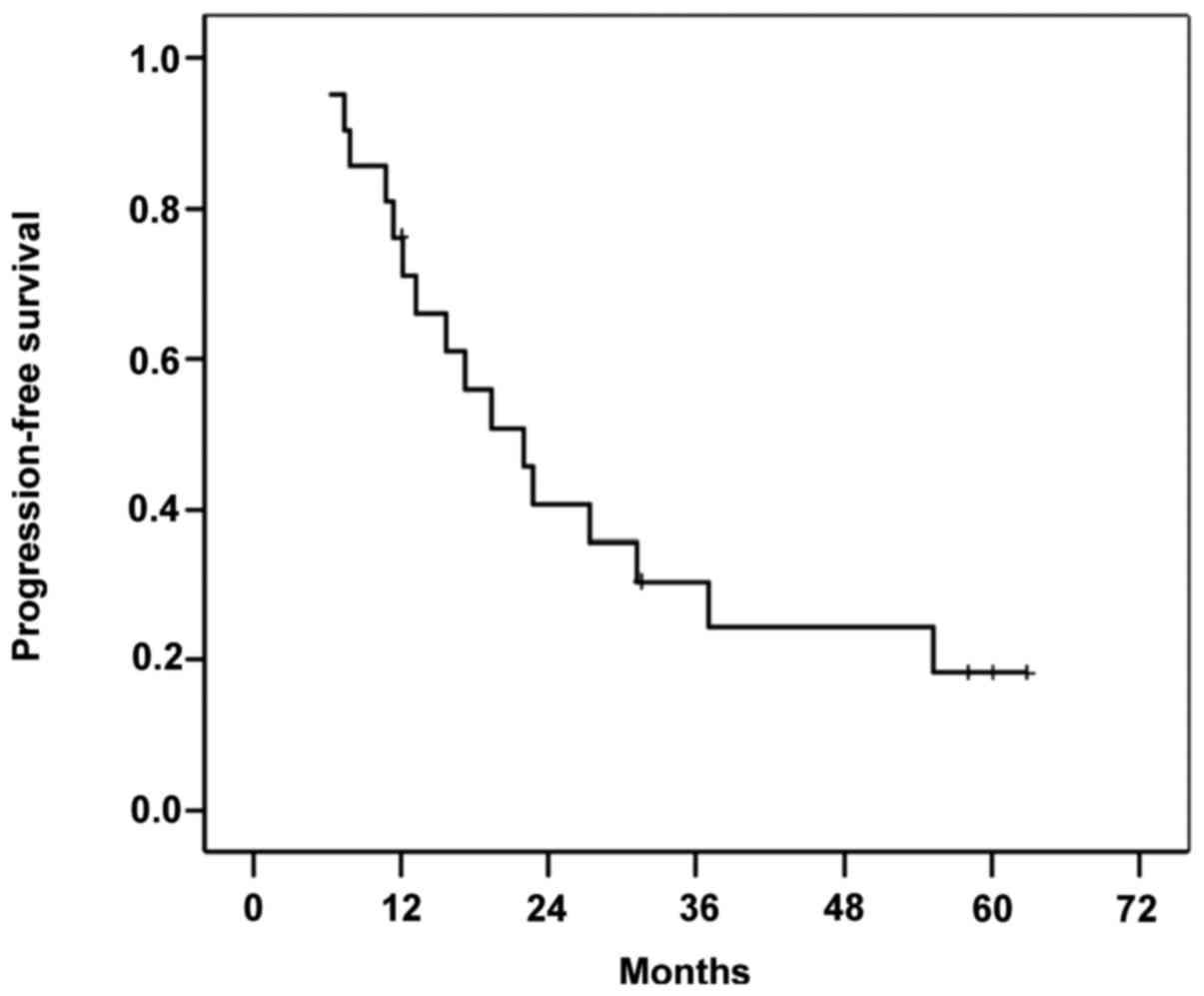
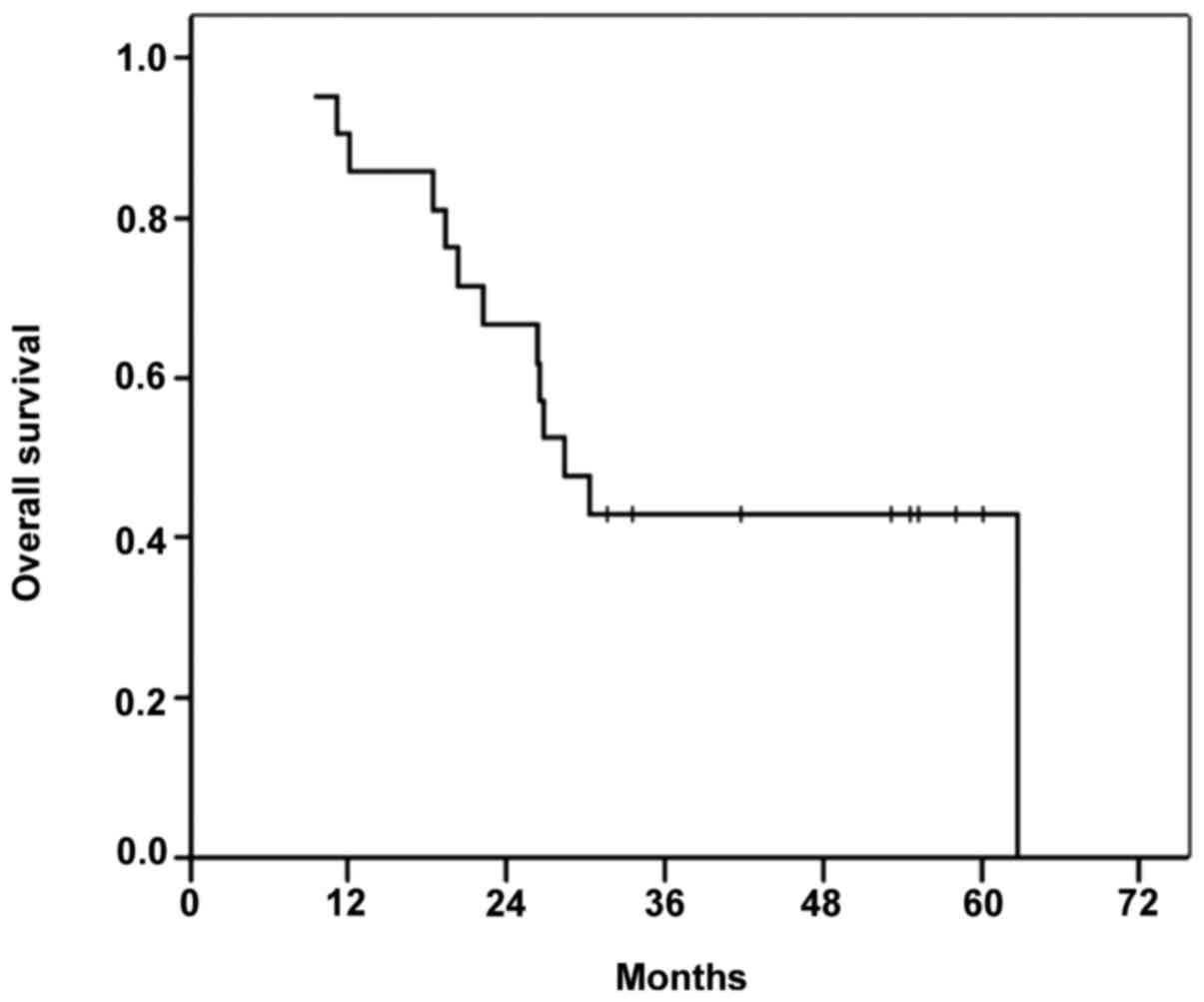
|
1
|
American Cancer Society, . Lung cancer
(Non-small cell). Atlanta, GA, USA: 2016
|
|
2
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim E, Harris G, Patel A, Adachi I,
Edmonds L and Song F: Preoperative versus postoperative
chemotherapy in patients with resectable non-small cell lung
cancer: Systematic review and indirect comparison meta-analysis of
randomized trials. J Thorac Oncol. 4:1380–1388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arriagada R, Dunant A, Pignon JP, Bergman
B, Chabowski M, Grunenwald D, Kozlowski M, Le Péchoux C, Pirker R,
Pinel MI, et al: Long-term results of the international adjuvant
lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy
in resected lung cancer. J Clin Oncol. 28(1): 35–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, et al: SEER Cancer Statistics Review. 1975–2013,
National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013Accessed
September 1, 2016.
|
|
6
|
NSCLC Meta-analysis Collaborative Group, .
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Depierre A, Milleron B, Moro-Sibilot D,
Chevret S, Quoix E, Lebeau B, Braun D, Breton JL, Lemarié E, Gouva
S, et al: Preoperative chemotherapy followed by surgery compared
with primary surgery in resectable stage I (except T1N0), II and
IIIa non-small-cell lung cancer. J Clin Oncol. 20:247–253. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albain KS, Rusch VW, Crowley JJ, Rice TW,
Turrisi AT III, Weick JK, Lonchyna VA, Presant CA, McKenna RJ,
Gandara DR, et al: Concurrent cisplatin/etoposide plus chest
radiotherapy followed by surgery for stages IIIA (N2) and IIIB
non-small-cell lung cancer: Mature results of southwest oncology
group phase II study 8805. J Clin Oncol. 13:1880–1892.
1995.PubMed/NCBI
|
|
9
|
Vokes EE, Herndon JE II, Crawford J,
Leopold KA, Perry MC, Miller AA and Green MR: Randomized phase II
study of cisplatin with gemcitabine or paclitaxel or vinorelbine as
induction chemotherapy followed by concomitant chemoradiotherapy
for stage IIIB non-small-cell lung cancer: Cancer and leukemia
group B study 9431. J Clin Oncol. 20:4191–4198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Brien ME, Splinter T, Smit EF, Biesma B,
Krzakowski M, Tjan-Heijnen VC, Van Bochove A, Stigt J,
Smid-Geirnaerdt MJ, Debruyne C, et al: Carboplatin and paclitaxol
(Taxol) as an induction regimen for patients with biopsy-proven
stage IIIA N2 non-small cell lung cancer. An EORTC phase II study
(EORTC 08958). Eur J Cancer. 39:1416–1422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zarogoulidis K, Kontakiotis T,
Hatziapostolou P, Fachantidou E, Delis D, Goutsikas J,
Constantinidis TC and Athanasiadis A: A Phase II study of docetaxel
and carboplatin in the treatment of non-small cell lung cancer.
Lung Cancer. 32:281–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
World Medical Association, . Declaration
of Helsinki: WMA Declaration of Helsinki - Ethical Principles for
Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/Accessed.
September 1–2016.
|
|
13
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martini N and Flehinger BJ: The role of
surgery in N2 lung cancer. Surg Clin North Am. 67:1037–1049. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe Y, Shimizu J, Oda M, Hayashi Y,
Watanabe S, Tatsuzawa Y, Iwa T, Suzuki M and Takashima T:
Aggressive surgical intervention in N2 non-small cell cancer of the
lung. Ann Thorac Surg. 51:253–261. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naruke T, Goya T, Tsuchiya R and Suemasu
K: The importance of surgery to non-small cell carcinoma of lung
with mediastinal lymph node metastasis. Ann Thorac Surg.
46:603–610. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mountain CF: Expanded possibilities for
surgical treatment of lung cancer. Survival in stage IIIa disease.
Chest. 97:1045–1051. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chemotherapy in non-small cell lung
cancer, . A meta-analysis using updated data on individual patients
from 52 randomised clinical trials. Non-small cell lung cancer
collaborative group. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosell R, Gómez-Codina J, Camps C, Maestre
J, Padille J, Cantó A, Mate JL, Li S, Roig J, Olazábal A, et al: A
randomized trial comparing preoperative chemotherapy plus surgery
with surgery alone in patients with non-small-cell lung cancer. N
Engl J Med. 330:153–158. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosell R, Gómez-Codina J, Camps C, Sánchez
J Javier, Maestre J, Padilla J, Cantó A, Abad A and Roig J:
Preresectional chemotherapy in stage IIIA non-small-cell lung
cancer: A 7-year assessment of a randomized controlled trial. Lung
Cancer. 26:7–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roth JA, Atkinson EN, Fossella F, Komaki
R, Ryan M Bernadette, Putnam JB Jr, Lee JS, Dhingra H, De Caro L,
Chasen M and Hong WK: Long-term follow-up of patients enrolled in a
randomized trial comparing perioperative chemotherapy and surgery
with surgery alone in resectable stage IIIA non-small-cell lung
cancer. Lung Cancer. 21:1–6. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roth JA, Fossella F, Komaki R, Ryan MB,
Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, McGavran M,
et al: A randomized trial comparing perioperative chemotherapy and
surgery with surgery alone in resectable stage IIIA non-small-cell
lung cancer. J Natl Cancer Inst. 86:673–680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pass HI, Pogrebniak HW, Steinberg SM,
Mulshine J and Minna J: Randomized trial of neoadjuvant therapy for
lung cancer: Interim analysis. Ann Thorac Surg. 53:992–998. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin J, Ginsberg RJ, Venkatraman ES,
Bains MS, Downey RJ, Korst RJ, Kris MG and Rusch VW: Long-term
results of combined-modality therapy in resectable non-small-cell
lung cancer. J Clin Oncol. 20:1989–1995. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirn DH, Lynch TJ, Mentzer SJ, Lee TH,
Strauss GM, Elias AD, Skarin AT and Sugarbaker DJ: Multimodality
therapy of patients with stage IIIA, N2 non-small-cell lung cancer.
Impact of preoperative chemotherapy on resectability and
downstaging. J Thorac Cardiovasc Surg. 106:696–702. 1993.PubMed/NCBI
|
|
28
|
Sugarbaker DJ, Herndon J, Kohman LJ,
Krasna MJ and Green MR: Results of cancer and leukemia group B
protocol 8935. A multiinstitutional phase II trimodality trial for
stage IIIA (N2) non-small-cell lung cancer. Cancer and leukemia
group B thoracic surgery group. J Thorac Cardiovasc Surg.
109:473–483; discussion 483–485. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Meerbeeck JP, Kramer GW, Van Schil PE,
Legrand C, Smit EF, Schramel F, Tjan-Heijnen VC, Biesma B, Debruyne
C, van Zandwijk N, et al: European Organisation for Research and
Treatment of Cancer-Lung Cancer Group: Randomized controlled trial
of resection versus radiotherapy after induction chemotherapy in
stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst.
99:442–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garrido P, González-Larriba JL, Insa A,
Provencio M, Torres A, Isla D, Sanchez JM, Cardenal F, Domine M,
Barcelo JR, et al: Long-term survival associated with complete
resection after induction chemotherapy in stage IIIA (N2) and IIIB
(T4N0-1) non small-cell lung cancer patients: The Spanish Lung
Cancer Group Trial 9901. J Clin Oncol. 25:4736–4742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martini N, Kris MG, Flehinger BJ, Gralla
RJ, Bains MS, Burt ME, Heelan R, McCormack PM, Pisters KM, Rigas
JR, et al: Preoperative chemotherapy for stage IIIa (N2) lung
cancer: The Sloan-Kettering experience with 136 patients. Ann
Thorac Surg. 55:1365–1373; discussion, 1373–1374. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar P, Herndon J, Elias AD, Sugarbaker
DJ and Green MR: Comparison of pre-operative thoracic radiation
therapy (TRT) to pre-operative chemotherapy (CT) in surgically
staged IIIA(N2) non-small cell lung cancer (NSCLC): Initial results
of cancer and leukemia group B (CALGB) phase III protocol 9134. Int
J Radiation Oncol Biol Phys. 39:1951997. View Article : Google Scholar
|
|
33
|
Andre F, Grunenwald D, Pignon JP, Dujon A,
Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V and Le
Chevalier T: Survival of patients with resected N2 non-small-cell
lung cancer: Evidence for a subclassification and implications. J
Clin Oncol. 18:2981–2989. 2000.PubMed/NCBI
|
|
34
|
Choi YS, Shim YM, Kim J and Kim K:
Recurrence-free survival and prognostic factors in resected pN2
non-small cell lung cancer. Eur J Cardiothorac Surg. 22:695–700.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rami-Porta R, Mateu-Navarro M, Freixinet
J, de la Torre M, Torres-García AJ, Pun YW and Armengod AC:
Bronchogenic Carcinoma Cooperative Group of the Spanish Society of
Pneumology and Thoracic Surgery (GCCB-S): Type of resection and
prognosis in lung cancer. Experience of a multicentre study. Eur J
Cardiothorac Surg. 28:622–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garrido P, González-Larriba JL, Insa A,
Provencio M, Torres A, Isla D, Sanchez JM, Cardenal F, Domine M,
Barcelo JR, et al: Long-term survival associated with complete
resection after induction chemotherapy in stage IIIA (N2) and IIIB
(T4N0-1) non small-cell lung cancer patients: The Spanish lung
cancer group trial 9901. J Clin Oncol. 25:4736–4742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krzakowski M, Provencio M, Utracka-Hutka
B, Villa E, Codes M, Kuten A, Henke M, Lopez M, Bell D, Biti G, et
al: Oral vinorelbine and cisplatin as induction chemotherapy and
concomitant chemo-radiotherapy in stage III non-small cell lung
cancer: Final results of an international phase II trial. J Thorac
Oncol. 3:994–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagai K, Tsuchiya R, Mori T, Tada H,
Ichinose Y, Koike T and Kato H: Lung Cancer Surgical Study Group of
the Japan Clinical Oncology Group: A randomized trial comparing
induction chemotherapy followed by surgery with surgery alone for
patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209).
J Thorac Cardiovasc Surg. 125:254–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu Y-L, Gu L-J, Weng Y-M, Feng W-N and
Cheng C: Neo-adjuvant chemotherapy with docetaxel plus carboplatin
for non-small cell lung cancer. Ann Oncol. 13:(Suppl 5).
1402002.PubMed/NCBI
|
|
40
|
Yang X, Wu Y and Gu L: A randomized trial
comparing neoadjuvant gemcitabine plus carboplatin or cisplatin
followed by surgery with surgery alone in Clinical Stage IIIA
non-small-cell lung cancer (NSCLC). Lung Cancer. 49:S2882005.
View Article : Google Scholar
|