Introduction
Currently, breast cancer treatment is progressing on
a daily basis, and is divided into subtypes based on the hormone
receptors human epidermal growth factor receptor type 2 (HER2) and
Ki-67 (1). However, treatment
algorithms do not necessarily result in satisfactory clinical
outcomes. Therefore, to further improve the breast cancer
prognosis, predictive factors are required in order to arrive at
more accurate prognoses and improve treatment efficacy. If such
biomarkers could be identified, it would be possible to provide
appropriate treatments to relevant subjects, resulting in excellent
clinical outcomes. To date, prognostic prediction has been based on
older studies of morphological characteristics (2). More recent research has concentrated on
molecular biomarkers (3). The present
study reports the findings of a systematic review of prognosis for
patients with breast cancer based on molecular biomarkers. The
correlations between these biomarkers, prognosis and the treatment
response may be useful for all breast cancer patients.
Literature search
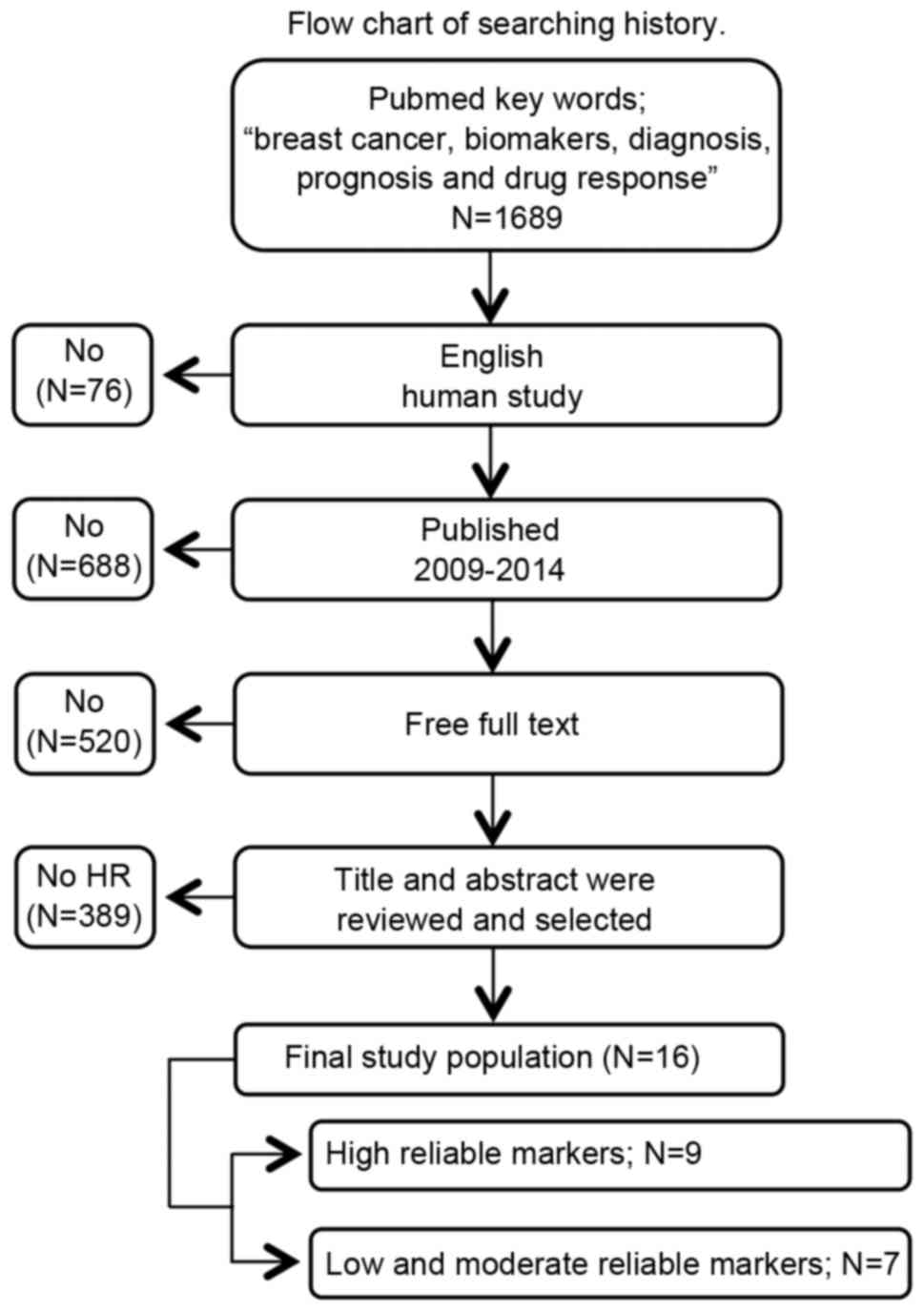
A search of the PubMed database (National Center for
Biotechnology Information, Bethesda, MD, UDA) using the key words
‘breast cancer,’ ‘biomarkers,’ ‘diagnosis,’ ‘prognosis’ and ‘drug
response’ retrieved 1,689 potential studies. Subsequent to
filtering for studies involving humans and written in English, 76
studies were excluded. When the remaining reports were limited to
the period between 2009 and 2014, an additional 688 were excluded.
Of the remainder, 520 studies were excluded, as they did not
contain the full text. Finally, the abstracts of 405 studies were
evaluated and those that contained insufficient descriptions of
diagnostic performance, prognosis and drug response were excluded,
resulting in a total of 16 studies for analysis (Fig. 1).
Highly reliable biomarkers (Table I)
Retinoic acid receptor α (RARA)
Approximately 1/3 of estrogen receptor α
(ERα)-positive breast cancer patients treated with tamoxifen
experienced a relapse of the disease (4). RARA is a potential biomarker for
tamoxifen resistance (5). The
anti-tumor properties of RARA can be explained in association with
the interaction of the receptor with ERα and their joint genomic
binding site (6). The association
between ERα resistance and RARA resistance was confirmed using
tamoxifen-susceptible and -resistant cell lines (MCF7 and LCC2,
respectively). The tamoxifen-resistant cells were found to express
high levels of RARA (7).
Patients with ERα-positive breast cancer tumors with
high internal levels of RARA protein that were treated with
tamoxifen as adjuvant therapy exhibited shorter recurrence-free
survival (RFS) than patients with low internal levels of RARA
protein (7). Johansson et al
(7) performed an investigation into
serum RARA levels using ELISA, and found significantly higher RFS
rates in patients with high RARA expression levels compared with
patients with low levels: Hazard ratio (HR)=4.1; 95% confidence
interval (CI)=1.55–11.0; P=0.0046. Therefore, RARA may potentially
be a useful target of new treatment regimens and a biomarker to
predict the effectiveness of tamoxifen adjuvant treatment in
ERα-positive breast cancer.
Aromatase expression
Aromatase expression by breast cancer cells has been
shown to influence the effectiveness of endocrine treatments for
breast cancer (8,9). Ellis et al (10) conducted a study using a sample from a
clinical trial comparing tamoxifen and letrozole as neoadjuvant
endocrine therapies, and found that aromatase expression levels in
tumor and somatic cells was correlated with treatment-induced
changes in Ki-67, RFS, and breast cancer-specific survival (BCSS)
(11–13).
Aromatase expression was correlated with a smaller
tumor size (P=0.01), a higher Allred score of estrogen receptor
(P=0.006) (14) and lower Ki-67
levels (P=0.003). In addition, aromatase expression by tumor cells
was a significant prognostic factor of the independent variables
RFS (HR=2.3; 95% CI=1.2–4.6; P=0.01) and BCSS (HR=3.76; 95%
CI=1.4–10.0; P=0.008) (15). The
aforementioned data supports the use of aromatase blockers as the
first choice treatment for post-menopausal, hormone-positive breast
cancer.
Osteopontin
Osteopontin is a secreted extracellular matrix
adhesion protein associated with tumor cell invasion and metastasis
(16,17). Pang et al (18) examined the clinical and pathological
effects of the adhesion molecules osteopontin-c, E-cadherin and
β-catenin in breast cancer, and found higher expression levels of
all the aforementioned adhesion molecules in breast cancer compared
with normal tissue. The expression of osteopontin-c was associated
with lymph node metastasis, and higher tumor node-metastasis
classification (19) and histological
grade (19). In addition, high
expression levels of osteopontin-c have been correlated with tumor
recurrence and metastasis, as well as triple negative subtypes,
which are predictive factors of the independent variables
disease-free survival (DFS; HR=3.094; 95% CI, 1.229–7.789; P=0.016)
and overall survival (OS; HR=2.558; 95% CI, 1.048–6.243; P=0.039)
(20). Therefore, the development of
treatments targeting osteopontin-c may be beneficial for the
treatment of breast cancer.
Ki-67
Ohno et al (21) examined the role of Ki-67 as a
predictive biomarker of treatment response in a randomized,
multicenter study to compare the effectiveness of docetaxel
subsequent to treatment with
fluorouracil/epirubicin/cyclophosphamide with or without
capecitabine, in patients with operable breast cancer. The endpoint
was the rate of pathological complete response (pCR). Analysis of
hormone receptors and the Ki-67 labeling index (Ki-67LI) by
multivariate logistic regression analysis identified Ki-67 as an
independent prognostic factor (HR=2.718; 95% CI=1.331–5.549;
P=0.0061). In addition, the aforementioned results also suggest
that the Ki-67LI prior to treatment was a predictor for the
response to preoperative docetaxel treatment and preoperative
capecitabine treatment in early-stage breast cancer.
Denkert et al (22) obtained 1,166 breast cancer bioassay
specimens from a large-scale cohort study established to
investigate neoadjuvant treatment (the GeparTrio trial) and
evaluated pre-treatment Ki-67 levels by immunohistochemical
analysis. The study used the standardized, 3-endpoint, cut-off
algorithm (pCR, DFS and OS) (23).
The Ki-67 index and preoperative chemotherapy variables were
divided into 3 subgroups each: ≤15, 15.1–35 and ≥35%, and pCR rates
were 4.2, 12.8 and 29.0%, respectively (P<0.0005). The HR for
prognosis also increased in response to Ki-67 (HR=1, 3.32 and 9.20,
respectively), indicating that Ki-67 is a prognostic predictor for
hormone receptor-positive, but not triple-negative, breast cancer.
The aforementioned findings regarding Ki-67 may provide important
information for the development of other quantitative
biomarkers.
DeCensi et al (24) examined postoperative remission and
prognosis in response to Ki-67 in early stage ERα-positive breast
cancer patients treated with tamoxifen for 4 weeks as a short-term
neo-adjuvant therapy and reported that post-treatment levels of
Ki-67 in the second (14–19%), third (20–29%), and top (≥30%)
quartiles had recurrence HRs of 2.92 (95% CI, 0.95–8.96), 4.37
(1.56–12.25) and 6.05 (2.07–17.65), respectively, compared with
those in the bottom quartile (<14%; P=0.001). The mortality
risks were 5.5-fold higher when Ki-67 levels were ≥20% (95%
CI=1.26–23.16; P<0.006) when compared to those with Ki-67 levels
<20% (P=0.006). The authors concluded that the level of Ki-67
subsequent to short-term neoadjuvant tamoxifen is a good predictor
of RFS and OS, supporting the use of Ki-67 as a surrogate biomarker
to personalize adjuvant treatment and to cost-effectively screen
novel drugs.
Carcinoembryonic antigen-related cell
adhesion molecule 6 (CEACAM6)
CEACAM6 is a human carcinoembryonic antigen that
functions as a multi-functional regulatory protein and is
overexpressed in various cell processes associated with cancer
(25,26). CEACAM6 expression in atypical ductal
hyperplasia has been suggested to serve an important role in the
development of breast cancer (27).
CEACAM6 has also been associated with invasive and
treatment-resistant breast cancer (28). However, in a large-scale cohort study,
CEACAM6 expression in luminal breast cancer exhibited no effect on
OS or correlation with prognosis, although an association between
CEACAM6 expression and prognosis in breast cancer overexpressing
HER2 was revealed, as the high expression group tended to exhibit
poorer OS (28). The aforementioned
results appear to indicate that treatment is required for breast
cancer patients with HER2 overexpression and the presence of
CEACAM6.
Phosphatidylinositol-4,5-bisphosphate
3-kinase, catalytic subunit α (PIK3CA)
PIK3CA is a cancer gene coding for 1 of 2
phosphoinositide 3-kinase (PI3 K) subunits (29), which is a gain-of-function mutation in
certain types of cancer, and is present in 20–40% patients with
breast cancer (30). Cizkova et
al (31) identified PIK3CA
mutations in 17 (21.3%) tumors among 80 HER2-positive patients
treated with trastuzumab for 1 year. Patients exhibiting wild-type
PIK3CA demonstrated an improved DFS compared with patients
exhibiting the PIK3CA mutations. The prognosis for HER2-positive
patients with PIK3CA mutations treated with trastuzumab was
significantly worse than for patients exhibiting the wild-type
variation, which is considered to occur since the P13K/protein
kinase B pathway is adversely affected by PIK3CA mutations,
resulting in the lower efficacy of trastuzumab. Thus, the detection
of PIK3CA mutations is only required in HER2-positive patients.
Tissue inhibitor of
metalloproteinases-1 (TIMP-1)
Paclitaxel is the first chemotherapy treatment of
choice for patients with lymph node metastasis (32,33).
However, there are currently no biomarkers to predict
susceptibility to chemotherapy. TIMP-1 has been shown to protect
cells from apoptosis (34). A
previous epidemiological study demonstrated an association between
high levels of TIMP-1 and reduced responsiveness to
cyclophosphamide/methotrexate/5-fluorouracil and
anthracycline-based chemotherapy regimens (35).
In a retrospective study of 99 breast cancer
patients, Zhu et al (36)
reported a correlation between TIMP-1 expression levels in primary
tumors and improved responsiveness to paclitaxel-based
chemotherapy. Kaplan-Meier survival analysis revealed that patients
with high TIMP-1 levels had poorer 5-year DFS that those with lower
TIMP-1 levels (71.1 vs. 88.5%, respectively; P=0.020). The 5-year
OS was also lower (78.9 and 96.7%, respectively, P=0.004). The
responsiveness to paclitaxel-based chemotherapy was significantly
worse when the TIMP-1 expression levels were high. The
aforementioned findings indicate that TIMP-1 may be a useful
predictive biomarker for chemotherapy resistance.
Low and moderately reliable biomarkers
(Table II)
Ferritin light chain (FTL)
Ferritin is a ubiquitous iron-binding protein. In
vertebrates, there are 2 types of apoferritin, which are assembled
from 24 subunits including light and heavy chain types. The ratio
between the ferritin heavy chain and FTL can vary greatly,
depending on the tissue type and cellular conditions (37). The increase in ferritin from different
cancer tissue samples exhibited a close correlation with disease
onset (38). Ricolleau et al
(39) investigated the utility of FTL
as a prognostic marker for lymph node metastasis-positive breast
cancer and determined an FTL cut-off level in tumors of 2.4. The
high FTL level group had a significantly lower metastasis-free
survival rate, indicating that FTL was an independent prognostic
marker (HR=1.30; 95% CI=1.10–1.50; P=0.001) (40).
Urokinase-type plasminogen activator
(uPA) and plasminogen activator type 1 inhibitor (PAI-1)
uPA, as a tumor-associated proteolytic factor, and
PAI-1 serve important roles in tumor invasion and metastasis
(41), and cell signaling, adhesion,
migration and proliferation (42). In
the final Chemo-N0 trial for the validation of The American Society
of Clinical Oncology-recommended biomarkers (1993–1998; n=647; 12
centers; median follow-up period 113 months; range between 5 and
167 months), high uPA/PAI-1 levels were correlated with
significantly lower DFS among breast cancer patients who did not
receive adjuvant treatment (HR=1.84; 95% CI=1.1–3.0; P=0.017) and
OS (HR=1.84; 95% CI=1.1–3.1; P=0.02). uPA/PAI-1 was also identified
as a prognostic factor for breast cancer in other studies (43).
C-reactive protein (CRP)
Serum CRP is a marker of acute inflammatory response
and is considered to be a prognostic indicator in breast cancer
(44,45). The Women's Healthy Eating and Living
study was a randomized comparative study examining the effect of a
diet high in vegetables and low in fat on the prevention of
premature mortality in women diagnosed with breast cancer. Serum
protein analysis of 2,023 of 3,088 eligible women showed that acute
inflammation (CRP ≥10 mg/l) was markedly suppressed by a high
vegetable/low fat diet, resulting in improved long-term survival
(46). Although the anti-oncological
effects remain unclear, CRP was identified as an independent
biomarker for prognosis of survival in breast cancer (HR=1.96; 95%
CI=1.22–3.13).
Chromosome 17 centromere enumeration
probe (Ch17CEP)
Chromosome 17 is the second densest chromosome in
the human genome and codes for several genes, including BRCA1 and
HER2 with important roles in breast cancer, as well as the
housekeeping DNA repair genes TP53, RAD51C and RAD52B (47). Ch17 centromeric region duplication
(Ch17CEP) is closely associated with HER2 amplification (48). Ch17CEP overlap is also a powerful
marker for genome instability in breast cancer and is correlated
with susceptibility to chemotherapy (48,49). In
novel endovascular access trial/BR9601 clinical trials, prognostic
factors were analyzed and categorized according to breast cancer
subtype. Although numerous factors were not associated with
subtype, Ch17CEP overlap was an independent prognostic factor for
DFS and OS for all subtypes. Ch17CEP overlap was identified as a
prognostic biomarker for breast cancer treated with
cyclophosphamide, methotrexate and fluorouracil therapy in
combination with epirubicin (HR=0.80; 95% CI=0.68–0.95; P=0.009)
(50).
Soluble human epidermal growth factor
receptor 2 (sHER2)
HER2 is a 185-kDa protein arising from the
intracellular, transmembrane and extracellular domains (ECD)
(51). The ECD is occasionally
spliced by metalloprotease, resulting in the release of sHER2 into
the peripheral circulation (52).
sHER2 is an important biomarker for HER2-positive breast cancer at
any stage (53). In the N9831
adjuvant breast cancer trials, early stage HER2-positivity was
identified as a biomarker of clinical outcome and disease
progression (54). Moreno-Aspitia
et al (55) evaluated sHER2
levels at the time of relapse using a sample from the N9831
clinical trial and found that DFS was lower in patients with sHER2
levels >15 ng/ml, compared with patients with lower levels of
sHER2 (HR=2.36; 95% CI=1.19–4.70; P=0.01). Therefore, in early
stage HER2-positive breast cancer, sHER2 was found to be a suitable
prognostic biomarker for relapse, and survival in relapsed
patients.
Mitotic arrest deficient like 1
(MAD1L1)
MAD1L1 is a checkpoint gene associated with
chromosomal instability. Abnormalities in MAD1L1 have been observed
in a number of cancer types, including colon and lung cancer
(56). Sun et al (57) analyzed MADL1 expression in breast
cancer tissues from 461 patients and normal breast tissue to
identify correlations between MAD1L1 expression and clinical
pathological characteristics. The results of the aforementioned
study revealed that OS was particularly worse in the high MAD1L1
expression group (HR=1.825; 95% CI=1.073–3.107; P=0.027). In
patients with high nuclear MAD1L1 expression subsequent to taxol
treatment, prognosis was poorer in the non-treated patients
(P=0.026). Nuclear MAD1L1 expression therefore appears to have
enhanced the treatment resistance and affected the prognosis of
breast cancer, demonstrating that MAD1L1-positive breast cancer was
not susceptible to taxol treatment.
Methylation of paired-like homeodomain
2 (PITX2P2)
C-phosphate-G islands located within the gene
regulatory site are associated with the suppression of gene
expression. The methylation of DNA dinucleotides in this gene is a
common early event subsequent to the onset of cancer (58–60).
Methylation patterns specific to tumor subtypes, including breast
cancer, are reportedly associated with clinical outcomes (61–63).
Several studies reported that PITX2 DNA methylation was associated
with a high risk of relapse in lymph node metastasis-positive,
hormone receptor-positive breast cancer patients undergoing whole
body adjuvant tamoxifen therapy (64,65). In a
cohort study of 241 lymph node metastasis-positive breast cancer
patients with a history of anthracycline treatment, PITX2P2
methylation (a subtype of PITX2 methylation) was associated with an
increased long-term relapse (HR=1.66; 95% CI=1.21–2.28; P=0.002)
and reduced survival rates (HR=1.47; 95% CI=1.11–1.96; P=0.0084)
(66).
Conclusions
In the present review of studies between 2009 and
2014, biomarkers were grouped according to reliability (high,
medium, or low). A total of 3 studies were retrieved from the
literature that classified Ki-67 as a high reliability biomarker.
The utility of Ki-67 as a biomarker has been re-evaluated in the
present study.
Of the high reliability biomarkers referred to in
the 9 studies included in the present review, 0 were assessed by
bioassays and only 1 mentioned biomarker measurement in peripheral
blood. Although the evaluation of proteins in peripheral blood is
relatively simple, in 2013, Johansson (7) reported that the identification of
biomarkers is difficult, as measurements of biomarkers require too
much quantification data. Therefore, the development of biomarkers
from peripheral blood presents a challenge for future studies.
Although a number of molecules were identified in
the present review, other markers, such as hormone receptors, were
not widely evaluated. The ratio of ER-α/ER-β expression,
βIII-tubulin and thyroid-stimulating hormone were identified as
oncological indicators in breast cancer. Thus, additional studies
of the correlations between the aforementioned biomarkers and
prognosis and treatment response may be useful.
This study hypothesizes that the most important
biomarkers of breast cancer are found in the blood, and that RARA,
uPA/PAI-1, CRP, sHER2 are good biomarkers in routine examination.
The authors highlight RARA in particular as an important biomarker
in breast cancer. Future studies on biomarkers are likely to
progress the understanding of the topic. For all biomarkers,
reliability is important, but for the development of useful
biomarkers, cost and ease of monitoring are crucial
considerations.
Acknowledgements
The authors would like to thank Drs. Tousei Ohmura,
Hidekazu Kameshima, and Yasuyo Suzuki for their help with preparing
this study and participating in valuable discussions. The present
study was partially supported by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology, Japan (grant nos. 26461921, 25293289, 24659592 and
50175634).
References
|
1
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thurlimann B and Senn HJ: Panel members:
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henderson IC and Patek AJ: The
relationship between prognostic and predictive factors in the
management of breast cancer. Breast Cancer Res Treat. 52:261–288.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lang JE, Wecsler JS, Press MF and Tripathy
D: Molecular markers for breast cancer diagnosis, prognosis and
targeted therapy. J Surg Oncol. 111:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niederreither K and Dollé P: Retinoic acid
in development: Towards an integrated view. Nat Rev Genet.
9:541–553. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hua S, Kittler R and White KP: Genomic
antagonism between retinoic acid and estrogen signaling in breast
cancer. Cell. 137:1259–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johansson HJ, Sanchez BC, Mundt F, Forshed
J, Kovacs A, Panizza E, Hultin-Rosenberg L, Lundgren B, Martens U,
Máthé G, et al: Retinoic acid receptor alpha is associated with
tamoxifen resistance in breast cancer. Nat Commun. 4:21752013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteban JM, Warsi Z, Haniu M, Hall P,
Shively JE and Chen S: Detection of intratumoral aromatase in
breast carcinomas. An immunohistochemical study with
clinicopathologic correlation. Am J Pathol. 140:337–343.
1992.PubMed/NCBI
|
|
9
|
Miki Y, Suzuki T and Sasano H:
Controversies of aromatase localization in human breast
cancer-stromal versus parenchymal cells. J Steroid Biochem Mol
Biol. 106:97–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellis MJ, Coop A, Singh B, Mauriac L,
Llombert-Cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady
C, et al: Letrozole is more effective neoadjuvant endocrine therapy
than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen
receptor-positive primary breast cancer: Evidence from a phase III
randomized trial. J Clin Oncol. 19:3808–3816. 2001.PubMed/NCBI
|
|
11
|
Eiermann W, Paepke S, Appfelstaedt J,
Llombart-Cussac A, Eremin J, Vinholes J, Mauriac L, Ellis M, Lassus
M, Chaudri-Ross HA, et al: Preoperative treatment of postmenopausal
breast cancer patients with letrozole: A randomized double-blind
multicenter study. Ann Oncol. 12:1527–1532. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ellis MJ, Tao Y, Luo J, A'Hern R, Evans
DB, Bhatnagar AS, Ross HA Chaudri, von Kameke A, Miller WR, Smith
I, et al: Outcome prediction for estrogen receptor-positive breast
cancer based on postneoadjuvant endocrine therapy tumor
characteristics. J Natl Cancer Inst. 100:1380–1388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellis MJ, Coop A, Singh B, Tao Y,
Llombart-Cussac A, Jänicke F, Mauriac L, Quebe-Fehling E,
Chaudri-Ross HA, Evans DB and Miller WR: Letrozole inhibits tumor
proliferation more effectively than tamoxifen independent of HER1/2
expression status. Cancer Res. 63:6523–6531. 2003.PubMed/NCBI
|
|
14
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
15
|
Ellis MJ, Miller WR, Tao Y, Evans DB, Ross
HA Chaudri, Miki Y, Suzuki T and Sasano H: Aromatase expression and
outcomes in the P024 neoadjuvant endocrine therapy trial. Breast
Cancer Res Treat. 116:371–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senger DR, Wirth DF and Hynes RO:
Transformed mammalian cells secrete specific proteins and
phosphoproteins. Cell. 16:885–893. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown LF, Papadopoulos-Sergiou A, Berse B,
Manseau EJ, Tognazzi K, Perruzzi CA, Dvorak HF and Senger DR:
Osteopontin expression and distribution in human carcinomas. Am J
Pathol. 145:610–623. 1994.PubMed/NCBI
|
|
18
|
Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu
N, Lan F, Liu Y, Yan S, Dong X and Cai L: Prognostic values of
osteopontin-c, E-cadherin and β-catenin in breast cancer. Cancer
Epidemiol. 37:985–992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ortiz-Martínez F, Perez-Balaguer A,
Ciprián D, Andrés L, Ponce J, Adrover E, Sánchez-Payá J, Aranda FI,
Lerma E and Peiró G: Association of increased osteopontin and
splice variant-c mRNA expression with HER2 and
triple-negative/basal-like breast carcinomas subtypes and
recurrence. Hum Pathol. 45:504–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bramwell VH, Tuck AB, Chapman JA, Anborgh
PH, Postenka CO, Al-Katib W, Shepherd LE, Han L, Wilson CF,
Pritchard KI, et al: Assessment of osteopontin in early breast
cancer: Correlative study in a randomised clinical trial. Breast
Cancer Res. 16:R82014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohno S, Chow LW, Sato N, Masuda N, Sasano
H, Takahashi F, Bando H, Iwata H, Morimoto T, Kamigaki S, et al:
Randomized trial of preoperative docetaxel with or without
capecitabine after 4 cycles of
5-fluorouracil-epirubicin-cyclophosphamide (FEC) in early-stage
breast cancer: Exploratory analyses identify Ki67 as a predictive
biomarker for response to neoadjuvant chemotherapy. Breast Cancer
Res Treat. 142:69–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denkert C, Loibl S, Muller BM, Eidtmann H,
Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et
al: Ki67 levels as predictive and prognostic parameters in
pretherapeutic breast cancer core biopsies: A translational
investigation in the neoadjuvant GeparTrio trial. Ann Oncol.
24:2786–2793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff finder: A
comprehensive and straightforward web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeCensi A, Guerrieri-Gonzaga A, Gandini S,
Serrano D, Cazzaniga M, Mora S, Johansson H, Lien EA, Pruneri G,
Viale G and Bonanni B: Prognostic significance of Ki-67 labeling
index after short-term presurgical tamoxifen in women with
ER-positive breast cancer. Ann Oncol. 22:582–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuroki M, Matsuo Y, Kinugasa T and
Matsuoka Y: Three different NCA species, CGM6/CD67, NCA-95 and
NCA-90, are comprised in the major 90 to 100-kDa band of
granulocyte NCA detectable upon SDS-polyacrylamide gel
electrophoresis. Biochem Biophys Res Commun. 182:501–506. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC cancer. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Poola I, Shokrani B, Bhatnagar R, DeWitty
RL, Yue Q and Bonney G: Expression of carcinoembryonic antigen cell
adhesion molecule 6 oncoprotein in atypical ductal hyperplastic
tissues is associated with the development of invasive breast
cancer. Clin Cancer Res. 12:4773–4783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsang JY, Kwok YK, Chan KW, Ni YB, Chow
WN, Lau KF, Shao MM, Chan SK, Tan PH and Tse GM: Expression and
clinical significance of carcinoembryonic antigen-related cell
adhesion molecule 6 in breast cancers. Breast Cancer Res Treat.
142:311–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
German S, Aslam HM, Saleem S, Raees A,
Anum T, Alvi AA and Haseeb A: Carcinogenesis of PIK3CA. Hered
Cancer Clin Pract. 11:52013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanker AB, Pfefferle AD, Balko JM, Kuba
MG, Young CD, Sánchez V, Sutton CR, Cheng H, Perou CM, Zhao JJ, et
al: Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors
and induces resistance to combinations of anti-HER2 therapies. Proc
Natl Acad Sci USA. 110:14372–14377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cizkova M, Dujaric ME, Lehmann-Che J,
Scott V, Tembo O, Asselain B, Pierga JY, Marty M, de Cremoux P,
Spyratos F and Bieche I: Outcome impact of PIK3CA mutations in
HER2-positive breast cancer patients treated with trastuzumab. Br J
Cancer. 108:1807–1809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hortobagyi GN and Holmes FA: Single-agent
paclitaxel for the treatment of breast cancer: An overview. Semin
Oncol. 23(1): Suppl 1. S4–S9. 1996.
|
|
33
|
Bergh J, Jönsson PE, Glimelius B and
Nygren P: SBU-group. Swedish Council of Technology Assessment in
Health Care. A systematic overview of chemotherapy effects in
breast cancer. Acta Oncol. 40:253–281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chromek M, Tullus K, Lundahl J and Brauner
A: Tissue inhibitor of metalloproteinase 1 activates normal human
granulocytes, protects them from apoptosis, and blocks their
transmigration during inflammation. Infect Immun. 72:82–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schrohl AS, Meijer-van Gelder ME,
Holten-Andersen MN, Christensen IJ, Look MP, Mouridsen HT, Brünner
N and Foekens JA: Primary tumor levels of tissue inhibitor of
metalloproteinases-1 are predictive of resistance to chemotherapy
in patients with metastatic breast cancer. Clin Cancer Res.
12:7054–7058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu D, Zha X, Hu M, Tao A, Zhou H, Zhou X
and Sun Y: High expression of TIMP-1 in human breast cancer tissues
is a predictive of resistance to paclitaxel-based chemotherapy. Med
Oncol. 29:3207–3215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arosio P, Yokota M and Drysdale JW:
Structural and immunological relationships of isoferritins in
normal and malignant cells. Cancer Res. 36:1735–1739.
1976.PubMed/NCBI
|
|
38
|
Levi S, Yewdall SJ, Harrison PM,
Santambrogio P, Cozzi A, Rovida E, Albertini A and Arosio P:
Evidence of H- and L-chains have co-operative roles in the
iron-uptake mechanism of human ferritin. Biochem J. 288:591–596.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ricolleau G, Charbonnel C, Lodé L,
Loussouarn D, Joalland MP, Bogumil R, Jourdain S, Minvielle S,
Campone M, Déporte-Fety R, et al: Surface-enhanced laser
desorption/ionization time of flight mass spectrometry protein
profiling identifies ubiquitin and ferritin light chain as
prognostic biomarkers in node-negative breast cancer tumors.
Proteomics. 6:1963–1975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jézéquel P, Campion L, Spyratos F,
Loussouarn D, Campone M, Guérin-Charbonnel C, Joalland MP, André J,
Descotes F, Grenot C, et al: Validation of tumor-associated
macrophage ferritin light chain as a prognostic biomarker in
node-negative breast cancer tumors: A multicentric 2004 national
PHRC study. Int J Cancer. 131:426–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duffy MJ: Urokinase plasminogen activator
and its inhibitor, PAI-1, as prognostic markers in breast cancer:
From pilot to level 1 evidence studies. Clin Chem. 48:1194–1197.
2002.PubMed/NCBI
|
|
42
|
Mengele K, Napieralski R, Magdolen V,
Reuning U, Gkazepis A, Sweep F, Brünner N, Foekens J, Harbeck N and
Schmitt M: Characteristics of the level-of-evedence-1 disease
forcast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev
Mol Diagn. 10:947–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harbeck N, Schmitt M, Meisner C, Friedel
C, Untch M, Schmidt M, Sweep CG, Lisboa BW, Lux MP, Beck T, et al:
Ten-year analysis of the prospective multicentre Chemo-N0 trial
validates American society of clinical oncology (ASCO)-recommended
biomarkers uPA and PAI-1 for therapy decision making in
node-negative breast cancer patients. Eur J Cancer. 49:1825–1835.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Favaro E, Amadori A and Indraccolo S:
Cellular interactions in the vascular niche: Implications in the
regulation of tumor dormancy. APMIS. 116:648–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Allin KH and Nordestgaard BG: Elevated
C-reactive protein in the diagnosis, prognosis, and cause of
cancer. Crit Rev Clin Lab Sci. 48:155–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Villaseñor A, Flatt SW, Marinac C,
Natarajan L, Pierce JP and Patterson RE: Postdiagnosis C-reactive
protein and breast cancer survivorship: Findings from the WHEL
study. Cancer Epidemiol Biomarkers Prev. 23:189–199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zody MC, Garber M, Adams DJ, Sharpe T,
Harrow J, Lupski JR, Nicholson C, Searle SM, Wilming L, Young SK,
et al: DNA sequence of human chromosome 17 and analysis of
rearrangement in the human lineage. Nature. 440:1045–1049. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Watters AD, Going JJ, Cooke TG and
Bartlett JM: Chromosome 17 aneusomy is associated with poor
prognostic factors in invasive breast carcinoma. Breast Cancer Res
Treat. 77:109–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Corzo C, Bellosillo B, Corominas JM,
Salido M, Coll MD, Serrano S, Albanell J, Solé F and Tusquets I:
Does polysomy of chromosome 17 have a role in ERBB2 and
topoisomerase IIalpha expression? Gene, mRNA and protein
expression: A comprehensive analysis. Tumor Biol. 28:221–228. 2007.
View Article : Google Scholar
|
|
50
|
Earl HM, Hiller L, Dunn JA, Vallier AL,
Bowden SJ, Jordan SD, Blows F, Munro A, Bathers S, Grieve R, et al:
Adjuvant epirubicin followed by cyclophosphamide, methotrexate and
fluorouracil (CMF) vs CMF in early breast cancer: Results with over
7 years median follow-up from the randomised phase III NEAT/BR9601
trials. Br J Cancer. 107:1257–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sias PE, Kotts CE, Vetterlein D, Shepard M
and Wong WL: ELISA for quantitation of the extracellular domain of
p185HER2 in biological fluids. J Immunol Methods. 132:73–80. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Codony-Servat J, Albanell J,
Lopez-Talavera JC, Arribas J and Baselga J: Cleavage of the HER2
ectodomain is a pervanadate-activable process that is inhibited by
the tissue inhibitor of metalloproteases-1 in breast cancer cells.
Cancer Res. 59:1196–1201. 1999.PubMed/NCBI
|
|
53
|
Tse C, Gauchez AS, Jacot W and Lamy PJ:
HER2 shedding and serum HER2 extracellular domain: Biology and
clinical utility in breast cancer. Cancer Treat Rev. 38:133–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Moreno-Aspitia A, Hillman DW, Dyar SH,
Tenner KS, Gralow J, Kaufman PA, Davidson NE, Lafky JM, Reinholz
MM, Lingle WL, et al: Soluble human epidermal growth factor
receptor 2 (HER2) levels in patients with HER2-positive breast
cancer receiving chemotherapy with or without trastuzumab: Results
from north central cancer treatment group adjuvant trial N9831.
Cancer. 119:2675–2682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tsukasaki K, Miller CW, Greenspun E,
Eshaghian S, Kawabata H, Fujimoto T, Tomonaga M, Sawyers C, Said JW
and Koeffler HP: Mutations in the mitotic check point gene, MAD1L1,
in human cancers. Oncogene. 20:3301–3305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun Q, Zhang X, Liu T, Liu X, Geng J, He
X, Liu Y and Pang D: Increased expression of mitotic arrest
deficient-like 1 (MAD1L1) is associated with poor prognosis and
insensitive to Taxol treatment in breast cancer. Breast Cancer Res
Treat. 140:323–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Baylin SB and Herman JG: DNA
hypermethylation in tumorigenesis: Epigenetics joins genetics.
Trends Genet. 16:168–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jones PA: DNA methylation errors and
cancer. Cancer Res. 56:2463–2467. 1996.PubMed/NCBI
|
|
60
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Frühwald MC: DNA methylation patterns in
cancer: Novel prognostic indicators? Am J Pharmacogenomics.
3:245–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Laird PW: The power and the promise of DNA
methylation markers. Nat Rev Cancer. 3:253–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maier S, Nimmrich I, Koenig T,
Eppenberger-Castori S, Bohlmann I, Paradiso A, Spyratos F, Thomssen
C, Mueller V, Nährig J, et al: DNA-methylation of the homeodomain
transcription factor PITX2 reliably predicts risk of distant
disease recurrence in tamoxifen-treated, node-negative breast
cancer patients-Technical and clinical validation in a multi-centre
setting in collaboration with the European Organisation for
Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J
Cancer. 43:1679–1686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Martens JW, Nimmrich I, Koenig T, Look MP,
Harbeck N, Model F, Kluth A, Bolt-de Vries J, Sieuwerts AM,
Portengen H, et al: Association of DNA methylation of phosphoserine
aminotransferase with response to endocrine therapy in patients
with recurrent breast cancer. Cancer Res. 65:4101–4117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Widschwendter M, Siegmund KD, Müller HM,
Fiegl H, Marth C, Müller-Holzner E, Jones PA and Laird PW:
Association of breast cancer DNA methylation profiles with hormone
receptor status and response to tamoxifen. Cancer Res.
64:3807–3813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hartmann O, Spyratos F, Harbeck N,
Dietrich D, Fassbender A, Schmitt M, Eppenberger-Castori S,
Vuaroqueaux V, Lerebours F, Welzel K, et al: DNA methylation
markers predict outcome in node-positive, estrogen
receptor-positive breast cancer with adjuvant anthracycline-based
chemotherapy. Clin Cancer Res. 15:315–323. 2009. View Article : Google Scholar : PubMed/NCBI
|