Introduction
Cancer cells commonly exhibit a malignant metabolic
phenotype, which is characterized by increased rates of glycolysis,
glutaminolysis and de novo synthesis of fatty acids (FAs)
compared with normal cells. These metabolic alterations result from
diverse gain-of-function mutations in oncogenes and
loss-of-function of tumor suppressor genes, which aid cancer cells
to thrive under various environmental conditions (1).
Glucose and glutamine supply the majority of the
necessary carbon and nitrogen for the synthesis of macromolecules,
energy and reducing equivalents to support cell growth through
glycolysis and glutaminolysis (2).
Lipogenesis is a third metabolic feature of cancer. In general,
malignant cells synthetize de novo FAs instead of taking
them up from the circulation, and malignant cells frequently
overexpress FA synthase (FASN) (3).
For de novo synthesis of FAs, glucose and glutamine supply
citrate. Glucose is converted to acetyl-coenzyme A (CoA) in the
mitochondrial matrix to synthesize citrate in the tricarboxylic
acid (TCA) cycle, whereas glutamine supplies carbon in the form of
mitochondrial oxaloacetate to maintain citrate production in the
first step of the TCA cycle (4).
Thus, the metabolism of glutamine and glucose is orchestrated to
support the production of acetyl-CoA and NADPH required for fatty
acid synthesis (4).
Despite the strong rationale for developing a
combination of drugs to simultaneously target these three key
processes in malignant cells, to the best of our knowledge, there
is no preclinical in vivo evidence supporting the antitumor
activity of this triple targeting, despite the availability of
well-characterized pharmacological inhibitors of these enzymes
(5). Among anti-glycolytic and
anti-glutaminolytic drugs, lonidamine (LND) and
6-diazo-5-oxo-L-norleucine (DON) are well-known inhibitors of
hexokinase II (HK-II) and glutaminase K, respectively, which have
previously been clinically evaluated (5). Regarding lipogenesis, a number of
experimental compounds have been developed; however, none have
reached clinical trials (6). Among
these, orlistat has shown promising activity in a number of
malignancies due to its ability to inhibit FASN, which is
responsible for the de novo synthesis of FA (7,8). It was
previously reported that LND, DON and orlistat inhibit cell
viability in a number of human cancer cell lines, and that these
drugs are synergistic in vitro (9). The present study demonstrated that this
triple combination is feasible and effective against tumor models
in mice.
Materials and methods
Cell lines, drugs and
preparations
Human colon cancer SW480 and mouse colon cancer
CT26.WT cell lines were obtained from the American Type Culture
Collection (Manassas, VA, USA) and cultured in DMEM-F15 and
RPMI-1640 respectively, supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), in a
humidified 5% CO2 atmosphere at 37°C. LND, DON and
orlistat were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Stock solutions were prepared using dimethyl
sulfoxide, water and ethanol for LND (11 mg/ml), DON (16 mg/ml) and
orlistat (200 mg/ml) respectively.
Doses
To investigate whether the combined administration
of these drugs was clinically feasible and following assessment of
existing pharmacokinetic data in clinical studies of LND and DON
(5), the human doses of LND and DON
used in clinical trials were translated to mouse doses using the
formula: Mouse equivalent dose=human dose (mg/kg) × human
Km/mouse Km, where the human and mouse
Km was 37 and 3, respectively, as reported by
Reagan-Shaw et al (10). As
shown in Table I, the human doses of
LND and DON, each used as a single agent, were as follows: 450
mg/daily (6.3 mg/kg day assuming a 70 kg patient) for LND and a 480
mg/m2 DON total dose (divided between days 1, 2 and 3).
DON is prescribed in humans by m2; therefore this dose
was first converted to mg/kg by assuming 1.7 m2 of body
surface area, which results in 825 mg/70 kg=11.8 mg/kg. Thus, using
these human doses to calculate the doses for mice weighting 20 g,
three different schedules were administered (Table I). For orlistat, which is used
systemically in cancer models, there exists only preclinical
information, and in the majority of cases it is used at 240
mg/m2 in mice (7,8). Therefore, this dose was used in
schedules 1 and 2, but a dose of 360 mg/m2 was used in
schedule 3 to gain insight into its tolerability beyond common
doses used in mice.
 | Table I.Dose schedule of LND, DON and orlistat
used in mice. |
Table I.
Dose schedule of LND, DON and orlistat
used in mice.
| Drug | Schedule 1 | Schedule 2 | Schedule 3 | Human dose |
|---|
| LND | 25 mg/kg, 0.5
mg/day | 5 mg/kg, 0.1
mg/day | 25 mg/kg, 0.5
mg/day | 6.3 mg/kg, 450
mg/day |
| DON | 36.2 mg/kg, 0.75 mg
divided between days 1, 5 and 9 | 72.5 mg/kg, 1.5 mg
divided between days 1, 5 and 9 | 12.6 mg/kg, 0.25 mg
divided between days 1, 5 and 9 | 11.8 mg/kg divided
between days 1, 2 and 3 |
| Orlistat | 240 mg/kg daily | 240 mg/kg daily | 360 mg/kg
daily | Unknown |
Tolerability of the triple combination
in vivo
To study these schedules of the triple combination
that are tolerable when injected into healthy mice, groups of
6-week-old BALB/c female mice (6 mice per group, 36 mice in total;
Harlan Laboratories, Mexico City, Mexico) were treated with the
triple combination of LND, DON and orlistat for a 21-day cycle.
Mice were allowed to acclimatize for 1 week and kept in a 12:12
light-dark cycle with access to food and water ad libitum.
The three treatment schedules are shown in Table I. The three drugs were
intraperitoneally administered, with at least 3 h between each
injection and careful skin disinfection to avoid infectious
peritonitis. The total volume of injection was <20 µl for each
drug. The control group was injected with the vehicle of each drug,
at identical volumes to the treatment groups. Mice were weighed and
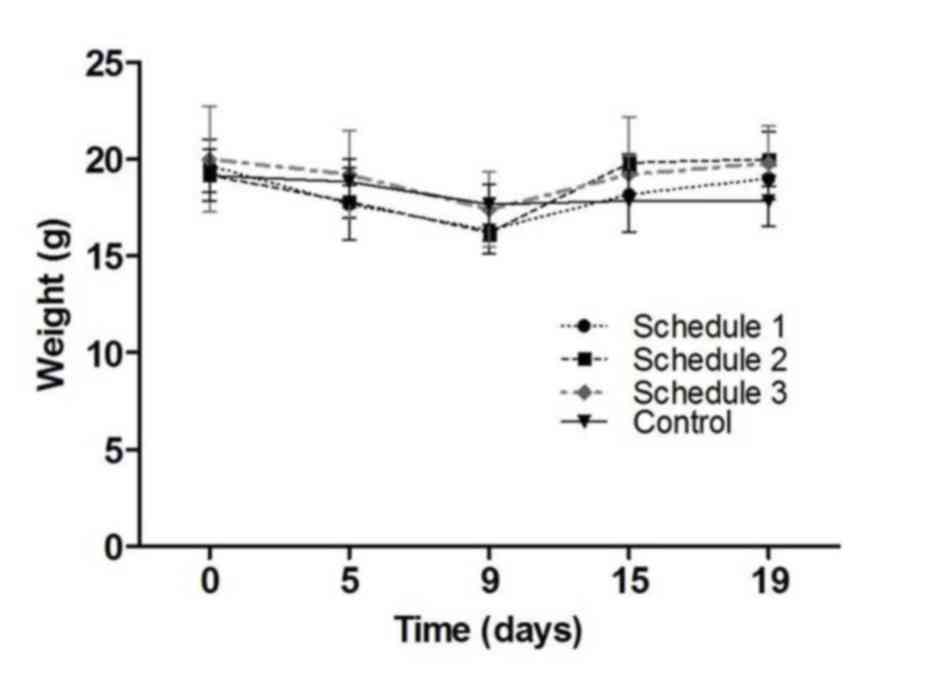
clinically inspected on days 0, 5, 9, 15 and 19.
Antitumor effect of the triple
combination in vivo in a syngeneic model
Six week-old BALB/c female mice (6 mice per group,
12 in total) were obtained from Harlan Laboratories. Mice were
allowed to acclimatize for 1 week prior to starting the
experiments. Housing conditions included access to food and water
ad libitum in a 12:12 light-dark cycle. Handling was
performed inside a laminar flow cabinet, and a total of
4×105 CT26.WT cells were injected in one flank. The
treatment commenced 2 weeks subsequent to inoculation, when the
tumors measured ~100 mm3. The treatment consisted of
intraperitoneal injection of 0.5 mg LND daily (total dose), 0.25 mg
DON on days 1, 5 and 9 (total dose, 0.75 mg), and 240 mg/kg
orlistat daily, which constituted schedule 1 of the tolerability
experiment, in a cycle of 21 days. Drug administration was
performed as aforementioned. Animals were weighed, clinically
inspected and tumors were measured with electronic calipers, and
the tumor volume was estimated using the formula
axb2x(π/6)=V (mm3), where a is the major
diameter, b is the minor diameter and V is the volume. At the end
of treatment the mice were sacrificed in a CO2 chamber
and necropsied. Tumors were dissected and weighed. Visual
inspection of major organs was performed.
Antitumor effect of the triple
combination in an allogeneic model
Two groups six-week-old BALB/c nu/nu female mice,
with 6 mice per group, were obtained from Harlan Laboratories.
Acclimatization, housing, feeding and manipulation were performed
as aforementioned. Nude mice were injected with 1×106
SW480 cells in each flank (total dose, 2×106 cells). The
treatment was started 2 weeks subsequent to inoculation, when the
tumors were ~250 mm3 in size. The treatment consisted of
intraperitoneal injection of 0.5 mg LND daily (total dose), 0.25 mg
DON on days 1, 5 and 9 (total dose, 0.75 mg) and 240 mg/kg orlistat
daily in a 21-day cycle, which constituted schedule 1 of the
tolerability experiment. Drug administration was performed as
aforementioned. Animals were weighed, clinically inspected and
tumors were measured with electronic calipers, and the tumor volume
was estimated using the formula axb2x(π/6)=V
(mm3), where a is the major diameter, b is the minor
diameter and V is the volume. At the end of treatment the mice were
sacrificed in a CO2 chamber and necropsied. Tumors were
dissected and weighed. Visual inspection of major organs was
performed.
Ethics statement
All animal studies were designed to reduce the
suffering of the animals, and were performed in compliance with the
policies of the Institutional Research Ethics Board and Animal Care
Committee of the Instituto Nacional de Cancerología (Mexico City,
Mexico) (permit numbers, CA006/CB595/10 and INCAN/CC/010/10).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical differences in weight among the groups of mice treated
with different schedules were evaluated using analysis of variance,
and the tumor volumes at each time and the final weight of the
tumors between the control and treated groups were evaluated with
paired Student's t-test. Statistical analyses were conducted using
SPSS (SPSS, Inc., Chicago, IL, USA).
Results
Tolerability in vivo
Previous results from our laboratory (9) showed that in vitro treatment with
the combination of LND, DON and orlistat is highly synergistic and
has increased antitumor effects compared with treatment with each
drug alone. Additionally, it was found that total doses of 0.25 mg
LND and 0.25 mg DON plus 240 mg/m2 orlistat are well
tolerated in mice. To confirm these results, additional doses were
tested using the human equivalent dose in mice as a reference, as
shown in Table I. The three schedules
tested were shown to be well tolerated. There was a transient
decrease in weight during the first 9 days of treatment, but weight
was recuperated by day 19. No statistically significant differences
were found (P=0.788; Fig. 1). Mice
showed no hair frizzing or hypoactivity. No other clinical signs of
toxicity were observed.
Antitumor effects in the syngeneic
model
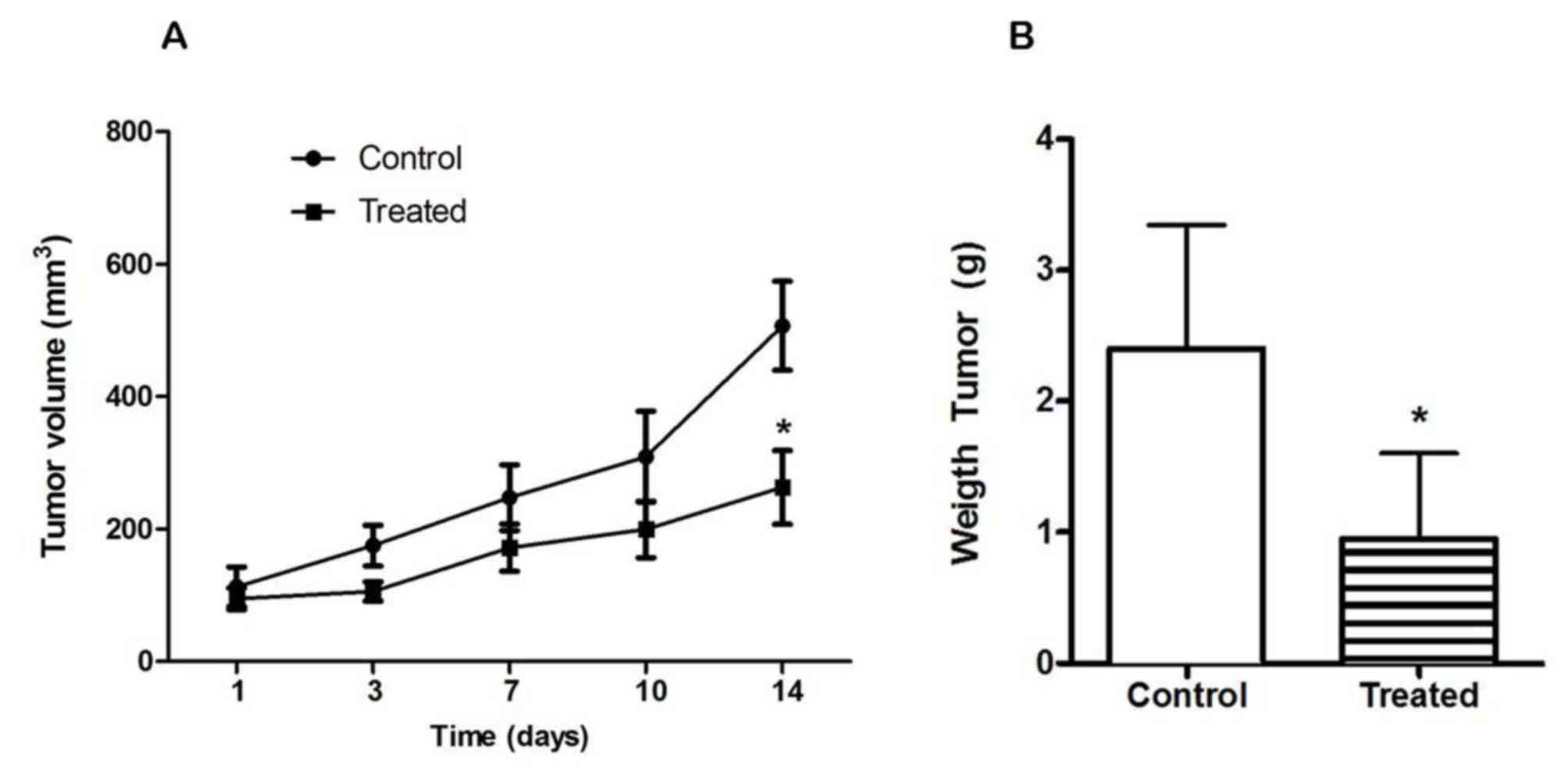
Based on these results, schedule 1 was chosen (total
doses of 0.5 mg LND daily and 0.75 mg DON divided over three days).
The treatment was well tolerated and tumor volumes (P=0.0455) and
tumor weights (P=0.0005) were significantly lower in the treated
animals; however, animals in the control group showed marked
hypoactivity, hair frizzing and weight loss after day 10.
Therefore, animals were sacrificed at day 14 (Fig. 2A and B).
Antitumor effects in the allogeneic
model
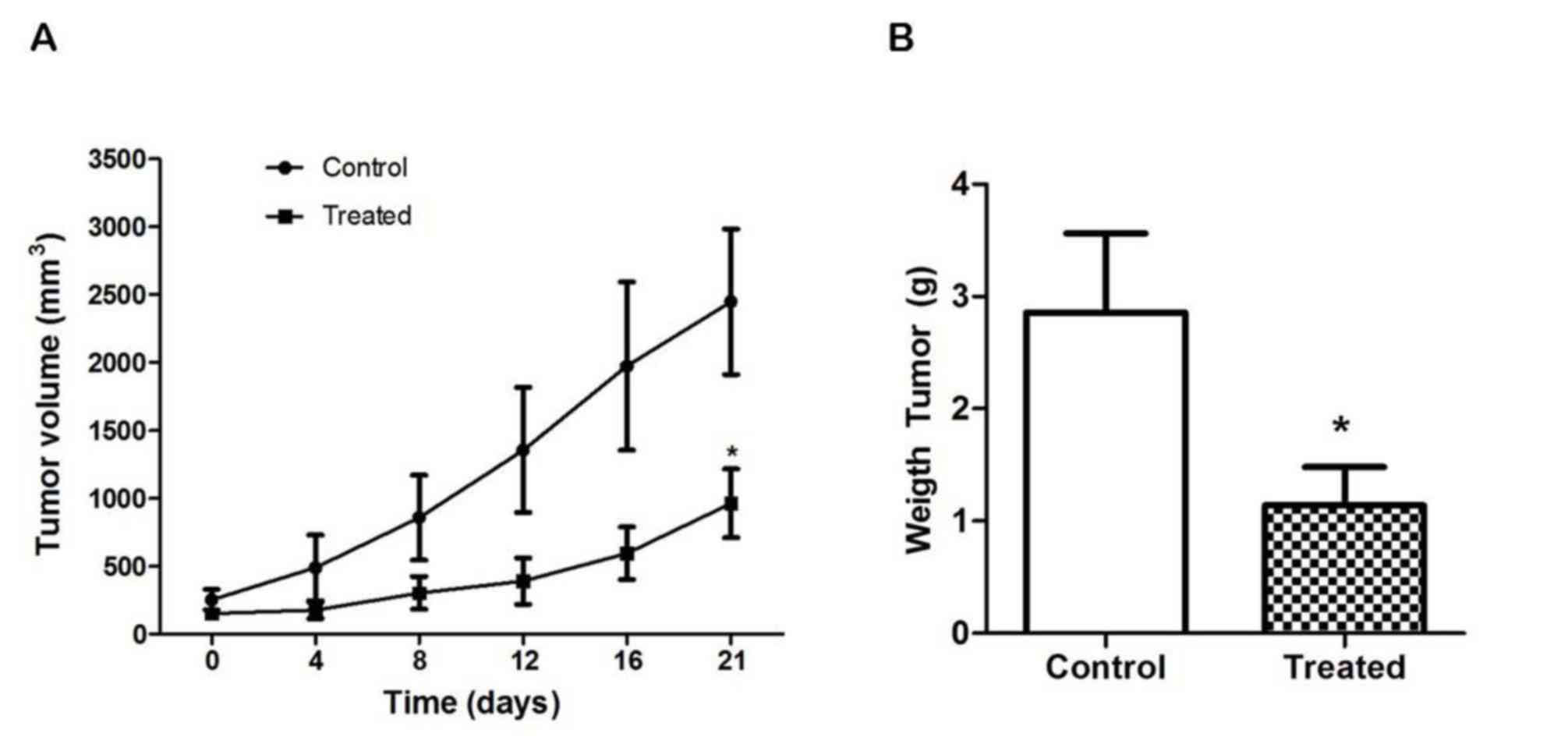
Treatment with schedule 1 was also well tolerated in
the nude mice injected with the human colon cancer SW480 cell line.
Total weight was not significantly different between the two
groups, and no evident signs of toxicity were noted. However, tumor
volumes were >2-fold lower in the treated animals and showed
extensive areas of necrosis. As shown in Fig. 3A and B, curves of volume began to
separate between day 4 and the end of treatment (P=0.0351), and the
tumor weights were significantly decreased in the treated animals
(P=0.0002).
Discussion
The results of the present study show that the
systemic administration of a pharmacological combination of
inhibitors of glycolysis, glutaminolysis and the de novo
synthesis of FAs is not only well tolerated, as demonstrated by no
changes in body weight and no evident signs of toxicity, but that
exerts antitumor effects in syngeneic mice injected with a murine
colon carcinoma and in nude mice bearing human colon carcinoma
cells.
The three most common, or at least most studied,
metabolic alterations of cancer cells are glycolysis,
glutaminolysis and the de novo synthesis of FAs (1–3). The
increased activities of these pathways are therefore natural
targets to attack the malignant metabolic phenotype. However,
antitumor strategies targeting the malignant metabolic phenotype
attempt to target these processes separately (11–13).
A number of preclinical studies using drugs to
target these pathways demonstrate that they are effective (14–17). Among
glycolytic inhibitors, a number of drugs are being evaluated in
experimental systems, as reviewed by Ganapathy-Kanniappan and
Geschwind (14). However, only LND,
2-deoxy-D-glucose and dichloracetate have reached clinical trials,
with modest results as single agents or in combination with
chemotherapy or radiation (15–17). In
particular, LND, the HK-II inhibitor used in the present study has
been widely investigated for the treatment of solid tumors with
encouraging results in phase II–III trials for the treatment of
advanced breast, ovarian and lung cancer (15). Regarding glutaminolysis inhibitors,
the 3 diazo analogs of L-glutamine, azaserine, DON and azotomycin,
showed clinical antitumor activity (18), but have not been further studied, with
the exception of DON, which was used with recombinant glutaminase
and showed promising results (19).
Newer selective agents against glutaminase are being developed. One
of these new agents, CB-839, has recently entered into clinical
trials (ClinicalTrial.gov identifiers
NCT02071862, NCT02071888 and NCT02071927). No clinical trials in
cancer have been undertaken with FASN inhibitors. Among FASN
inhibitors, orlistat shows promising activity in vitro and
in vivo in a number of malignancies due to its ability to
inhibit FASN, which is responsible for the de novo synthesis
of FAs (8).
To the best of our knowledge, no preclinical studies
have been performed using a drug combination concurrently targeting
these 3 metabolic alterations beyond our previous study (9). The most similar study was reported in
1993, in which the combination of DON and 2-deoxy-D-glucose led to
marked inhibition of glutamine oxidation and glycolysis, which was
accompanied by increased cytotoxicity against the human myeloid
TPH-1 cell line and freshly cultured myeloid blast cultures
obtained from a patient (20). Thus,
the results of the present study support the hypothesis that the
pharmacological blockade of the three main metabolic pathways is
feasible and exhibits antitumor activity.
The results of the present study regarding the doses
and schedule used may be observed as an approximation only. In
regard to orlistat, no preclinical pharmacokinetic studies have
been reported. Effective antitumor doses in vitro are
between 25 and 100 µM (8) and when
used in mice, the most frequently administered dose is 240 mg/kg.
Kridel et al (7) reported peak
blood levels of orlistat to be ~10 µM 2 h subsequent to a single
intraperitoneal administration at 155 mg/kg in mice (7). These results suggest that at 240 mg/kg,
therapeutic effective dose could be achieved in plasma; however,
this must be confirmed by pharmacokinetic studies. Notably, new
formulations of micellar nanoparticles of orlistat for cancer
treatment are in development (21),
as the systemic levels of orlistat used orally for obesity are
<10 ng/ml (0.02 µM) due to its poor absorption (22). For determining the dose of LND and DON
used in mice, the human dose (10)
was translated to a mouse dose, which is also an approximation.
However, pharmacokinetic analyses of the 3 drugs should be
performed to corroborate the appropriateness of the doses and to
determine potential pharmacokinetic interactions among them. It
should be noted that the doses used here for LND and DON are well
below those used in published preclinical trials (5). Thus, in the 3 schedules, doses of 25, 5
and 25 mg/kg LND were used compared with 50 and 100 mg/kg in the
literature (23,24). Similarly, doses of 36.2, 72.5 and 12.6
mg/kg DON were used, whereas in the literature the mean dose is
15.03 mg/kg (range, 0.02–100 mg/kg), with a mean of 13 days (range,
9–28 days) of administration in a 28-day cycle (25). The present data suggest that the
strong synergy observed in vitro at drug concentrations well
below those used separately (9) could
also occur in vivo. It also should be noted that the
combination was effective in the syngeneic (and low tumor burden)
and allogeneic (and high tumor burden) groups, suggesting that this
treatment is effective in murine and human colon carcinomas, as
well as in low and high tumor burdens. This was not unexpected,
since metabolic reprogramming in the tumor use of glucose,
glutamine and FAs, to different extents, is a common feature of
cancer cells.
In summary, the present results support the
hypothesis that targeting cancer metabolism by simultaneously
inhibiting 3 key metabolic pathways may actually have a wide
therapeutic window (26), as no
unacceptable effects in mice were observed when treated at
translated doses slightly below to those used in patients for LND
and DON administered separately. Notably, DON and LND are drugs
that are currently being re-studied (27–29), while
formulations of orlistat suitable for systemic administration have
been investigated (19). Thus, triple
pharmacological metabolic blockade of the malignant phenotype
appears feasible and promising for cancer therapy. However, despite
the target inhibition of the three drugs used here being strongly
demonstrated in other preclinical models (5,6),
additional studies are required to confirm whether the triple
metabolic blockade with this drug combination changes the rate of
oxidation of glucose, glutamine and fatty acids in tumors.
Acknowledgements
The present study was supported by CONACyT (grant
nos. 140654 and SB0771).
References
|
1
|
Chen JQ and Russo J: Dysregulation of
glucose transport, glycolysis, TCA cycle and glutaminolysis by
oncogenes and tumor suppressors in cancer cells. Biochim Biophys
Acta. 1826:370–384. 2012.PubMed/NCBI
|
|
2
|
Moncada S, Higgs EA and Colombo SL:
Fulfilling the metabolic requirements for cell proliferation.
Biochem J. 446:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–767. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan NL and Seyfried TN: Influence of serum
and hypoxia on incorporation of [(14)C]-D-glucose or
[(14)C]-L-glutamine into lipids and lactate in murine glioblastoma
cells. Lipids. 50:1167–1184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cervantes-Madrid D, Romero Y and
Dueñas-González A: Reviving lonidamine and
6-Diazo-5-oxo-L-norleucine to be used in combination for metabolic
cancer therapy. Biomed Res Int. 2015:6904922015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lupu R and Menendez JA: Pharmacological
inhibitors of fatty acid synthase (FASN)-catalyzed endogenous fatty
acid biogenesis: A new family of anti-cancer agents? Curr Pharm
Biotechnol. 7:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kridel SJ, Axelrod F, Rozenkrantz N and
Smith JW: Orlistat is a novel inhibitor of fatty acid synthase with
antitumor activity. Cancer Res. 64:2070–2075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flavin R, Peluso S, Nguyen PL and Loda M:
Fatty acid synthase as a potential therapeutic target in cancer.
Future Oncol. 6:551–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cervantes-Madrid D and Dueñas-González A:
Antitumor effects of a drug combination targeting glycolysis,
glutaminolysis and de novo synthesis of fatty acids. Oncol Rep.
34:1533–1542. 2015.PubMed/NCBI
|
|
10
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elf SE and Chen J: Targeting glucose
metabolism in patients with cancer. Cancer. 120:774–780. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin L, Alesi GN and Kang S: Glutaminolysis
as a target for cancer therapy. Oncogene. 35:3619–3625. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deepa PR, Vandhana S, Jayanthi U and
Krishnakumar S: Therapeutic and toxicologic evaluation of
anti-lipogenic agents in cancer cells compared with non-neoplastic
cells. Basic Clin Pharmacol Toxicol. 110:494–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Cosimo S, Ferretti G, Papaldo P,
Carlini P, Fabi A and Cognetti F: Lonidamine: Efficacy and safety
in clinical trials for the treatment of solid tumors. Drugs Today
(Barc). 39:157–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dwarakanath BS, Singh D, Banerji AK, Sarin
R, Venkataramana NK, Jalali R, Vishwanath PN, Mohanti BK, Tripathi
RP, Kalia VK and Jain V: Clinical studies for improving
radiotherapy with 2-deoxy-D-glucose: Present status and future
prospects. J Cancer Res Ther. 5:(Suppl 1). S21–S26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garon EB, Christofk HR, Hosmer W, Britten
CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S,
Kabbinavar F, et al: Dichloroacetate should be considered with
platinum-based chemotherapy in hypoxic tumors rather than as a
single agent in advanced non-small cell lung cancer. J Cancer Res
Clin Oncol. 140:443–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Catane R, Von Hoff DD, Glaubiger DL and
Muggia FM: Azaserine, DON, and azotomycin: Three diazo analogs of
L-glutamine with clinical antitumor activity. Cancer Treat Rep.
63:1033–1038. 1979.PubMed/NCBI
|
|
19
|
Unger C, Mueller C, Bausch MP,
Krzemieniecki K, Ochenduszko S, Wilk B, Jaeger E and Al-Batran S: A
phase I schedule optimization study of pegylated glutaminase
(PEG-PGA) plus 6-diazo-5-oxo-l-norleucine (DON) in patients (pts)
with advanced solid tumors. J Clin Oncol. 29:(Suppl) abstr 3049.
2011.
|
|
20
|
Griffiths M, Keast D, Patrick G, Crawford
M and Palmer TN: The role of glutamine and glucose analogues in
metabolic inhibition of human myeloid leukaemia in vitro. Int J
Biochem. 25:1749–1755. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paulmurugan R, Bhethanabotla R, Mishra K,
Devulapally R, Foygel K, Sekar TV, Ananta JS, Massoud TF and Joy A:
Folate receptor targeted polymeric micellar nanocarriers for
delivery of orlistat as a repurposed drug against triple negative
breast cancer. Mol Cancer Ther. 15:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhi J, Mulligan TE and Hauptman JB:
Long-term systemic exposure of orlistat, a lipase inhibitor, and
its metabolites in obese patients. J Clin Pharmacol. 39:41–46.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Cesare M, Pratesi G, Giusti A, Polizzi
D and Zunino F: Stimulation of the apoptotic response as a basis
for the therapeutic synergism of lonidamine and cisplatin in
combination in human tumour xenografts. Br J Cancer. 77:434–439.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nath K, Nelson DS, Heitjan DF, Leeper DB,
Zhou R and Glickson JD: Lonidamine induces intracellular tumor
acidification and ATP depletion in breast, prostate and ovarian
cancer xenografts and potentiates response to doxorubicin. NMR
Biomed. 28:281–290. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ovejera AA, Houchens DP, Catane R,
Sheridan MA and Muggia FM: Efficacy of 6-diazo-5-oxo-L-norleucine
and N-(N-gamma-glutamyl-6-diazo-5-oxo-norleucinyl)-6-diazo-5-
oxo-norleucine against experimental tumors in conventional and nude
mice. Cancer Res. 39:3220–3224. 1979.PubMed/NCBI
|
|
26
|
Heiden MG Vander: Targeting cancer
metabolism: A therapeutic window opens. Nat Rev Drug Discov.
10:671–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo L, Shestov AA, Worth AJ, Nath K,
Nelson DS, Leeper DB, Glickson JD and Blair IA: Inhibition of
mitochondrial complex II by the anti-cancer agent lonidamine. J
Biol Chem. 291:42–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Assanhou AG, Li W, Zhang L, Xue L, Kong L,
Sun H, Mo R and Zhang C: Reversal of multidrug resistance by
co-delivery of paclitaxel and lonidamine using a TPGS and
hyaluronic acid dual-functionalized liposome for cancer treatment.
Biomaterials. 73:284–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olsen RR, Mary-Sinclair MN, Yin Z and
Freeman KW: Antagonizing Bcl-2 family members sensitizes
neuroblastoma and Ewing's sarcoma to an inhibitor of glutamine
metabolism. PLoS One. 10:e01169982015. View Article : Google Scholar : PubMed/NCBI
|