level of small
non-coding RNAs derived from the first exon of protein-coding genes
is predictive of cancer status. EMBO Rep. 15:402–410. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mukherji S, Ebert MS, Zheng GX, Tsang JS,
Sharp PA and van Oudenaarden A: MicroRNAs can generate thresholds
in target gene expression. Nat Genet. 43:854–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Boren T, Xiong Y, Hakam A, Wenham R, Apte
S, Wei Z, Kamath S, Chen DT, Dressman H and Lancaster JM: MicroRNAs
and their target messenger RNAs associated with endometrial
carcinogenesis. Gynecol Oncol. 110:206–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wu W, Lin Z, Zhuang Z and Liang X:
Expression profile of mammalian microRNAs in endometrioid
adenocarcinoma. Eur J Cancer Prev. 18:50–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mauel S, Kruse B, Etschmann B, von der
Schulenburg AG, Schaerig M, Stövesand K, Wilcken B and Sterner-Kock
A: Latent transforming growth factor binding protein 4 (LTBP-4) is
downregulated in human mammary adenocarcinomas in vitro and in
vivo. APMIS. 115:687–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Burns MB, Temiz NA and Harris RS: Evidence
for APOBEC3B mutagenesis in multiple human cancers. Nat Genet.
45:977–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Roberts SA, Lawrence MS, Klimczak LJ,
Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL,
Saksena G, et al: An APOBEC cytidine deaminase mutagenesis pattern
is widespread in human cancers. Nat Genet. 45:970–976. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Genome Atlas Research Network Cancer.
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ren X, McHale CM, Skibola CF, Smith AH,
Smith MT and Zhang L: An emerging role for epigenetic dysregulation
in arsenic toxicity and carcinogenesis. Environ Health Perspect.
119:11–19. 2011. View Article : Google Scholar : PubMed/NCBI
|