Introduction
Burkitt lymphoma (BL) is a type of B cell
non-Hodgkin lymphoma (NHL), which most commonly presents in
children and young adults (1). Three
subtypes of BL have been defined: i) The endemic form occurring
primarily in Africa, which usually affects children and
adolescents, and is associated with Epstein-Barr virus (EBV)
infection; ii) the adult sporadic form; and iii) the
immunodeficiency-associated form, occurring primarily in patients
infected with the human immunodeficiency virus (HIV) (1,2).
BL is a clinically aggressive tumor with bulky
observable masses, B symptoms and frequent involvement of
extranodal sites (2). Bone marrow
infiltration and leptomeningeal central nervous system (CNS)
involvement are also common at the time of diagnosis (3). The association between BL and mature B
cell acute lymphoblastic leukemia (ALL) has previously been
recognized, and these two nomenclatures may represent different
manifestations of a single disease rather than two separate
diseases (3,4).
BL tumor cells are characterized by the expression
of B cell-specific surface markers, including immunoglobulin (Ig)
M, cluster of differentiation (CD) 19 and CD20, and by Burkitt-like
morphology according to the French-American-British classification
(4). The chromosomal translocation
t(8;14), which results in the juxtaposition of the IgH region on
chromosome 14 with the MYC gene from chromosome 8, has been
identified in nearly all cases of BL (5,6). BL is
prone to invade the CNS during the early stages of the disease, and
this invasion is usually accompanied by abnormalities in
cerebrospinal fluid (CSF) examinations and/or neuroradiography
(7,8).
The present study describes the symptoms and
treatment regime of an adult patient with BL and CNS invasion, who
presented with a sudden onset of right oculomotor nerve palsy
without abnormal neuroradiography, CSF examinations and initial
peripheral blood.
Case report
A 29-year-old man was admitted to the Department of
Hematology, The Second Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, China) on 6 November, 2013 with a
1-week history of a drooping right eyelid and impaired
eye-movement. The patient also suffered from double vision without
apparent etiology, but did not experience symptoms of fever,
chills, headache, numbness, vomiting, glare, halos, starbursts,
sweating, weight loss or trauma, and had no history of smoking,
alcohol or drug abuse. No significant abnormalities were reported
in the family medical history.
Physical examination revealed that the pupils were
round and symmetrical, with a diameter of 3.0 mm, and the patient
had normal vision, visual field and fundus, in addition to direct
and indirect light reflex. The right eye exhibited impaired
downside and abduction movements; however, normal closure action
and other neural functions, including facial sensation, hearing,
pronunciation and swallowing, were normal. The patient was able to
walk and move freely without numbness of limbs. No enlargement of
lymph nodes, liver or spleen was observed.
Laboratory tests revealed a normal blood count on
day 1, which was as follows: White blood cell (WBC) count,
5.8×109/l (normal range, 4.0–10.0×109/l);
hemoglobin (Hb) count, 133 g/l (normal range, 110–160 g/l);
platelet count, 150×109/l (normal range,
100–300×109/l); neutrophilic granulocyte percentage,
62.1% (normal range, 50.0–70.0%); lymphocyte percentage, 27.4%
(normal range, 20.0–40.0%); and monocyte percentage, 9.3% (normal
range, 4.0–12.0%). Serum lactate dehydrogenase (LDH) and
erythrocyte sedimentation rate were 907 U/l (normal range, 100–248
U/l) and 90 mm/h (normal range, 0–15 mm/h), respectively. Serum
albumin, Ig, acute phase reactive protein, renal and liver function
tests were all within normal limits. Viral serological
examinations, including hepatitis A, B and C virus, HIV, EBV and
cytomegalovirus were all negative. B-mode ultrasonography exhibited
no marked abnormalities in the liver, gallbladder, pancreas or
spleen, and no significant enlargement of lymph nodes was detected.
Magnetic resonance angiography of the brain with intravenous
gadolinium was unremarkable, and an enhanced computed tomography
(CT) scan indicated that orbital and optic nerve were normal.
Furthermore, cerebrovascular diseases, including thrombosis,
infraction and hemorrhagic apoplexy, were excluded by cerebral
angiography. Though there were no signs of meningeal irritation, a
lumbar puncture was performed and CSF examination was normal: WBC
count, 0/µl (normal range, 0–5/µl); red blood cell count, 0/µl
(normal range, 0–5/µl); glucose level, 3.7 mmol/l (normal range,
2.8–4.5 mmol/l); and protein level, 23.30 mg/dl (normal range,
15–40 mg/dl). Neither abnormal cells nor microorganisms were
identified in the centrifugal extraction of CSF.
The patient was initially diagnosed with abducens
diplopia which was considered to have developed from a non-specific
type of single cranial neuritis. The patient was subsequently
provided with methylprednisolone (5 mg, oral tid) and vitamin B12
(0.5 mg, intramuscular injection qd) to take for ~1 week. However,
the condition of the patient did not improve, and arthralgia and
bone pain developed in the sacrum and pelvis. A magnetic resonance
imaging (MRI) exam was performed, which revealed diffuse abnormal
signals in bones, although no marked bone destruction was
identified on X-ray or CT images. Re-examination of peripheral
blood 8 days later revealed slight decrease in Hb level to 122 g/l
(normal range, 110–160 g/l), while WBC and platelet counts remained
within normal limits. However, LDH levels had escalated rapidly to
2,452 U/l (normal range, 100–248 U/l). At day 13, symptoms of
anemia became more severe; however, WBC and platelet counts
remained normal (Table I). A
peripheral blood smear was subsequently performed revealing
atypical lymphocytes and immature cells. Bone marrow examination
identified extreme hyperplasia with excess blast cells >59%
(normal range, <5%) and inhibited normal hematopoiesis.
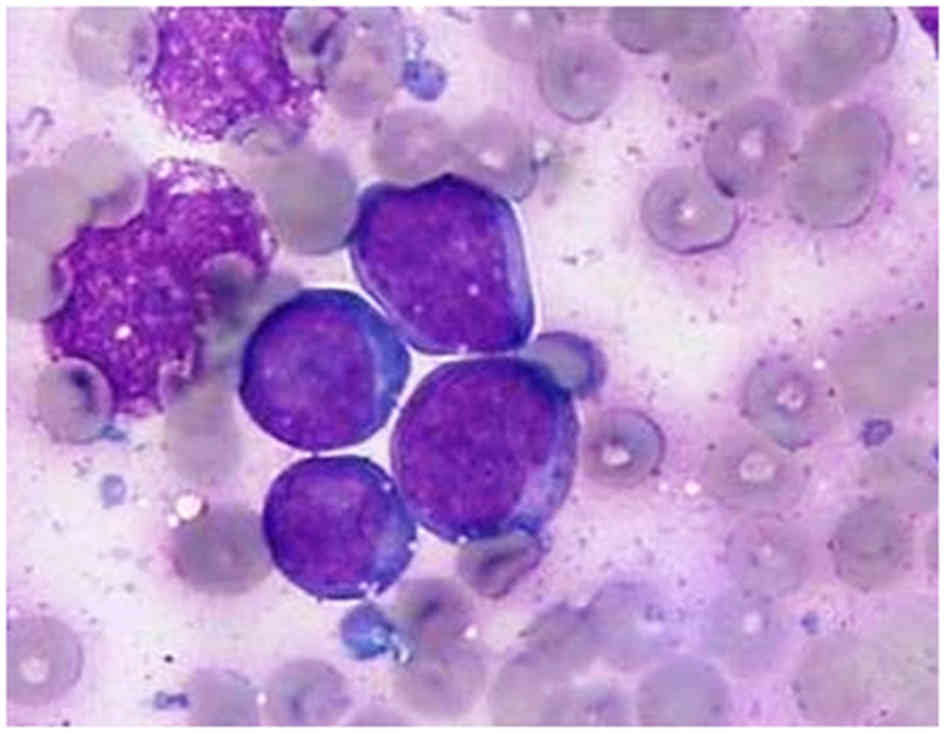
Microscopically, tumor cells were characterized by a diffuse
monotonous pattern of medium-sized, round nuclei with clumped
chromatin, deep basophilic cytoplasm and easily identified
cytoplasmic vacuoles, all hallmarks of Burkitt-like cell morphology
(Fig. 1). Immunophenotype analysis
demonstrated positive expression of membrane antigens, including
CD20, CD22, CD19, CD38, CD10, CD22, CD79a and human leukocyte
antigen-D related, which supported the diagnosis of mature B
lineage ALL with a Ki-67 index >90%. There was no significant
expression of the following markers: CD3, CD5, CD23, CD34, terminal
deoxynucleotidyl transferase (TdT), B cell lymphoma-2 (Bcl-2),
melanoma associated antigen-1 (MUM-1) or myeloperoxidase. The
expression of all available leukemia fusion genes was negative and
included breakpoint cluster region/ABL, myeloid/lymphoid or
mixed-lineage leukemia (MLL)-AFX, MLL-AF1p; MLL/AF4, dupMLL;
MLL/ENL, E2A/pre B-cell leukemia homeobox 1, SIL/T-cell acute
lymphocytic leukemia 1, homeobox 11, TEL/acute myeloid leukemia 1
and TEL/Abelson murine leukemia viral oncogene homolog 1.
Cytogenetic studies of 16 metaphases identified 46, XY and 4
chromosome abnormalities as 45, X, -Y, t(8;14)(q24;q32).
 | Table I.Complete blood counts and LDH of the
present patient at the time of initial diagnosis of abducens
diplopia, 8 days later during follow-up and at the established
diagnosis of BL. |
Table I.
Complete blood counts and LDH of the
present patient at the time of initial diagnosis of abducens
diplopia, 8 days later during follow-up and at the established
diagnosis of BL.
| BL parameter | Initial diagnosis of
abducens diplopia | Follow-up | At BL diagnosis |
|---|
| Time, days | 1 | 8 | 13 |
| Hemoglobin, g/l | 133 | 122 | 102 |
| Leukocytes,
×109/l | 5.8 | 5.4 | 5.4 |
| Lymphocyte, % | 27.4 | 29.5 | 22.4 |
| Neutrophils, % | 62.1 | 62.8 | 67.8 |
| Monocytes, % | 9.3 | 6.9 | 4.0 |
| Platelets,
×109/l | 150 | 149 | 120 |
| Peripheral blood
blasts, % | – | – | 4.0 |
| Serum LDH, U/l | 907 | 2452 | 3896 |
A bone marrow biopsy was subsequently performed to
check for unusual lumps and tangible swollen lymph nodes. Body
macrophages were dispersed among sheets of non-cohesive cells,
presenting a typical ‘starry sky’ morphological appearance.
Immunohistochemistry (IHC) was performed using antibodies purchased
from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) at a dilution of 1:100. These antibodies were used
including CD19 (#TA506234), CD20 (#TA800394), CD22 (#TA807826),
CD79a (#TA800688), CD3 (#TA320268), CD5 (#TA501335), CD23
(#TA801531), TdT (#TA327989), Bcl-2 (#ZA-0536), Bcl-6 (#TA804186),
MUM-1 (#ZA-0583) and cyclin D1 (#sc-753). The results demonstrated
positive expression of CD19, CD20, CD22 and CD79a, and negative
expression of CD3, CD5, CD23, TdT, Bcl-2, Bcl-6, MUM-1 and cyclin
D1. Therefore, the diagnosis of BL/mature B cell ALL with CNS
involvement was confirmed and the patient received combined
chemotherapy with hyperfractionated cyclophosphamide, vincristine,
therarubicin and dexamethasone, and an additional rituximab
(R-hyper-CVAD, rituximab 375 mg/m2 on day 1;
cyclophosphamide 600 mg/m2 on days 2–4; vincristine 1.4
mg/m2 on days 5 and 12; therarubicin 50 mg/m2
on day 5 and dexamethasone 40 mg on days 2–5 and 12–15). The
patient also underwent aggressive intrathecal chemotherapy with
methotrexate (5 mg), arabinocytidine (5 mg) and dexamethasone (5
mg) twice a week during intravenous chemotherapy. Following
treatment, CSF analysis exhibited a transparent appearance with
normal chemistry and cytological manifestation. Complete remission
and negative minimal residual disease (MRD) in the bone marrow were
achieved following induction chemotherapy, and palsy and double
vision of the right eye also improved. The patient was administered
3 further cycles of combined chemotherapy of R-hyper-CVAD and
intrathecal chemotherapy. However, no suitable donor could be
located in time for the patient to undergo the hematopoietic stem
cell transplantation required to prevent relapse. The patient
experienced relapse with recurrent positive MRD prior to the last
cycle of chemotherapy and finally succumbed to BL.
Discussion
Whether EBV-positive or negative, >80% of
patients with BL or mature B cell ALL are known to have the t(8;14)
(q24;q32) chromosome translocation, which juxtaposes the
MYC-encoding gene to an Ig enhancer element (IgEE) (9,10). As
IgEEs are specifically active in mature B cells, this translocation
to MYC results in aberrantly high MYC expression, which enhances B
cell proliferation regardless of whether EBV infection has occurred
(10). Other chromosomal aberrations
may also induce rapid cell proliferation, which results in a highly
aggressive clinical course (11).
Adults with BL frequently present with B symptoms,
bulky masses and laboratory evidence of tumor lysis; in addition,
CNS involvement occurs in ~70% of patients diagnosed (4). The pathology of CNS leukemia affects the
entire nervous system, including spinal cords, cranial nerves,
cerebral hemispheres and associated nerve roots. The diagnosis of
CNS leukemia is based on neurological dysfunction, leukemia cells
infiltrating the CSF, and/or abnormal neuroradiography on enhanced
CT and MRI scans (12,13). However, it is extremely rare that a
patient with neurological deficits does not exhibit any
abnormalities in either CSF or neuroradiography, and therefore BL
is easily neglected or misdiagnosed. Negative CSF cytology may
serve as a false negative signal or a possible former stage of the
disease.
Previous studies have demonstrated that a number of
patients with CNS infiltration exhibited normal CSF cytology
initially and required repeated lumbar punctures (14–16).
Therefore, multiple lumbar punctures and a flow cytometry analysis
of CSF may aid the diagnosis of patients with BL (17). CT and MRI scans have been the
principal imaging modalities for the staging and restaging of
lymphoma during the last few decades (7); however, they are not accurate at
identifying the early symptoms of lymphoma. Therefore, novel
neuroimaging methods that may improve early diagnosis of the
disease, thus improving patient prognosis, are required. In recent
years, a number of studies have demonstrated that information
provided by 18-fluorodeoxyglucose positron-emission tomography/CT
(FDG-PET/CT) may result in greater sensitivity compared with
anatomic imaging modalities (18,19).
In addition to oculomotor nerve palsy, the elevated
level of serum LDH and bone pain experienced by the current patient
is suggestive of early abnormal cell proliferation in bone marrow,
despite the initial results of the peripheral blood cell count
being normal. It is well established that serum LDH levels are an
independent prognostic factor in BL, and a higher LDH level, like
that experienced by the present patient, is associated with
advanced-stage disease and a less favorable outcome (20). Meanwhile, the abnormal MRI results of
the current patient indicated that sacrum and pelvis bone pain was
associated with bone marrow dysfunction. Though the peripheral
blood WBC and platelet counts were initially within the normal
ranges, a gradual change was observed from normal to anemia and,
finally, to anemia with blasts.
The survival rates of patients with BL/mature B cell
ALL, particularly for children and adolescents, have improved due
to the recent development of aggressive short-term chemotherapy,
with a 5-year event-free survival rate of 85–90% (11). By contrast, the majority of adults and
elderly patients cannot tolerate the adverse side effects of
aggressive chemotherapy, including drug toxicity and severe
infections. Therefore, optimum chemotherapeutic regimens for
high-grade BL have not yet been well established (21,22).
Several studies have recently reported high cure
rates following the use of similar chemotherapeutic protocols in
adults with BL. BL patients who received aggressive short-term
chemotherapy demonstrated better overall survival rate than those
who received chemotherapy with cyclophosphamide, doxorubicin,
vincristine and prednisolone (also known as CHOP), which is
routinely administered to patients with non-Hodgkin's lymphoma
(23). Prophylactic cranial
irradiation and prolonged maintenance have not demonstrated any
proven benefits and are not currently recommended for treatment of
the disease. Furthermore, introduction of the anti-CD20 monoclonal
antibody, rituximab, and/or allogeneic hematopoietic stem
transplantation has significantly improved outcomes of adult
patients, particularly those that are high-risk (24,25). The
poor prognosis of the current patient may have resulted from
several adverse prognostic factors, including CNS attacks,
complicated karyotype, a high level of LDH and relapse following
first-line treatment.
In conclusion, to the best of our knowledge, the
present case is the first to document oculomotor nerve palsy with
normal neuroradiography and CSF examination results as a preceding
symptom of adult sporadic BL with chromosomal translocation
t(8;14). Such atypical patients present major clinical challenges,
including harnessing novel biological technologies to improve risk
stratification, defining the value of prognostic factors and
developing innovative therapies, particularly for relapse and
refractory patients.
References
|
1
|
Turner JJ, Morton LM, Linet MS, Clarke CA,
Kadin ME, Vajdic CM, Monnereau A, Maynadié M, Chiu BC,
Marcos-Gragera R, et al: InterLymph hierarchical classification of
lymphoid neoplasms for epidemiologic research based on the WHO
classification (2008): Update and future directions. Blood.
116:e90–e98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK, Vardiman J, Lister TA and Bloomfield CD:
The World Health Organization classification of neoplastic diseases
of the hematopoietic and lymphoid tissues. Report of the clinical
advisory committee meeting, Airlie house, Virginia, November, 1997.
Ann Oncol. 10:1419–1432. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Besien K, Ha CS, Murphy S, McLaughlin
P, Rodriguez A, Amin K, Forman A, Romaguera J, Hagemeister F,
Younes A, et al: Risk factors, treatment, and outcome of central
nervous system recurrence in adults with intermediate-grade and
immunoblastic lymphoma. Blood. 91:1178–1184. 1998.PubMed/NCBI
|
|
4
|
Blum KA, Lozanski G and Byrd JC: Adult
Burkitt leukemia and lymphoma. Blood. 104:3009–3020. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hecht JL and Aster JC: Molecular biology
of Burkitt's lymphoma. J Clin Oncol. 18:3707–3721. 2000.PubMed/NCBI
|
|
6
|
ar-Rushdi A, Nishikura K, Erikson J, Watt
R, Rovera G and Croce CM: Differential expression of the
translocated and the untranslocated c-myc oncogene in Burkitt
lymphoma. Science. 222:390–393. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson KA, Tung K, Mead G and Sweetenham
J: The imaging of Burkitt's and Burkitt-like lymphoma. Clin Radiol.
53:835–841. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cairo MS, Gerrard M, Sposto R, Auperin A,
Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy
K, et al: Results of a randomized international study of high-risk
central nervous system B non-Hodgkin lymphoma and B acute
lymphoblastic leukemia in children and adolescents. Blood.
109:2736–2743. 2007.PubMed/NCBI
|
|
9
|
Klein G: Burkitt lymphoma-a stalking horse
for cancer research? Semin Cancer Biol. 19:347–350. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayday AC, Gillies SD, Saito H, Wood C,
Wiman K, Hayward WS and Tonegawa S: Activation of a translocated
human c-myc gene by an enhancer in the immunoglobulin heavy-chain
locus. Nature. 307:334–340. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miles RR, Arnold S and Cairo MS: Risk
factors and treatment of childhood and adolescent Burkitt
lymphoma/leukaemia. Br J Haematol. 156:730–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pui CH and Thiel E: Central nervous system
disease in hematologic malignancies: Historical perspective and
practical applications. Semin Oncol. 36(4): Suppl 2. S2–S16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang SJ, Yoon DH, Kim S, Yoon S, Kim DY,
Park CS, Huh J, Lee SW, Lee DH, Rvu JS and Suh C: A unique pattern
of extranodal involvement in Korean adults with sporadic Burkitt
lymphoma: A single center experience. Ann Hematol. 91:1917–1922.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crivii SM, Neamtu S and Bocsan G:
Neuroimmunitary profile estimation in cerebrospinal fluid and its
importance in childhood acute lymphoblastic leukaemia. Arch
Geschwulstforsch. 59:199–204. 1989.PubMed/NCBI
|
|
15
|
Yadav J and Jain V: Cushing syndrome
related to leukemic infiltration of the central nervous system. J
Pediatr Endocrinol Metab. 28:717–719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bromberg JE, Breems DA, Kraan J, Bikker G,
van der Holt B, Smitt PS, van den Bent MJ, van't Veer M and Gratama
JW: CSF flow cytometry greatly improves diagnostic accuracy in CNS
hematologic malignancies. Neurology. 68:1674–1679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quijano S, López A, Sancho J Manuel,
Panizo C, Debén G, Castilla C, García-Vela J Antonio, Salar A,
Alonso-Vence N, González-Barca E, et al: Identification of
leptomeningeal disease in aggressive B-cell non-Hodgkin's lymphoma:
Improved sensitivity of flow cytometry. J Clin Oncol. 27:1462–1469.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carrillo-Cruz E, Marín-Oyaga VA, Solé
Rodríguez M, Borrego-Dorado I, de la Cruz Vicente F, Quiroga
Cantero E, Manzanares Pérez M, Capote FJ, Ramírez Sánchez MJ,
Espigado Tocino I, et al: Role of 18F-FDG-PET/CT in the management
of Burkitt lymphoma. Eur J Haematol. 94:23–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Just PA, Fieschi C, Baillet G, Galicier L,
Oksenhendler E and Moretti JL: 18F-fluorodeoxyglucose positron
emission tomography/computed tomography in AIDS-related Burkitt
lymphoma. AIDS Patient Care STDS. 22:695–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mintzer DM, Andreeff M, Filippa DA,
Jhanwar SC, Chaganti RS and Koziner B: Progression of nodular
poorly differentiated lymphocytic lymphoma to Burkitt's-like
lymphoma. Blood. 64:415–421. 1984.PubMed/NCBI
|
|
21
|
Mead GM, Barrans SL, Qian W, Walewski J,
Radford JA, Wolf M, Clawson SM, Stenning SP, Yule CL, Jack AS, et
al: A prospective clinicopathological study of dose modified
CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined
using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10
trial). Blood. 112:2248–2260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smeland S, Blystad AK, Kvaløy SO, Ikonomou
IM, Delabie J, Kvalheim G, Hammerstrøm J, Lauritzsen GF and Holte
H: Treatment of Burkitt's/Burkitt-like lymphoma in adolescents and
adults: A 20-year experience from the Norwegian Radium Hospital
with the use of three successive regimens. Ann Oncol. 15:1072–1078.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Todeschini G, Bonifacio M, Tecchio C,
Balter R, Carli G, Stefani PM, Adami F, Zamò A, Dei Tos AP, Marino
F, et al: Intensive short-term chemotherapy regimen induces high
remission rate (over 90%) and event-free survival both in children
and adult patients with advanced sporadic Burkitt
lymphoma/leukemia. Am J Hematol. 87:22–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas DA, Faderl S, O'Brien S,
Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, Verstovsek S,
Wierda WG, Pierce SA, et al: Chemoimmunotherapy with hyper-CVAD
plus rituximab for the treatment of adult Burkitt and Burkitt-type
lymphoma or acute lymphoblastic leukemia. Cancer. 106:1569–1580.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barnes JA, Lacasce AS, Feng Y, Toomey CE,
Neuberg D, Michaelson JS, Hochberg EP and Abramson JS: Evaluation
of the addition of rituximab to CODOX-M/IVAC for Burkitt's
lymphoma: A retrospective analysis. Ann Oncol. 22:1859–1864. 2011.
View Article : Google Scholar : PubMed/NCBI
|