Introduction
Polysaccharides are currently the subject of
numerous biochemical and nutritional studies as modifiers of
biological responses, due to their various biological activities
and use in medicine and health foods (1). Numerous natural polysaccharides and
polysaccharide-protein complexes obtained from various organisms,
including algae, plants, microorganisms and animals (2,3), have been
demonstrated to possess significant antitumor, anti-radiation,
antioxidant, anti-human immunodeficiency virus and
immunostimulatory activities (4,5), as well
as relatively low toxicity (6).
Strongylocentrotus nudus (S. nudus) roe is a form of
seafood in China; according to traditional Chinese medicine,
consumption of S. nudus may prevent cardiovascular diseases
and enhance immunity (7,8). Previous studies have revealed that
polysaccharides in S. nudus eggs are able to activate
immunocytes, including lymphocytes, macrophages and natural killer
(NK) cells (9–13). A previous study isolated a
polysaccharide, known as SEP, from S. nudus eggs using
diethylaminoethyl cellulose (DEAE)-52 column purification and
elution with distilled water; SEP was well-characterized and
demonstrated to be a potent immunomodulatory agent (9). Notably, a polysaccharide fraction named
SEP-S was identified when the cellulose DEAE-52 anion exchange
column was eluted using NaCl solutions of increasing ionic
strength, following SEP purification and elution with distilled
water (9).
According to previous studies, SEP is a D-glucan
containing an α-1, 4-linked backbone and α-1, 6-linked branches; it
is able to activate splenocytes and prevent the growth of Sarcoma
180, histocompatibility complex-22 (H22) hepatocellular carcinomas
and Lewis lung cancer, by promoting T cell proliferation and
differentiation into cytotoxic T lymphocytes (CTLs) and enhanced
NK-mediated cytotoxicity to tumor cells in vivo (10,11).
Additionally, SEP exerts a variety of immune regulatory functions,
consisting of promotion of cytokine secretion and antibody
production (12,13).
In the present study, a salt-eluted polysaccharide
fraction (SEP-S) from S. nudus eggs was identified and it's
antitumor and immunoregulatory activities were investigated, to the
best of our knowledge, for the first time using the H22
tumor-bearing mouse model. SEP-S is a homogeneous polysaccharide of
α-D-glucan, with a reduced molecular weight of 9.33×105
Da, compared with SEP. The present study also assayed the
biological effects of SEP-S on murine hepatocarcinoma using H22
tumor-bearing mice, on the immune system, including T subsets and
toll-like receptors (TLRs) in spleen lymphocytes. The current study
therefore demonstrates the purification and characterization of a
polysaccharide component, SEP-S, which exerts effective
anti-hepatocarcinoma activity in vivo by enhancing the
function of the host immune system.
Materials and methods
Cell lines, mice and reagents
The cell lines used in the present study, consisting
of A549 human non-small cell lung cancer, HepG2 human
hepatocellular carcinoma, H22 mouse hepatocellular carcinoma, B16
mouse melanoma and MDCK Madin-Darby canine kidney, were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured at 37°C in a humidified atmosphere containing
5% CO2 in RPMI-1640 medium or Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100
U/ml of penicillin and 100 U/ml of streptomycin (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Male imprinting control region (ICR) mice between 6
and 8 weeks of age (weight, 18±2 g) were purchased from the
Laboratory Animal Center of Yangzhou University (Yangzhou, China)
and acclimatized for 1 week prior to use. Animals were provided
with continuous standard rodent chow and water and were housed in a
rodent facility at 22±1°C with a 12 h light-dark cycle. All
procedures involving animals and their care in this study were in
strict accordance with protocols of the Ethics Committee of China
Pharmaceutical University (Nanjing, China).
Concanavalin A and fluorescein-5-isothiocyanate
(FITC) were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Injectable cisplatin and 5-Fluorouracil (5-Fu)
were obtained from Qilu Pharmaceutical Co., Ltd. (Jinan, China) and
Tianjin Jinyao Amino Acid Co., Ltd. (Tianjin, China), respectively.
The antibodies specific to TLR2 (anti-TLR2; #16-9021; 1 mg/ml;
dilution, 1:100) and TLR4 (anti-TLR4; #14-9924; 0.5 mg/ml;
dilution, 1:50) were obtained from eBioscience, Inc. (San Diego,
CA, USA).
Isolation and purification of
SEP-S
S. nudus were collected from the Huang Hai
Sea, China and transported to the laboratory packed in ice. The
shell, spine and intestine were immediately removed, and the eggs
were stored at −20°C. Crude polysaccharide was isolated from the
S. nudus eggs and additionally purified as described
previously (9). Briefly, the dried
S. nudus eggs (60 g) were first treated with acetone (w/v,
1:1, 600 ml × 3) to remove fat and pigments. The pellets were
extracted with distilled water (600 ml) at 90°C every 6 h, a total
of 3 times. The supernatants were collected by centrifugation at
1,900 × g for 15 min and concentrated under vacuum. The
concentrated solution was deproteinated using the Sevag method
(14). The crude polysaccharide
fraction was obtained through precipitation with a 5-fold volume of
ethanol and desiccation in vacuo. The precipitate (0.2 g)
was dissolved in distilled water (5 ml) again, applied to a DEAE-52
(OH−) anion exchange chromatography column (2.6×30 cm)
and subsequently eluted stepwise with deionized water followed by a
NaCl gradient (0→1.0 mol/l) at a flow rate of 60 ml/h. The
fractions obtained by gradient NaCl elution were combined according
to the total carbohydrate content and quantified by the Dubois
et al (15) phenol-sulfuric
acid method. The main peak was additionally fractionated on a
Sephacryl S-400 column (1.6×80 cm, GE Healthcare Bio-Sciences AB,
Pittsburgh, PA, USA) and eluted with distilled water at a flow rate
of 20 ml/h to yield a new fraction. The fractions were collected
using the phenol-sulfuric acid method (15), with their absorption determined at 280
nm in the UV spectrum. The salt-eluted fraction was dialyzed and
lyophilized, resulting in a white purified polysaccharide from
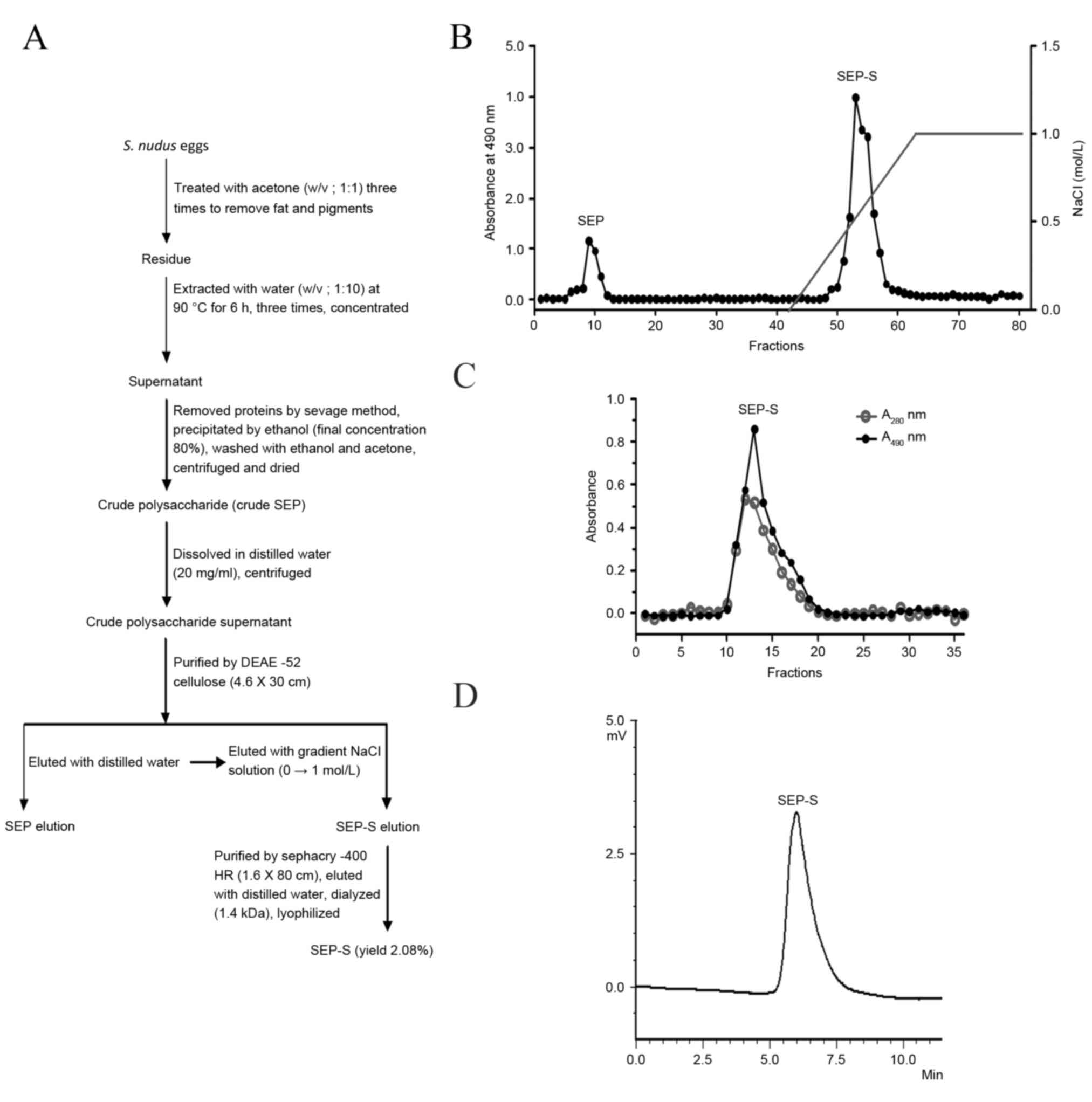
S. nudus eggs, named SEP-S. A flowchart describing the
isolation of the 2 polysaccharide fractions is presented in
Fig. 1A. SEP-S is a homogeneous
polysaccharide component composed of α-D-glucan, with a low
molecular weight of 9.33×105 Da, which differs from SEP.
The endotoxin levels of SEP-S were determined using the E-TOXATE™
kit (Sigma-Aldrich; Merck Millipore). Test samples used in the
study did not exhibit detectable levels of endotoxin within the
sensitivity limit of the kit (0.1–1.0 EU/ml).
In vitro cell viability assay
Splenocyte proliferation was assayed using the MTT
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) method
(16). The splenocyte proliferation
level of the control group was designated as 1, and the levels of
the other groups were compared with it. The MTT method was
additionally used to evaluate the antitumor properties of SEP-S
against four cancer cell lines in vitro. Tumor A549, HepG2,
H22 and B16 cells or MDCK cells were seeded in 96-well plates at a
concentration of 5×103 cells/well, and cultured in
RPMI-1640 or DMEM supplemented with 10% fetal calf serum.
Subsequently, the cancer cells or MDCK cells were inoculated with
SEP-S or cisplatin (at concentrations ranging from 0–500 µg/ml) for
48 h. The absorbance at 570 nm was determined using an ELISA reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Cell viabilities
were calculated using the following formula: Cell viability
(%)=(drug treatment-background)/(control-background) ×100%.
In vivo antitumor activity assay
To establish the murine solid tumor transplantation
model, H22 ascites tumor cells (2×107 cells in 0.2 ml
normal saline) were subcutaneously injected into the right axillary
region of ICR mice in all groups (17). Subsequently, 24 h following
inoculation, the mice were divided randomly into 5 groups, each
containing 12 mice. SEP-S was administered to each group at various
dosages (5, 10 and 20 mg/kg body weight). The positive control
group received 5-Fu at a dosage of 25 mg/kg body weight, and the
vehicle control group was treated with normal saline. All the
solutions were dissolved in saline, filtered through a 0.22 µm
millipore filter and administered daily via intravenous injection
(200 µl) for 12 days. The mouse weights were recorded following
each drug administration. Mice were sacrificed by cervical
dislocation 24 h subsequent to the final drug administration.
Spleen, thymus and tumor weights in the mice were determined. The
tumor inhibitory rate was calculated using the following formula:
Tumor inhibitory rate (%) = (WControl -
WTreated) / WControl × 100%;
WTreated and WControl were the average tumor
weight of the treated and vehicle control mice, respectively. The
thymus or spleen index was calculated as thymus or spleen weight
divided by the body weight, respectively (18). Tumor and spleen samples from each
group were fixed in 10% formalin and embedded in paraffin.
Subsequently, 5 mm sections were stained with hematoxylin and
eosin. Histological examinations were performed using an inverted
fluorescence microscope (Olympus Corporation, Tokyo, Japan). To
determine the life-prolonging effect of SEP-S, H22-bearing mice
treated for 12 days with 5, 10 and 20 mg/kg/day of SEP-S were
observed during the subsequent 17 days.
Cluster of differentiation (CD)
4+ and CD8+ T lymphocytes determination in
spleens and tumors from SEP-S-treated H22-bearing mice
Splenic lymphocytes from tumor-bearing ICR mice were
prepared and incubated with 10 µl anti-CD3-FITC (#11-0032; 0.5
mg/ml; dilution, 1:200), anti-CD4-FITC (#11-0041; 0.5 mg/ml;
dilution, 1:200) or anti-CD8-FITC antibodies (#12-0081; 0.2 mg/ml;
dilution, 1:80), all purchased from eBioscience Inc. (San Diego,
CA, USA), for 30 min at room temperature. The cells were then
washed twice with PBS and resuspended in 1% paraformaldehyde. The
counts of CD3+, CD4+ and CD8+ T
lymphocytes were determined using a flow cytometer (BD Biosciences,
San Jose, CA, USA) (10).
Cytotoxic activity assay of splenic
CTLs from SEP-S-treated H22-bearing mice
The cytotoxic activities of the CTLs were assayed
using the MTT method as previously described (19). Briefly, for CTL activity, splenic
lymphocytes in each group were prepared as effector cells, and the
ratios of effector cells to target H22 cells (E:T) were 40:1, 20:1
and 10:1. Subsequent to the cells being incubated at 37°C for 4 h,
the cellular cytotoxicity was determined as CTL activity
(%)=ODT-(ODS-ODE)/ODT
×100%, where the optical density values were ODT for the
target cell controls, ODS for the samples and
ODE for the effector cell controls.
Anti-TLR2/4 antibody-blocking
assays
To determine whether splenocyte proliferation
stimulated by SEP-S was associated with the surface receptor
TLR2/4, anti-TLR2/4 antibody-blocking assays were performed as
previously described (11). Mouse
splenocyte suspensions (1×106 cells/ml) cultivated in
96-well plates were pre-treated with 5 µg/ml of the TLR2 or TLR4
monoclonal antibodies at 37°C for 1 h, and then 200 µg/ml SEP-S was
added for 48 h. Subsequent to incubation, the inhibition of
splenocyte proliferation by anti-TLR2 and anti-TLR4 antibodies was
determined using the MTT method.
Statistical analyses
The data were statistically analyzed using a
student's t-test with Graphpad Prism 4.0 (Graphpad Software, Inc.
La Jolla, CA). The data were analyzed using one-way analysis of
variance, followed by Dunnett's test, to identify any differences
among the control, drug-treated and antibody-treated groups. The
results are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Isolation and purification of
SEP-S
Crude polysaccharide was prepared from S.
nudus eggs by hot-water extraction, deproteination and ethanol
precipitation. The crude polysaccharide was initially fractionated
on a DEAE-52 cellulose column (Fig.
1A). The elution curve indicated that the crude polysaccharide
was composed of two major fractions (Fig.
1B). SEP-S was eluted with a linear gradient of NaCl (0–1.0
mol/l); it was a novel polysaccharide component, distinct from the
previously reported SEP fraction eluted with distilled water
(8). The purification procedure
indicated that these two polysaccharide components had different
binding properties toward the DEAE-52 column, possibly due to
surface charge properties. SEP-S was additionally purified on a
Sephacryl-400 column, and the elution curve was a single peak, as
presented in Fig. 1C. The data
revealed that SEP-S was a single and symmetrical peak on the
high-performance liquid chromatography profile (Fig. 1D), with a purity of 99.0%, indicating
that it is a homogeneous polysaccharide. The yield of final
purified SEP-S was 2.1% of the dry weight. Therefore, by using the
SEP purification protocol with certain modifications, the present
study identified a polysaccharide component, SEP-S, which may
possess a negative charge, causing strong binding to the DEAE-52
column. In addition, SEP-S is a homogeneous polysaccharide
component composed of α-D-glucan, with a lower molecular weight of
9.33×105 Da, which differs from SEP.
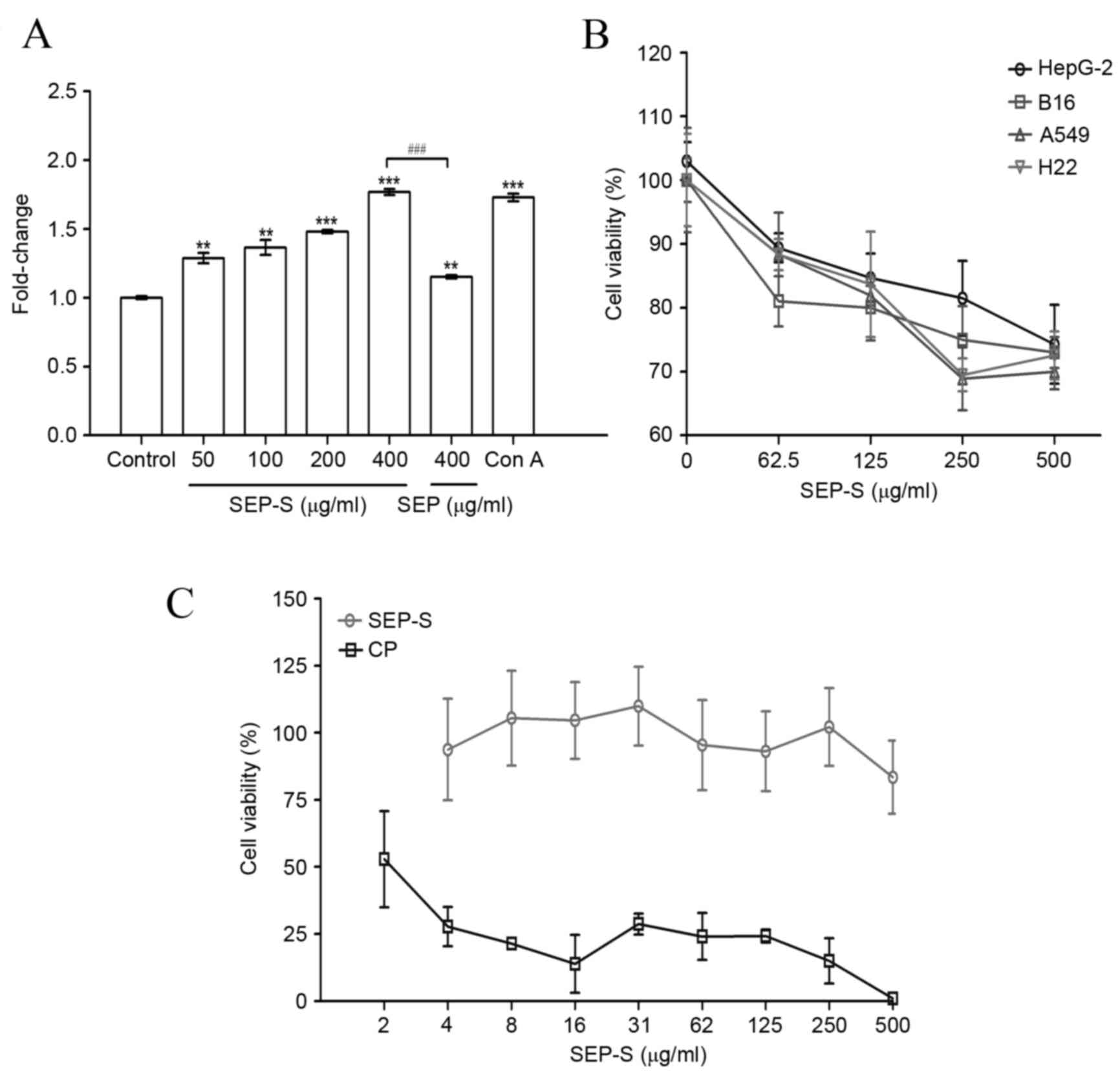
SEP-S stimulates the proliferation of
splenocytes and suppressed the growth of tumor cells in vitro
The mitogenic effect of SEP-S was initially examined
using total spleen cells. As presented in Fig. 2A, SEP-S notably enhanced the
proliferation of splenocytes at doses of 50 to 400 µg/ml. At the
dose of 400 µg/ml, SEP-S stimulated splenocyte proliferation by
1.8-fold, compared with the model control (P=0.0007), suggesting
that SEP-S exerted a direct mitogenic effect on mouse splenocytes,
which is increased, compared with that of SEP (P=0.0009). In
addition, SEP-S (400 µg/ml) stimulated splenocyte proliferation in
accordance to concanavalin A.
In total, the four A549, HepG2, H22 and B16 cancer
cell lines were selected for assessing the potential antitumor
activity of SEP-S in vitro using the MTT method. The tumor
cells were treated with various concentrations of SEP-S (62.5, 125,
250 and 500 µg/ml), and the viability of all decreased in a
dose-dependent manner, as presented in Fig. 2B. The inhibitory levels with H22 and
A549 were 30.5% and 31.1%, respectively, in response to SEP-S
treatment of, ≤250 µg/ml, and the inhibitory level with the HepG2
cancer cells was 25.7% at a concentration of 500 µg/ml. Notably,
SEP-S exhibited no direct cytotoxicity toward MDCK cells, as
presented in Fig. 2C. These data
indicate that SEP-S is a biological response modifier and may
suppress the proliferation of various cancer cells while exhibiting
no toxic effects toward normal cells, including MDCK, in
vitro.
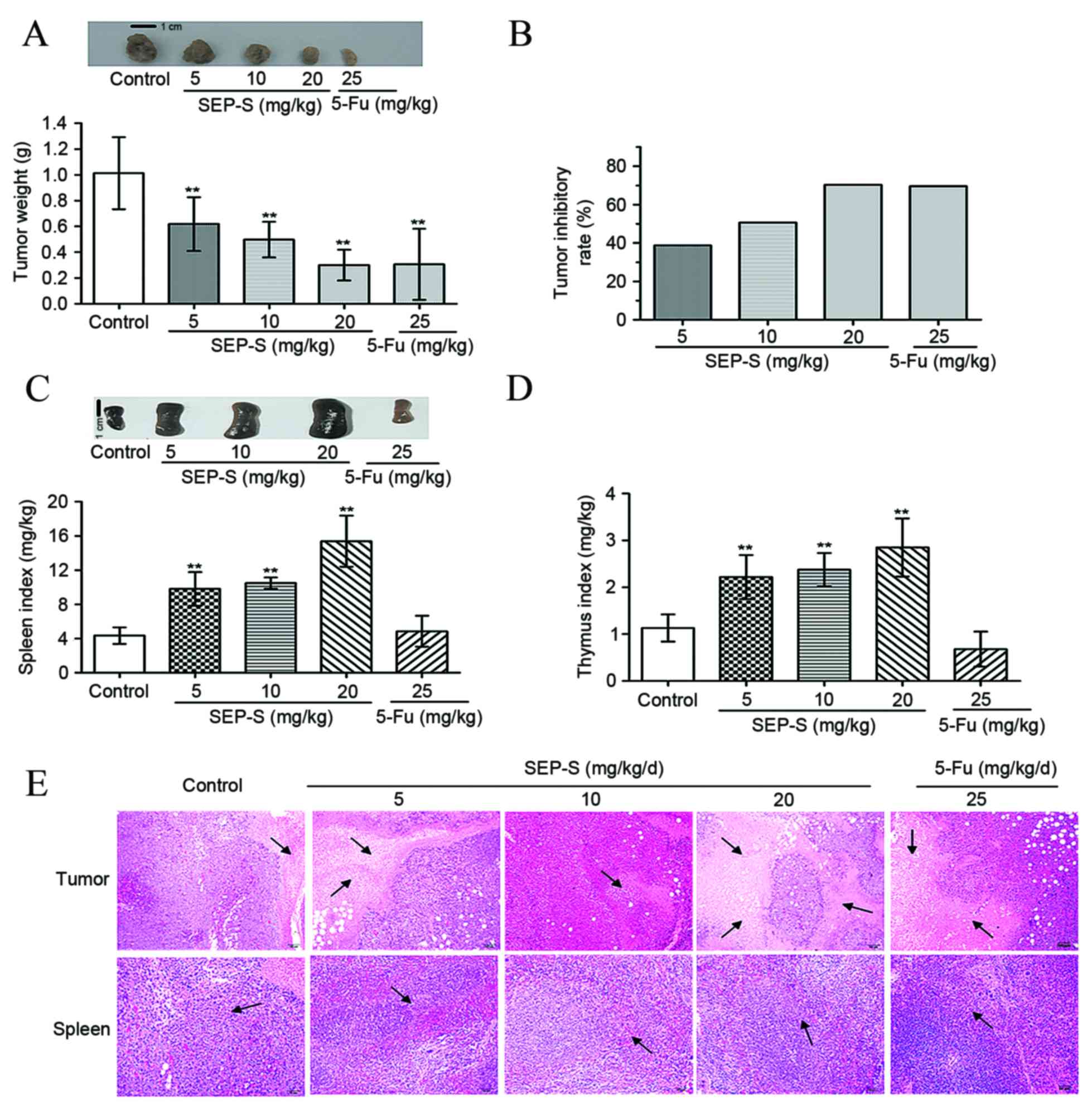
SEP-S effectively inhibits tumor
growth in H22-bearing mice
To investigate whether SEP-S has in vivo
antitumor activity, H22 xenograft tumors were excised from mice and
weighed following 12 days of SEP-S pretreatment. As evident from
Fig. 3A and B, injection with SEP-S
at 5, 10 and 20 mg/kg/day significantly reduced tumor weight in a
dose-dependent manner, with inhibition values of 38.8, 50.7 and
70.3%, respectively. The marked inhibition of tumor growth in mice
treated with 20 mg/kg SEP-S was comparable to that observed with
5-Fu treatment (69.6%). Additionally, the relative spleen and
thymus indices of the SEP-S-treated groups were increased compared
with those of the model groups [thymus indices: Control vs.
SEP-S-treated group (5 mg/kg), P=0.0057; control vs SEP-S-treated
group (10 mg/kg), P=0.0062; control vs. SEP-S-treated group (20
mg/kg), P=0.0073; spleen indices: Control vs. SEP-S-treated group
(5 mg/kg), P=0.0053; control vs. SEP-S-treated group (10 mg/kg),
P=0.0055; control vs. SEP-S-treated group (20 mg/kg), P=0.0064].
Consistent with the antitumor activity, at a dose of 20 mg/kg/day,
these 2 indices increased by ~3.6- and 2.5-fold, respectively,
compared with the model group (Fig. 3C
and D). To additionally assess the antitumor and
immunoregulatory effect of SEP-S on H22-bearing mice, tumor and
spleen tissues from each group were subjected to histochemical
examination. As presented in Fig. 3E,
the tumor cells from the model control were arranged tightly with a
large nucleus. By contrast, in the SEP-S-treated group, the tumor
cells were arranged loosely with anomalous shapes and were either
fragmented or lightly stained, which indicated necrotic morphology.
In addition, the tumors of the SEP-S-treated group exhibited
increased infiltration of lymphocyte populations, compared with the
model control. Furthermore, SEP-S facilitated splenocyte
multiplication, compared with the model control, particularly at
the dose of 20 mg/kg/day, suggesting that SEP-S protects the immune
organs against atrophy; this was coincident with an increase in the
spleen index in SEP-S-treated mice.
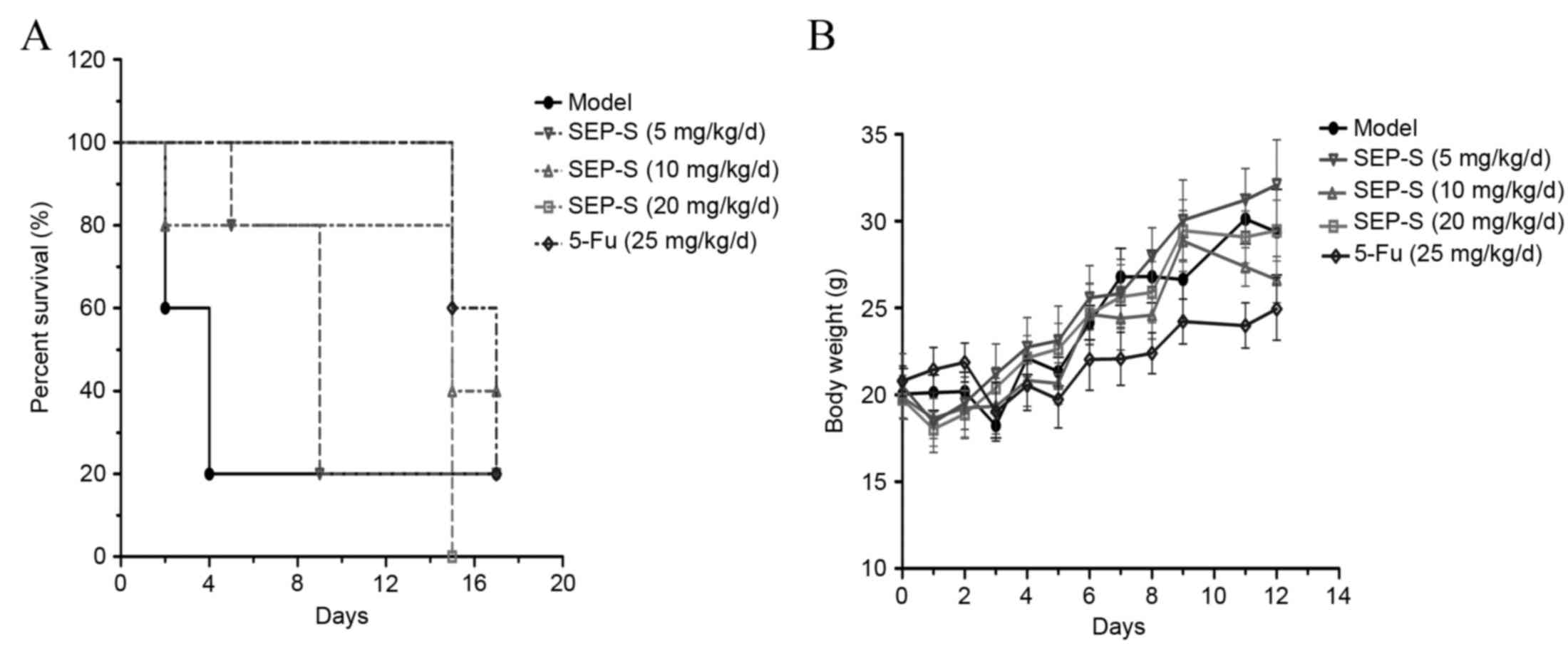
SEP-S prolongs the survival period and
maintains the body weight of H22-bearing mice
SEP-S treatment effectively increased the survival
time of the H22 tumor-bearing mice. The average survival time of
H22-bearing mice treated with SEP-S was extended ~3-fold, compared
with that of the model group. The group treated with 20 mg/kg/day
of SEP-S was almost comparable to that treated with 5-Fu in terms
of prolonging the survival period of H22 tumor-bearing mice
(Fig. 4A). As for body weight, there
was a protective effect in the SEP-S-treated groups (Fig. 4B), particularly at a dose of 5
mg/kg/day. Collectively, these results indicate that SEP-S
treatment exerted beneficial effects in causing regression of tumor
growth, enhancing immune function, extending the survival period
and increasing the body weight of H22 tumor-bearing mice.
SEP-S upregulates T lymphocyte subsets
from the spleen and tumor and enhances CTL activity in H22-bearing
mice
To investigate the effects of SEP-S on cellular
immunity, the counts of CD3+, CD4+ and
CD8+ T lymphocytes from the spleen and tumor were
determined using flow cytometry. As illustrated in Fig. 5A and B, the percentage of the
CD4+ and CD8+ T lymphocytes was increased in
the spleen and the tumor of the SEP-S-treated groups. The 20
mg/kg/day SEP-S-treated group had significantly elevated
proportions of CD4+ and CD8+ T lymphocyte
subsets in the spleen (≤29.5% and 24.8%, respectively;
CD4+, P=0.041; CD8+, P=0.0061) and a
significantly elevated proportion of CD4+ T lymphocytes
in the tumor (≤2.7%; P=0.0063), whereas the CD8+ T
lymphocyte proportion was not significantly elevated in the tumor
(3.7%; P=0.531). The activity of CTLs from spleens of SEP-S-treated
mice was markedly elevated in a ratio- and dose-dependent manner.
In the 20 mg/kg/day group, the CTL activities were increased to
33.6, 50.2 and 70.8% at ratios of 10:1, 20:1 and 40:1 (E:T),
respectively (Fig. 5C). These data
indicated that SEP-S stimulated T lymphocyte immunity in
vivo.
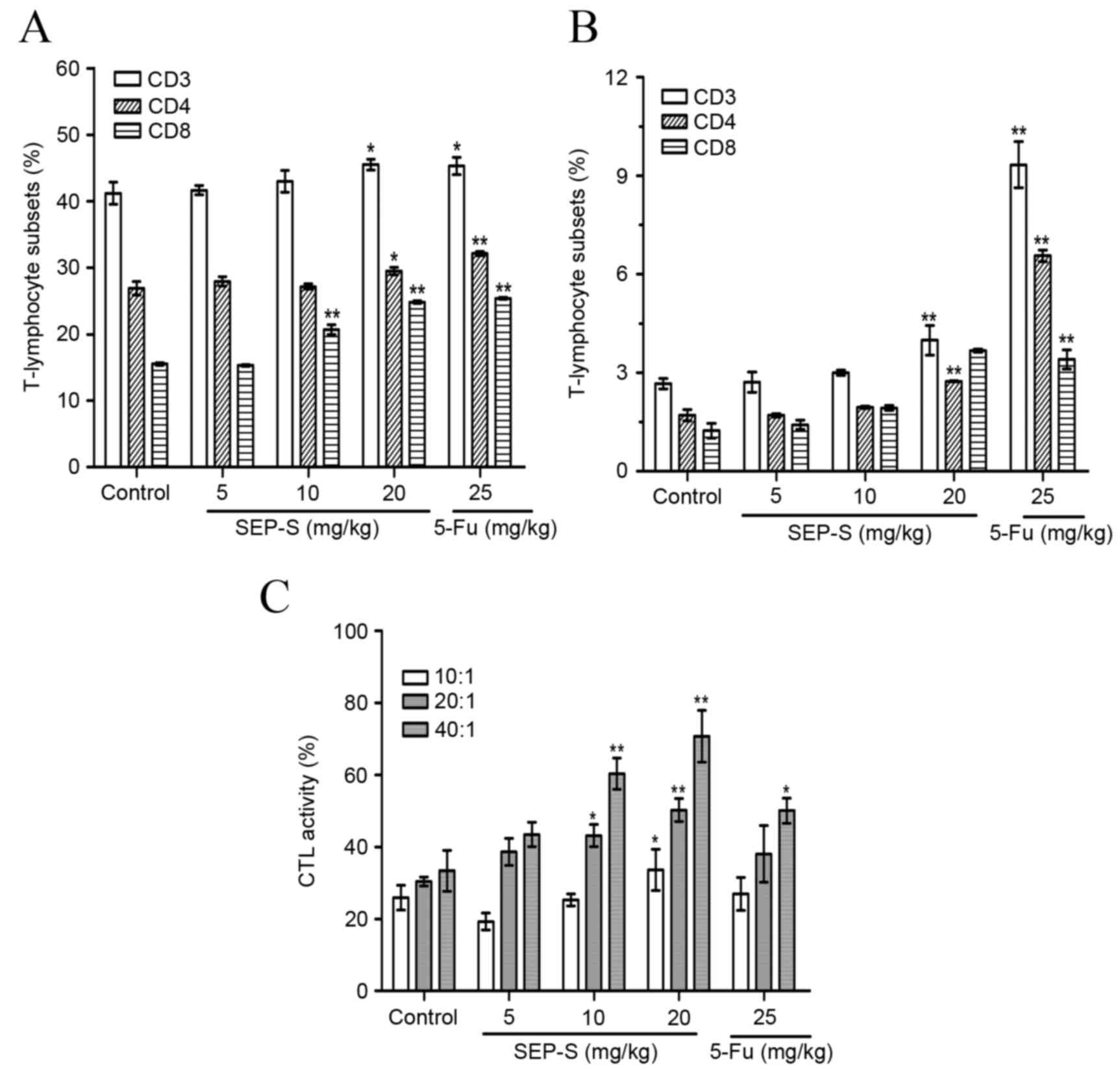 | Figure 5.SEP-S upregulates T lymphocyte
subsets from the spleen and tumor of H22-bearing mice. SEP-S (5, 10
and 20 mg/kg/day) increased the percentage of the CD4+
and CD8+ T-lymphocytes from (A) the spleen and (B) tumor
in a dose-dependent manner, compared with the model control. (C)
SEP-S enhanced the activity of CTLs from the spleen of H22-bearing
mice. SEP-S (5, 10 and 20 mg/kg/day) significantly enhanced the
cytotoxic activity of splenocytes from H22-bearing mice at effector
cell to target H22 cell ratios of 10:1, 20:1 and 40:1. Splenocytes
were prepared and assayed for CTL activity using the MTT method.
The values are presented as the mean ± standard deviation from
three separate experiments (n=12). *P<0.05 and **P<0.01,
compared with the model group. SEP-S, an eluant of NaCl solution
gradient elution of Strongylocentrotus nudus egg
polysaccharide; H22, histocompatibility 22; CD, cluster of
differentiation; CTL, cytotoxic T lymphocyte; 5-Fu,
5-Fluorouracil. |
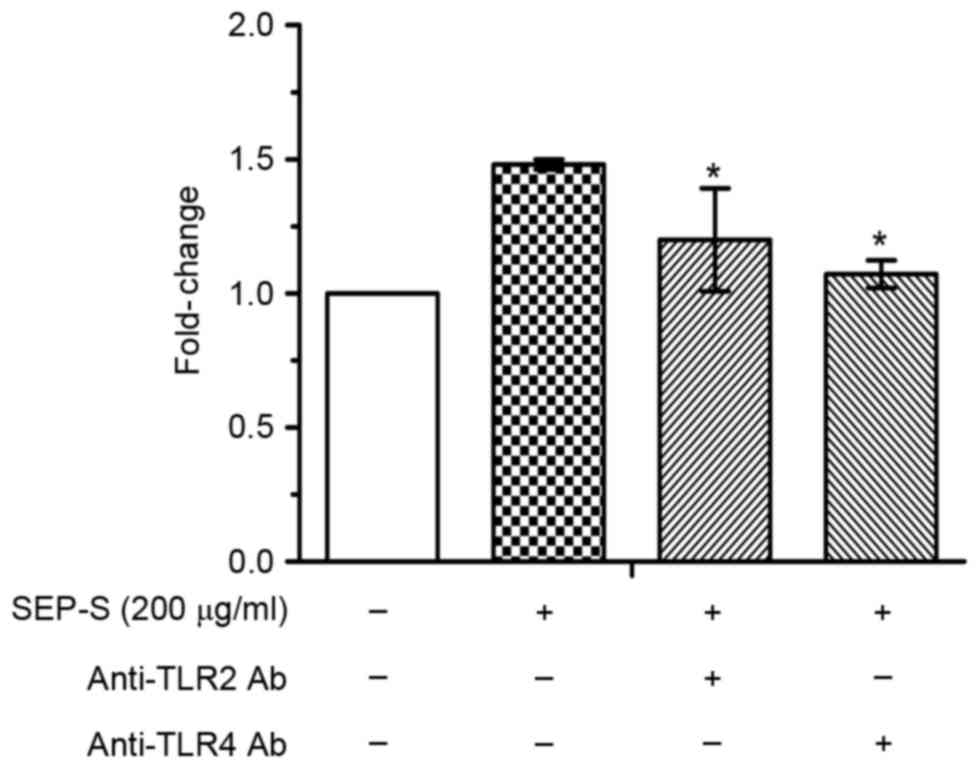
Splenocyte proliferation induced by
SEP-S is inhibited by the TLR2 and TLR4 monoclonal antibodies
TLRs have been revealed to be expressed on various
lymphocytes, and TLR2 and TLR4 are expressed on T cells; TLR2/4 is
generally considered to be the main receptor of polysaccharides
(20,21). To determine whether TLR2 or TLR4 was
involved in the enhancement of splenocyte proliferation by SEP-S,
blocking experiments were performed using anti-TLR2 and anti-TLR4
antibodies. As illustrated in Fig. 6,
mouse splenocyte proliferation in vitro was enhanced
1.5-fold by 200 µg/ml SEP-S, compared with the control group, and
it was significantly reduced by the anti-TLR2 or anti-TLR4 blocking
antibody (anti-TLR2 vs. control, P=0.041; anti-TLR4 vs. control,
P=0.034). These data suggest that TLR2 and TLR4 are involved in the
stimulation of splenocyte proliferation by SEP-S.
Discussion
As a component of traditional Chinese medicine,
S. nudus is able to prevent cardiovascular disease and
enhance immunity (22,23). In the present study, a novel
salt-eluted polysaccharide component, SEP-S, with an average
molecular weight of 9.33×105 Da, was successfully
isolated and purified from S. nudus. Monosaccharide
component analysis indicated that SEP-S was composed of glucose.
The material obtained by NaCl gradient elution from the DEAE-52
cellulose column was additionally purified using Sephacryl S-400 to
obtain a fraction, which was named SEP-S. In our previous study,
the distilled water-eluted fraction of the DEAE-52 cellulose
column, named SEP, had a molecular weight of 1.95×106 Da
and contained no protein or uronic acid (9). The immunoregulatory activities of
polysaccharide depend on its structure, molecular weight and the
number of branches (24). Varying
molecular weights may lead to varying steric hindrances of the
polysaccharides, resulting in altered receptor binding ability on
the immune cells and differing immunological activities (24,25). In
certain cases, polysaccharides with larger molecular weights are
less beneficial for crossing multiple membrane barriers to exert
biological activities due to the greater volume (24,25). It
was observed that polysaccharides with varying molecular weights
from instant coffee had differing in vitro immunostimulatory
properties, and lower molecular weight fractions exhibited improved
activity (25). As a lower molecular
weight polysaccharide, which differs from SEP, SEP-S may possess
increased biological activities.
The anti-hepatocarcinoma activity exhibited by SEP-S
was an attractive property. SEP-S possessed antitumor activity
in vitro while having no cytotoxicity toward normal cells,
including MDCK. The present study additionally observed that SEP-S
effectively inhibited H22 tumor growth in vivo with no
cytotoxic effect, and the inhibitory rate was ≤70.3% at the dose 20
mg/kg/day. The tumor-inhibitory effect of SEP-S was more robust,
compared with other polysaccharides, including SEP (10), schizophyllan and polysaccharide-K
(26), the endo-polysaccharide of
Phellinus igniarius (27) and
the exopolysaccharide fraction of Cordyceps sinensis
(28).
The currently proposed underlying mechanisms by
which polysaccharides exert antitumor effects consist of direct
tumor inhibition and immuno-enhancement (29). In the current study, SEP-S had a
higher antitumor activity, compared with SEP, potentially due to
its substantial cytotoxicity toward tumor cells and
immune-regulation activity. The antitumor activity of
polysaccharides may be affected by their monosaccharide
composition, protein content, molecular mass and chain conformation
(30). The in vitro antitumor
assay revealed that SEP-S exhibited antitumor activity against the
growth of HepG2 cells to a certain extent. In addition, the
cellular immune response effected by T cells has a central role in
the generation and regulation of the immune response to tumor
antigens (31). T cells are divided
into subsets of CD4+ or CD8+, where CD4 T
helper cells (Th) are important regulators of the immune system
(32) and CD8 CTLs are major
effectors of cell-mediated immunity (innate and adaptive immunity)
and eliminate target tumor cells (33). SEP-S exerted a direct mitogenic effect
on mouse splenocytes in a dose-dependent manner, which was marked,
compared with that of SEP. Additionally, SEP-S not only
significantly increased the percentages of CD4+ and
CD8+ T cells, but markedly enhanced the cytotoxic
activity of CTLs in H22-bearing mice. The enhanced percentage of
CD4+ and CD8+ T cells additionally indicates
that Th cells and CTLs were activated by SEP-S.
Polysaccharides isolated from a variety of sources
were reported to activate immune cells via the TLR, CR3 and
dectin-1 receptors. However, CR3 and dectin-1 are β-glucan-specific
receptors (34–36). In the present study, it was observed
that SEP-S mediated splenocyte proliferation via TLR2 and TLR4,
similar to SEP (11). It is important
to further study the underlying mechanisms by which SEP-S activates
the immune system, particularly as SEP-S may be used as a
protective immunotherapeutic agent for the treatment of liver
cancer.
Acknowledgements
The present study was supported by The National
Science and Technology Major Project Foundation of China (grant no.
2012ZX09102301-003), The Scientific and Technological Support &
Social Development Plan of Jiangsu Province (grant no. BE2011784)
and The Priority Academic Program Development of Jiangsu Higher
Education Institutions (PADA).
References
|
1
|
Sun YX: Structure and biological
activities of the polysaccharide from the leaves, roots and fruits
of Panax ginseng C.A. Meyer: An overview. Carbohydr Polym.
85:490–499. 2011. View Article : Google Scholar
|
|
2
|
Li S, Pan C, Xia W, Zhang W and Wu S:
Structural characterization of the polysaccharide moiety of an
aqueous glycopeptide from mannatide. Int J Biol Macromol.
67:351–359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang CX, Wang MC, Liu J, Gan D and Zeng
XX: Extraction, preliminary characterization, antioxidant and
anticancer activities in vitro of polysaccharides from Cyclina
sinensis. Carbohydr Polym. 84:851–857. 2011. View Article : Google Scholar
|
|
4
|
Huang Y, Jiang C, Hu Y, Zhao X, Shi C, Yu
Y, Liu C, Tao Y, Pan H, Feng Y, et al: Immunoenhancement effect of
rehmannia glutinosa polysaccharide on lymphocyte proliferation and
dendridtic cell. Carbohydr Polym. 96:516–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang GX, Prasad KN, Jiang YM, Yang B, Jia
YX and Sun J: Extraction and structural identification of
alkali-soluble polysaccharides of longan (Dimocarpus longan Lour.)
fruit pericarp. Innov Food Sci Emerg Technol. 10:638–642. 2009.
View Article : Google Scholar
|
|
6
|
Yang B, Zhao MM, Prasad KN, Jiang GX and
Jiang YM: Effect of methylation on the structure and radical
scavenging activity of polysaccharides from longan (Dimocarpus
longan Lour.) fruit pericarp. Food Chem. 118:364–368. 2010.
View Article : Google Scholar
|
|
7
|
Swann JB and Smyth MJ: Immune surveillance
of tumors. J Clin Invest. 117:1137–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Y, Xing Y, Mi H, Guo Z, Lu Y and Xi T:
Extraction, preliminary characterization and immunostimulatory
activity in vitro of a polysaccharide isolated from
Strongylocentrotus nudus eggs. Carbohydr Polym. 111:576–583. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CH, Lin Q, Gao Y, Ye L, Xing YY and Xi
T: Characterization and antitumor activity of a polysaccharide from
Strongylocentrotus nudus eggs. Carbohydr Polym. 67:313–318. 2007.
View Article : Google Scholar
|
|
10
|
Wang M, Wang H, Tang Y, Kang D, Gao Y, Ke
M, Dou J, Xi T and Zhou C: Effective inhibition of a
Strongylocentrotus nudus eggs polysaccharide against hepatocellular
carcinoma is mediated via immunoregulation in vivo. Immunol Lett.
141:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ke M, Wang H, Zhang M, Tian Y, Wang Y, Li
B, Yu J, Dou J, Xi T and Zhou C: The anti-lung cancer activity of
SEP is mediated by the activation and cytotoxicity of NK cells via
TLR2/4 in vivo. Biochem Pharma. 89:119–130. 2014. View Article : Google Scholar
|
|
12
|
Liu C, Xi T, Lin Q, Xing Y, Ye L, Luo X
and Wang F: Immunomodulatory activity of polysaccharide isolated
from Strongylocentrotus nudus eggs. Int Immunopharmacol.
8:1835–1841. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Wang M, Chen J, Tang Y, Dou J, Yu
J, Xi T and Zhou C: A polysaccharide from Strongylocentrotus nudus
eggs protects against myelosuppression and immunosuppression in
cyclophosphamide-treated mice. Int Immunopharmacol. 11:1946–1953.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sevag MG, Lackman DB and Smolens J: The
isolation of the components of streptococcal nucleoproteins in
serologically active form. J Biol Chem. 124:425–436. 1938.
|
|
15
|
Dubois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substance. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
16
|
Lee SM, Yoon MY and Park HR: Protective
effects of paeonia lactiflora pall on hydrogen peroxide-induced
apoptosis in PC12 cells. Biosci Biotechnol Biochem. 72:1272–1277.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rashid S, Unyayar A, Mazmanci MA, McKeown
SR, Banat IM and Worthington J: A study of anti-cancer effects of
Funalia trogii in vitro and in vivo. Food Chem Toxicol.
49:1477–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun LQ, Wang L and Zhou Y:
Immunomodulation and antitumor activities of
different-molecular-weight polysaccharides from Porphyridium
cruentum. Carbohydr Polym. 87:1206–1210. 2012. View Article : Google Scholar
|
|
19
|
Xu HS, Wu YW, Xu SF, Sun HX, Chen FY and
Yao L: Antitumor and immunomodulatory activity of polysaccharides
from the roots of Actinidia eriantha. J Ethnopharmacol.
125:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoon YD, Han SB, Kang JS, Lee CW, Park SK,
Lee HS, Kang JS and Kim HM: Toll-like receptor 4-dependent
activation of macrophages by polysaccharide isolated from the radix
of latycodon grandiflorum. Int Immunopharmacol. 3:1873–1882. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav M and Schorey JS: The β-glucan
receptor dectin-1 functions together with TLR2 to mediate
macrophage activation by mycobacteria. Blood. 108:3168–3175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mourão PA and Pereira MS: Searching for
alternatives to heparin: Sulfated fucans from marine invertebrates.
Trends Cardiovasc Med. 9:225–232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahara H, Ishikawa M, Takahashi N, Ohtani
S, Sato N, Gasa S, Akino T and Kikuchi K: In vivo ant-tumour effect
of 3′-sulphonoquinovosyl 1′-monoacylglyceride isolated from sea
urchin (Strongylocentrotus intermedius) intestine. Br J Cancer.
75:324–332. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miura NN, Adachi Y, Yadomae T, Tamura H,
Tanaka S and Ohno N: Structure and biological activities of
beta-glucans from yeast and mycelial forms of candida albicans.
Microbiol Immunol. 47:173–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Passos CP, Cepeda MR, Ferreira SS, Nunes
FM, Evtuguin DV, Madureira P, Vilanova M and Coimbra MA: Influence
of molecular weight on in vitro immunostimulatory properties of
instant coffee. Food Chem. 161:60–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu M, Li J, Kong F, Lin J and Gao Y:
Induction of immunomodulating cytokines by a new
polysaccharide-peptide complex from culture mycelia of Lentinus
edodes. Immunopharmacology. 40:187–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Pan JZ, Li X, Zhou Y, Meng Q and
Wang Q: Endo-polysaccharide of Phellinus igniarius exhibited
anti-tumor effect through enhancement of cell mediated immunity.
Int Immunopharmacol. 11:255–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Li J, Qiu S, Chen J and Zheng Y:
Effects of the exopolysaccharide fraction (EPSF) from a cultivated
Cordyceps sinensis on immunocytes of H22 tumor bearing mice.
Fitoterapia. 79:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan L, Ding S, Ai L and Deng K: Antitumor
and immunomodulatory activity of water-soluble polysaccharide from
Inonotus obliquus. Carbohydr Polym. 90:870–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang M, Cui SW, Cheung PCK and Wang Q:
Antitumor polysaccharides from mushrooms: A review on their
isolation process, structural characteristic and antitumor
activity. Trends Food Sci Tech. 18:4–19. 2007. View Article : Google Scholar
|
|
31
|
Hong F, Yan J, Baran JT, Allendorf DJ,
Hansen RD, Ostroff GR, Xing PX, Cheung NK and Ross GD: Mechanism by
which orally administered beta-1, 3-glucans enhance the tumoricidal
activity of antitumor monoclonal antibodies in murine tumor models.
J Immunol. 173:797–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiku H: Importance of CD4+
helper T-cells in antitumor immunity. Int J Hematol. 77:435–438.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sandel MH, Speetjens FM, Menon AG,
Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA,
van de Velde CJ and Kuppen PJ: Natural killer cells infiltrating
colorectal cancer and MHC class I expression. Mol Immunol.
42:541–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han SB, Yoon YD, Ah HJ, Lee HS, Lee CW,
Yoon WK, Park SK and Kim HM: Toll-like receptor-mediated activation
of B cells and macrophages by polysaccharide isolated from cell
culture of Acanthopanax senticosus. Int Immunopharmacol.
3:1301–1312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nair PK, Melnick SJ, Ramachandran R,
Escalon E and Ramachandran C: Mechanism of macrophage activation by
(1, 4)-alpha-D-glucan isolated from Tinospora cordifolia. Int
Immunopharmacol. 6:1815–1824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brown GD, Herre J, Williams DL, Willment
JA, Marshall AS and Gordon S: Dectin-1 mediates the biological
effects of beta-glucan. J Exp Med. 197:1119–1124. 2003. View Article : Google Scholar : PubMed/NCBI
|