Introduction
Anemone raddeana of the family Ranunculaceae
is a traditional medicine, which was widely used by the Chinese in
ancient times to promote the excretion of urine and expel excess
gas. At present, with the development of separation and
purification techniques, several constituents of Anemone
raddeana have been isolated and identified (1), which is convenient for examining the
mechanism of single components.
Raddeanin A, a primary triterpenoid saponin
extracted from Ranunculaceae Anemone raddeana rhizome
(2), has shown antiproliferative
effects on human hepatic cancer cells (3). Previous studies have also shown that
Raddeanin A induces apoptosis and inhibits invasion in human
gastric cancer cells (4), and
Raddeanin A has a significant inhibitory effect on the growth of
tumor cells, including S180, H22 and U14 cells, in vivo
(5). All these activities suggest
that Raddeanin A may be a potential compound for the treatment of
cancer, and a focus is required on its further investigation and
therapeutic application.
However, the inhibitory effect of Raddeanin A on
HCT-116 colon cancer cells has not been reported, and few
investigations have been performed on the pharmacokinetics and
tissue distribution in mice following oral administration of
Raddeanin A, which are important in the development of novel drugs
(6).
The present study aimed to investigate the
inhibitory activity of Raddeanin A on the growth of HCT-116 colon
cancer cells. In addition, detailed investigations of the in
vivo pharmacokinetic and tissue distribution of Raddeanin A
were performed.
Materials and methods
Chemicals and instruments
Raddeanin A (purity >98%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). Digoxin, used as internal standard (IS;
purity >98.0%) was obtained from Sichuan Weikeqi Biological
Technology Company (Chengdu, China). Formic acid of a liquid
chromatography-mass spectrometry (LC-MS) grade was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). High
performance liquid chromatography (HPLC) gradient grade methanol
and acetonitrile were purchased from Merck Millipore. Milli-Q water
was produced using a Millipore purification system (EMD Millipore,
Billerica, MA, USA). Trypsin-ethylene diamine tetraacetic acid
(EDTA) solution (0.25%) was obtained from Biosharp (Hefei, China)
and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide
(MTT) was purchased from Sigma-Aldrich. An AnnexinV-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) detection kit was
supplied by BD Biosciences (Franklin Lakes, NJ, USA).
An LC-MS/MS system was used in the present study,
the HPLC system consisted of two LC-20AD pumps, a DGU-20A3
degasser, an SIL-20AC auto sampler and a CTO-20AC column oven
(Shimadzu Corporation, Kyoto, Japan). The MS was achieved on an API
4000 Q-trap MS/MS system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) equipped with a Turbo Ion Spray
inlet in the negative ion mode. Quantification was performed using
multiple reaction monitoring mode.
Cell experiments
Cell line and cell culture
The HCT-116 human colorectal carcinoma cells,
obtained from American Type Culture Collection (Manassas, VA, USA),
were cultured at 37°C in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% heat-treated
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml streptomycin.
The cells were incubated in an atmosphere of 95% air and 5%
CO2. The cells were stained by the addition of 0.04%
trypan blue to the culture medium to determine cell viability.
MTT assay
The cells were seeded in a 96-well plate at a
density of 1×104 cells/0.2 ml/well. Following
stabilization overnight, the cells were suspended in serum-free
medium with a range of concentrations of Raddeanin A (0.1, 0.5, 1,
5, 10, 50, 100 and 200 µM). Following treatment for 12, 24 and 48
h, MTT (20 µl; 5 mg/ml; Sigma-Aldrich; Merck Millipore) was added
into each well and the plates were placed in a 37°C incubator for 3
h. Following incubation, the medium was discarded and 150 µl DMSO
(Sigma-Aldrich; Merck Millipore) was added. The absorbance at a
wavelength of 490 nm was measured using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Cellular uptake
The HCT-116 cells were seeded at a density of
2×104/ml in a 6-well plate and incubated with Raddeanin A (0.25,
0.5, 1 and 2 µM) for 12, 24 and 48 h at 37°C. On harvesting, the
cell culture media were collected for analysis. The cells were
harvested using 0.25% Trypsin-EDTA Solution following being washed
twice with cooled PBS. The solution was then centrifuged at 1,000 ×
g for 10 min at 4°C to obtain the precipitates. All samples were
stored at −70°C until analysis.
DAPI staining
DAPI, a blue fluorescent dye, preferentially stains
double-stranded DNA and produces an enhanced florescence. In the
present study, the cells were seeded at a density of 2×104/ml and
incubated with Raddeanin A (1 and 3 µM) for 24 h at 37°C.
Subsequently, 4% paraformaldehyde was added to fix the cells and,
30 min later, DAPI (5 µg/ml) was added and the plate was incubated
at room temperature for 15 min. The cells were then observed under
a fluorescent microscope in bright field with a DAPI filter at 200x
magnification.
Flow cytometric analysis of levels of apoptosis
using AnnexinV-FITC/PI double staining
Following treatment of the cells with or without
Raddeanin A (1 and 3 µM), ~3×105 cells were harvested.
Subsequent to being washed twice with cold PBS, the cells were
re-suspended in 500 µl binding buffer, containing 10 mM Hepes/NaOH
(pH 7.4), 140 mM NaCl and 2.5 mM CaCl2. Annexin-V (5 µl)
and PI (5 µl) were then added, and incubated at room temperature
for 15 min in the dark for flow cytometric analysis.
Pharmacokinetic investigations in
mice
Sample preparation
The analytical method used was developed and
validated in a previous study (7) and
was successfully applied in the present study. Samples of plasma
from mice used for pharmacokinetic investigation (100 µl) were
spiked with 10 µl IS solution (10 µg/ml) and then vortexed for 3
min. Subsequently, 400 µl methanol was added and immediately
vortexed for 5 min, followed by centrifugation for 10 min at 12,000
× g at 4°C. A 350 µl volume of the supernatant was then transferred
into a separate 1.5 ml centrifuge tube and evaporated to dryness in
a vacuum desiccator. The residue was reconstituted in 100 µl
methanol-water (50:50, v/v), vortexed for 5 min and then
centrifuged at 12,000 × g for 10 min at 4°C. A 5 µl aliquot of the
solution was injected into the LC-MS/MS system. Data acquisition
was performed using Analyst 1.5.1 software (Applied Biosystems;
Thermo Fisher Scientific, Inc.).
Pharmacokinetics investigation
A total of 60 BALB/c mice (24–30 g; 30 females and
30 males) aged 6 weeks were used for the pharmacokinetic analyses.
Mice were supplied bu the Experimental Animal Center of Yangzhou
University (Jiangsu, China). All animals had free access to food
and water. All animals were housed in an environmentally controlled
breeding room at a temperature of ~25°C under a 12 h light/12 h
dark cycle for at least 1 week prior to the start of the
experiments. The mice were fasted for 12 h with free access to
water prior to each experiment. The present study was approved by
the Animal Ethical Committee of Nanjing Tech University (Nanjing,
China). The 60 mice were orally administered once with Raddeanin A
(1.5 mg/kg). Blood samples were collected into heparinized tubes at
0 h (pro-drug) and at 0.08, 0.17, 0.33, 0.5, 1, 1.5, 2, 3, 4, 6 and
8 h (n=5). The plasma samples were acquired by centrifugation at
3,500 × g for 10 min at 4°C and then stored at −80°C until
analysis.
Tissue distribution in mice
For investigating the tissue distribution of
Raddeanin A, an additional 20 BALB/c mice (10 females and 10 males;
24–30 g; aged 6 weeks; free access to food and water; Experimental
Animal Center of Yangzhou University) were assigned into four
groups. Samples of the heart, liver, spleen, lung, kidney, stomach,
dodecadactylon, jejunum, ileum, colon, caecum and rectum (~0.2 g)
were collected at 0, 0.4, 1 and 4 h (following sacrifice by cervial
dislocation) following an oral administration of Raddeanin A
solution (1.5 mg/kg). The tissue samples were rinsed with ice-cold
saline solution (0.9% NaCl) to remove the blood. All samples were
stored at −20°C until LC-MS/MS analysis.
Data analysis
Pharmacokinetic parameters were calculated using DAS
version 2.0 software (Mathematical Pharmacology Professional
Committee of China, Shanghai, China). Data are expressed as the
mean ± standard deviation. The significance of differences in the
data was evaluated using student's t test. P<0.05 was considered
to indicate a statistically significant difference. Statistical
analysis was performed using SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Cell experiments
MTT assay
Following treatment of the HCT-116 cells with
Raddeanin A for 12, 24 and 48 h, the viability of the cells was
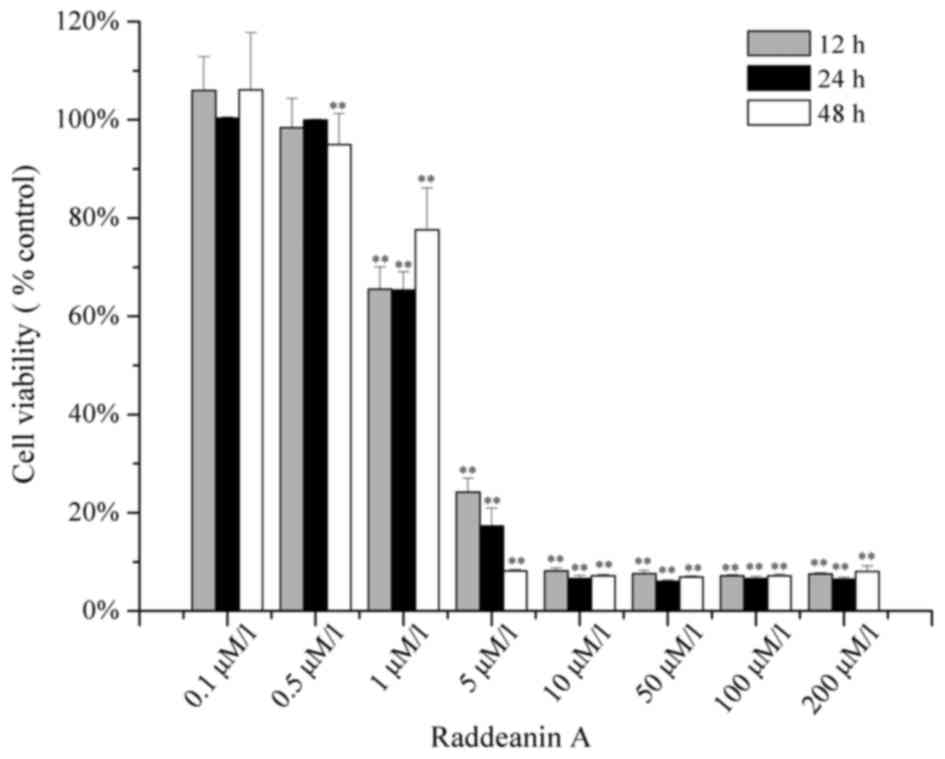
assessed using an MTT assay. The results, as shown in Fig. 1, indicated that the inhibitory effect
of Raddeanin A on the HCT-116 was dose-dependent and
time-independent. When treated with the dose of 5 µM Raddeanin A
for 12, 24 and 48 h, the percentages of viable cells declined to
24.19, 17.31 and 8.18%, respectively. The half maximal inhibitory
concentration (IC50) values of Raddeanin A on the HCT-16
cells treated for 12, 24 and 48 h were 1.376, 1.441 and 1.424 µM,
respectively. According to the results of the MTT assay, 1 and 3 µM
of Raddeanin A were selected to for the subsequent DAPI staining
and flow cytometric analysis experiments.
Cellular uptake
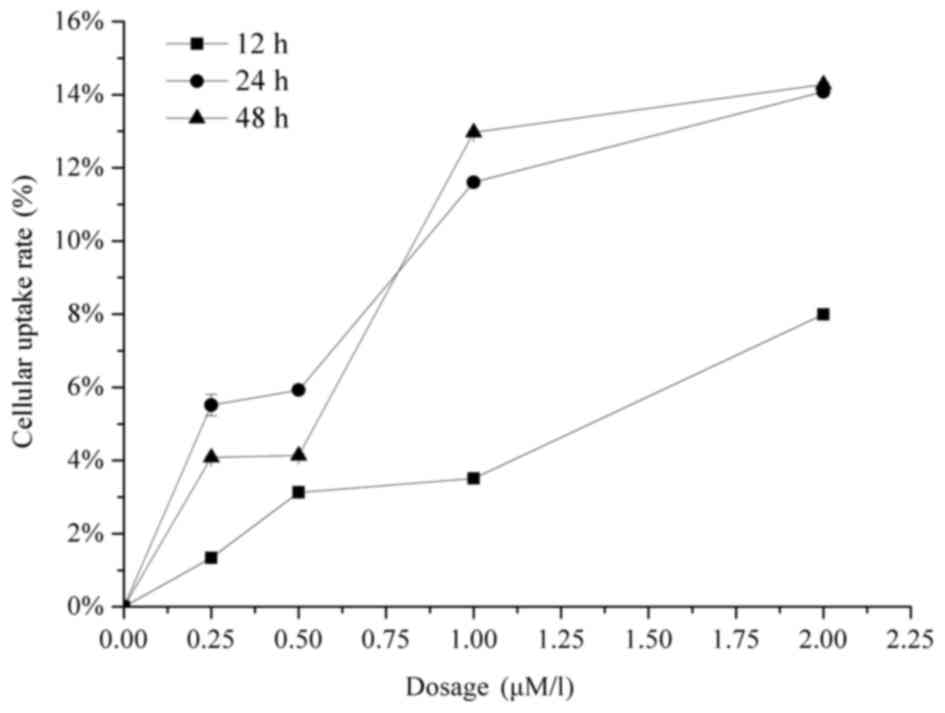
The cellular uptake of Raddeanin A was evaluated by
co-incubating HCT-116 cells with a range of drug concentrations for
12, 24 and 48 h. As shown in Fig. 2,
the results demonstrated that the uptake of Raddeanin A in the
HCT-116 cells was dose-dependent, however no time-dependency was
observed.
DAPI staining
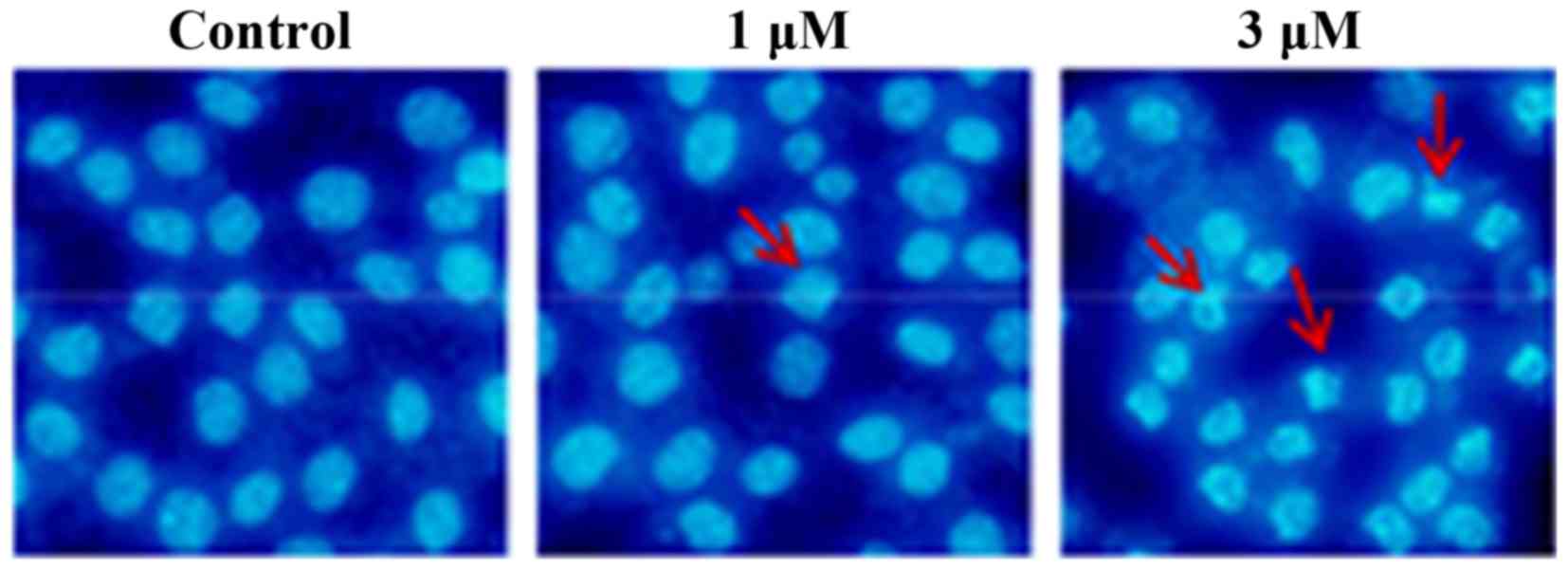
As shown in Fig. 3,
compared with the nuclei of the untreated cells, which exhibited a
round or oval shape and were used as a control, Raddeanin A exerted
a marked effect on the induction of cell apoptosis, visible from
the occurrence of distinct nuclear morphological changes of
apoptosis, including chromatin condensation, shrinkage and
apoptotic body formation.
Flow cytometric analysis of apoptosis using
AnnexinV-FITC/PI double staining
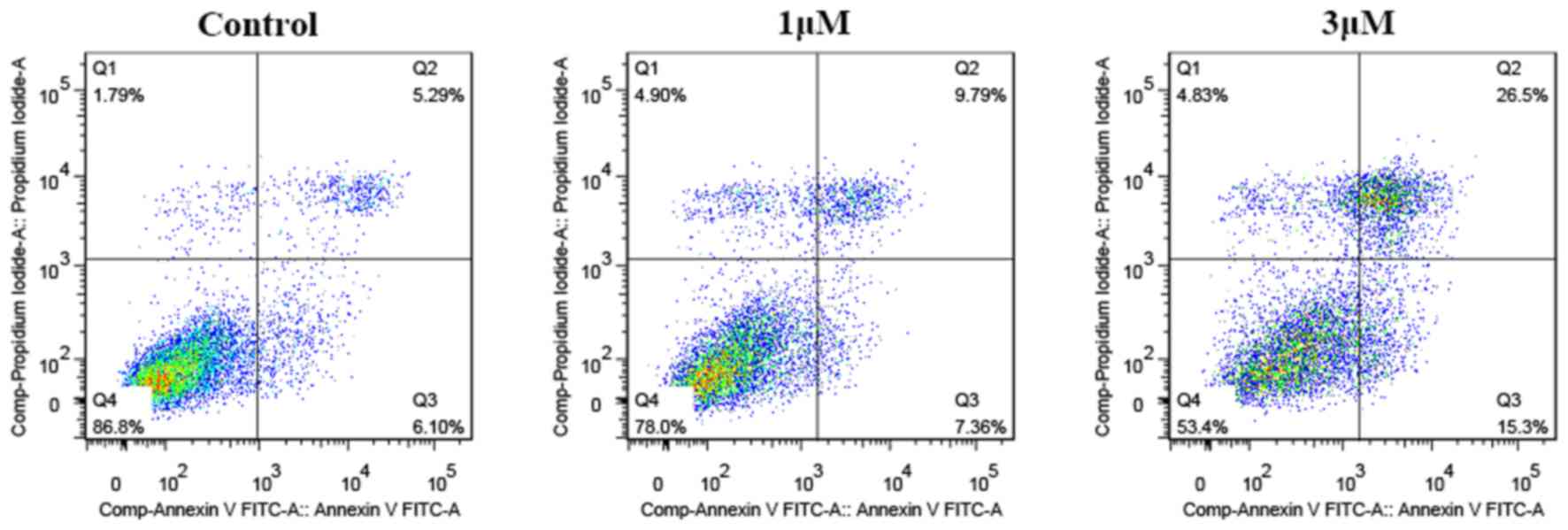
To further examine the apoptosis-inducing capability
of Raddeanin A quantitatively, flow cytometric analysis was
performed. The results (Fig. 4)
showed that the percentages of viable, early apoptotic, late
apoptotic and necrotic cells altered significantly following
treatment with different concentrations of Raddeanin A for 24 h.
The total apoptotic ratio reached 41.8% at the concentration of 3
µM, as evidenced by a clear shift from the live cell population to
early and late apoptotic cell populations.
Pharmacokinetic investigations in
mice
Pharmacokinetic investigations
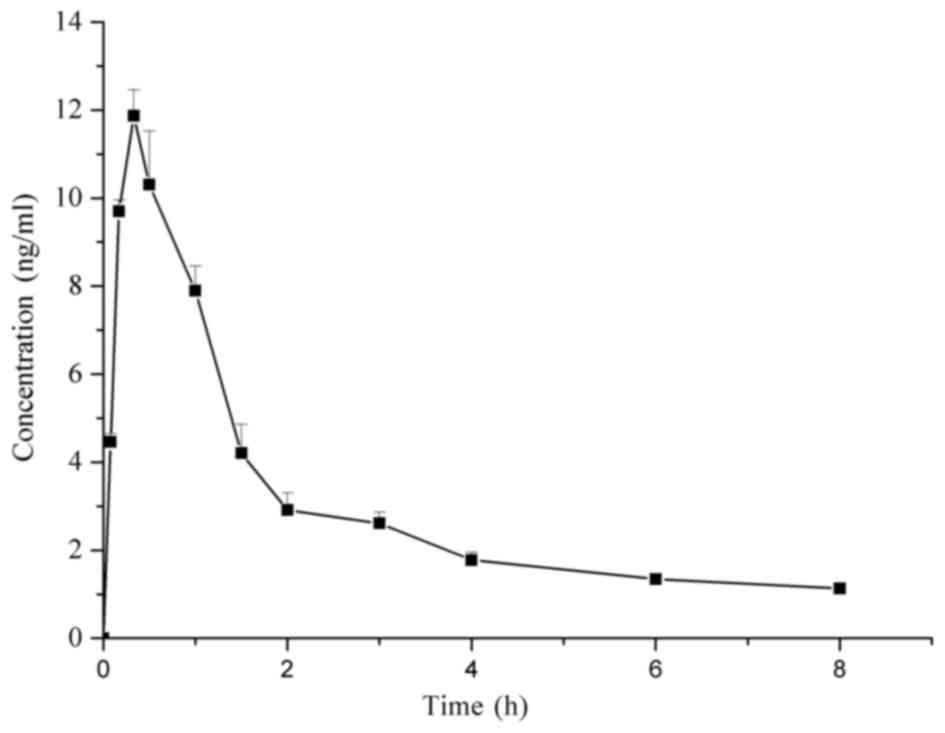
The mean plasma concentration-time graph of
Raddeanin A is shown in Fig. 5. The
major pharmacokinetic parameters of Raddeanin A calculated using a
non-compartmental model are presented in Table I. Raddeanin A was absorbed rapidly
in vivo, with a time to maximum concentration of 0.33 h. The
concentrations detected in the plasma were low, with a maximum
concentration of 12.326 µg/l. This was consistent with a previous
study (7), in which low
bioavailability of Raddeanin A was demonstrated. In addition,
Raddeanin A showed fast elimination with a half-life of 3.542±0.158
h and was undetectable in the plasma at 6 h.
 | Table I.Pharmacokinetic parameters of
Raddeanin A in mouse plasma following oral administration. |
Table I.
Pharmacokinetic parameters of
Raddeanin A in mouse plasma following oral administration.
| Parameter | Value |
|---|
| AUC(0-t)
(µg/l*h) | 24.247±0.458 |
| AUC(0-∞)
(µg/l*h) | 28.760±0.592 |
| MRT(0-t) (h) | 2.413±0.022 |
| t1/2 (h) | 3.542±0.158 |
| Tmax (h) | 0.330±0.000 |
| CL (l/h/kg) | 69.564±1.435 |
| V (l/kg) | 355.429±14.054 |
| Cmax (µg/l) | 12.326±0.598 |
Tissue distribution
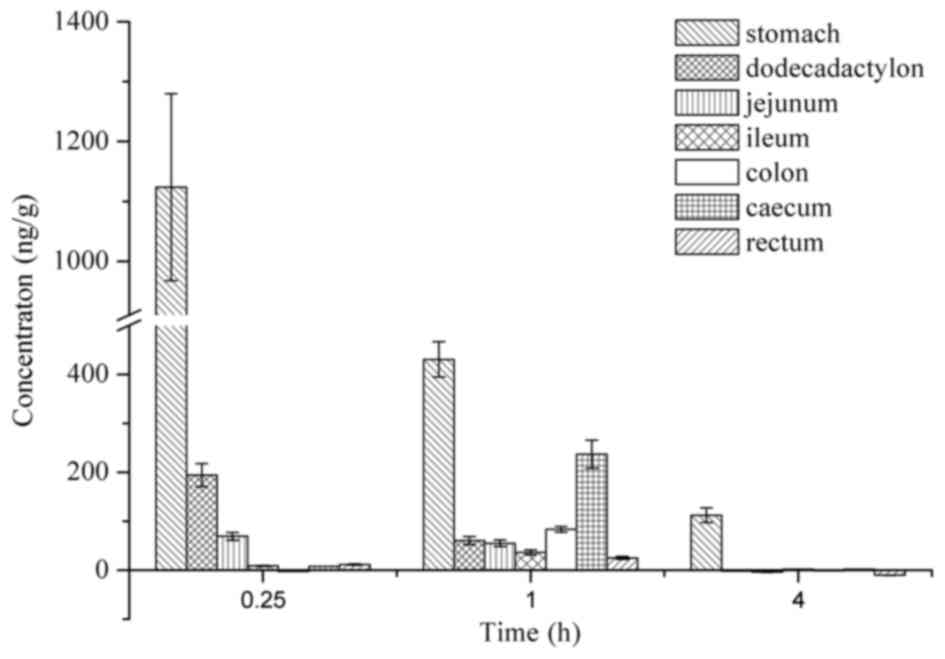
The in vivo distribution of Raddeanin A was
examined by the quantitative detection of the levels of Raddeanin A
in different tissues (Fig. 6).
Following oral administration, Raddeanin A was detected in various
gastrointestinal tract tissues, namely the stomach, dodecadactylon,
jejunum, ileum, colon, caecum and rectum. The highest level of
Raddeanin A was observed in the stomach, followed by the colon and
the caecum. At 4 h, Raddeanin A was almost undetectable in any of
the intestinal tract. In terms of the distribution of Raddeanin A
in the blood perfused organs, including the heart, liver, spleen,
lung and kidney, the drug levels were not sufficient to be detected
using the method used. This was predominantly due to the low
absorption of Raddeanin A in the plasma following oral
administration, as described above.
Discussion
In ancient time, traditional Chinese medicines were
widely used. Investigations have demonstrated the association
between the structure of triterpene saponin and its anticancer
activities (8) and Raddeanin A, as a
triterpene saponin, was one of the primary active components.
However, there are no reports on its apoptosis-inducing effect on
HCT-116 human colon cancer cells. In addition the cellular uptake
patterns of Raddeanin A have not been reported. In the present
study, a validated LC-MS/MS method was used to examine cellular
uptake, and pharmacokinetic investigations were performed.
In the cell experiments, Raddeanin A was observed to
inhibit the proliferation of HCT-116 cells in a dose dependent
manner. The IC50 was 1.413 µM, which was lower, compared
with that reported in a previous study on human gastric cancer
cells (4). DAPI staining and flow
cytometric analysis of apoptosis demonstrated the
apoptosis-inducing effect of Raddeanin A on the HCT-116 cells. The
cellular uptake of Raddeanin A in the HCT-116 cells occurred in a
dose-dependent manner.
The pharmacokinetic and tissue distribution
characteristics of Raddeanin A were measured in mice. In the
pharmacokinetic investigations, a previously developed validated
analytical method was utilized to successfully detect the
concentrations in mice plasma. The results showed the rapid
distribution and elimination of Raddeanin A, which was in agreement
with a previous study, which indicated that low bioavailability
leads to low concentrations in plasma (7). By contrast, examination of the tissue
distribution in the present study demonstrated that Raddeanin A was
predominantly distributed in the gastrointestinal tract.
Considering its effective antiproliferative activity on human colon
cancer cells, Raddeanin A is a promising candidate for the
treatment of superficial gastrointestinal cancer.
The present study demonstrated that the Raddeanin A
from Ranunculaceae Anemone raddeana induced the apoptosis of
HCT-116 cells, which, to the best of our knowledge, has not been
reported previously. The uptake of Raddeanin A in HCT-116 was
investigated, and the result showed dose-dependency. Additionally,
the determination of Raddeanin A in mouse intestinal tissues
demonstrated that Raddeanin A was predominantly distributed in the
stomach, followed by the cecum and colon. The cell experiments and
pharmacokinetic investigations performed support the use of
Raddeanin A as a potential oral drug for the treatment of
superficial colon cancer in the future. As saponin injection has
several harmful adverse effects, including hemolysis (9), the exploitation of a non-injectable
formulation for Raddeanin A is necessary.
Acknowledgements
This study was supported by the State Key Laboratory
of Materials-Oriented Chemical Engineering, Nanjing Tech University
(grant no. KL14-08) and the National Science and Technology Major
Projects for ‘Major New Drugs Innovation and Development’ (grant
no. 2013ZX09103001-004).
References
|
1
|
Cao P, Wu FE and Ding LS: Advances in the
studies on the chemical constituents and biologic activities for
anemone species. Nat Prod Res Dev. 16:581–584, 520. 2004.
|
|
2
|
Zhang JM, Cao L and Wu ZM: Studies on
anticancer activities of triterpenoid in Anemone raddeana Regel.
Chinese J New Drugs. 12:191–193. 2003.(In Chinese).
|
|
3
|
Ma M, Li DL, Zhao DY, et al: Study the
effect of raddeanin A on the proliferation of human hepatic cancer
in nude mice. Drug Eval Res. 01:40–43. 2015.(In Chinese).
|
|
4
|
Xue G, Zou X, Zhou JY, Sun W, Wu J, Xu JL
and Wang RP: Raddeanin A induces human gastric cancer cells
apoptosis and inhibits their invasion in vitro. Biochem Biophys Res
Commun. 439:196–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang MK, Ding LS and Wu FE: Antitumor
effects of raddeanin A on S180, H22 and U14 cell xenografts in
mice. Ai Zheng. 27:910–913. 2008.(In Chinese). PubMed/NCBI
|
|
6
|
Sun C, Li Q, Pan L, Liu B, Gu P, Zhang J,
Ding L and Wu C: Development of a highly sensitive LC-MS/MS method
for simultaneous determination of rupatadine and its two active
metabolites in human plasma: Application to a clinical
pharmacokinetic study. J Pharm Biomed Anal. 111:163–168. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Ma B, Zhang Q, Ying H, Li J, Xu Q,
Wu D and Wang Y: Development and validation of a sensitive liquid
chromatography/tandem mass spectrometry method for the
determination of raddeanin A in rat plasma and its application to a
pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life
Sci. 912:16–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao J, Li W, Tang Y, Zhang X, Li W and
Zhao Y: Three new triterpene saponins from Actinostemma lobatum
MAXIM and their cytotoxicity in vitro. Phytochem Lett. 11:301–305.
2015. View Article : Google Scholar
|
|
9
|
Zhou HL, Shun YX, Li Y, Wang B and Liu DY:
Progress in Studies on Chemical Constituents and Pharmacological
Effect of Anemone raddeana Regel. Lisizhen Medicine and Materia
Medica Research. 05:1239–1241. 2007.(In Chinese).
|