Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common malignant tumors in South China (1). In 2010, an estimated 41,503 new cases
and 20,058 deaths were attributed to NPC in China, accounting for
1.03% of all cancer-related deaths that year in China (2). Among the head and neck tumors, NPC has
the highest propensity to metastasize to distant sites (3). Although the standard treatment has
improved the local control rate for NPC from 54 to 78%, regional
recurrences occur in 10–19% of patients (4). Most of the regional recurrences are due
to the metastasis and therapy resistance (1). It has been proved that cancer stem cells
(CSCs) play a major role in tumor recurrence, metastasis and
therapy resistance (5).
The CSC hypothesis proposes that a small subset of
cancer cells has the properties of self-renewal, differentiation
and resistance to chemotherapy or radiotherapy (6). Although the CSCs possess the capacity to
form the heterogeneous lineages of cancer cells that comprise the
tumor (6), the methods to divide the
CSCs from normal cancer cells have not been developed. However,
previous studies showed that CSCs expressed one or several surface
markers, including cluster of differentiation (CD) 24 (7), CD44 (8),
aldehyde dehydrogenase 1 (9) and
CD133 (10), in particular human
cancers. However, there is not a reliable CSC marker in NPC cells,
and the side population (SP) phenotype can be a marker of CSCs
(11) and gastrointestinal cancer
(12). Therefore, effective compounds
that target NPC CSCs may be helpful in clinical therapy.
Several studies have revealed that nigericin
(13), and Smac mimetics in
combination with TNF-related apoptosis-inducing ligand (14) could target NPC CSCs. Longikaurin A
(LK-A) is a natural ent-kaurene diterpenoid extracted from the
Isodon genus whose anti-tumor activity has been verified
(15,16). According to our previous research, at
a low concentration, LK-A could inhibit the colony formation
ability of NPC cells (15). In
addition, LK-A exhibits anti-tumor activity in CNE2 xenograft tumor
models (15). However, to the best of
our knowledge, no research has been conducted to examine the effect
of LK-A on CSCs. Here, we studied whether LK-A could suppress
stemness in NPC.
Materials and methods
Chemicals and antibodies
We used a previously described method to obtain LK-A
from the leaves of Isodon ternifolius (D. Don) Kudô
(15). Briefly, the dried and milled
plant material (10 kg) was extracted four times by incubation with
100 l of 70% aqueous Me2CO for 3 days at room
temperature and then filtered. The filtrate was evaporated under
reduced pressure, and partitioned with ethyl acetate (EtOAc) (4×60
l). The EtOAc partition (938.5 g) was applied to a silica gel
(200–300 mesh), and six fractions, termed A-F, were eluted with
CHCl3-Me2CO (1:0–0:1). Fraction B (618.5 g)
was decolorized on an MCI® GEL and eluted with 90%
methanol-H2O to yield fractions B1-B4. Fractions B1 (116
g) and B2 (135 g) were further separated by repeated silica gel
column chromatography to isolate LK-A (20 g). The LK-A powder was
dissolved in dimethyl sulfoxide (DMSO) at a concentration of 50 mM
and then stored at −20°C. Before each experiment, we would freshly
dilute the LK-A in medium to achieve the working concentrations in
this study. The DMSO concentration was kept below 0.1% when used in
cell culture and did not exert any detectable effect on cell growth
or death. Cell culture reagents, including RPMI 1640 medium,
Dulbecco's modified Eagle's medium (DMEM)/F12, recombinant human
basic fibroblast growth factor (bFGF), recombinant human epidermal
growth factor (EGF) and B-27® Supplement were purchased
from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The following monoclonal antibodies were used for western blotting:
Anti-c-myc (1:1,000; catalog no., 5605S; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-fibronectin (1:2,000; catalog no.,
610077; BD Biosciences, Franklin Lakes, NJ, USA), anti-β-actin
(1:1,000; catalog no., 66009-1-1g; Proteintech Group, Chicago, IL,
USA) and anti-α-tubulin (1:3,000; catalog no., sc-8035; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). All other chemicals,
including bovine serum albumin, protease inhibitor cocktail, PBS
and Tween-20, were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany).
Cell culture
S18 and S26 cells are clones of the human NPC cell
line CNE2. A stable radioresistant NPC cell line (Sune2-IR) and its
parental cell line (Sune2), and the 5–8F NPC cell line, were
supplied by and maintained in the State Key Laboratory of Oncology
in South China, Collaborative Innovation Center for Cancer
Medicine, Sun Yat-sen University Cancer Center (Guangzhou, China)
in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS)
(Invitrogen; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml)
and streptomycin (100 U/ml). The NPC cell lines were incubated at
37°C in 5% CO2/95% air.
Nasosphere formation assay
S18 and S26 cells were plated in triplicate at a
density of 200 cells per well in ultra-low attachment 6-well plates
(Corning Incorporated, Corning, NY, USA), and then cultured in
DMEM/F12 with 20 ng/ml recombinant human bFGF, 20 ng/ml recombinant
human EGF and B-27® Supplement. The spheres were
collected after 7 days and counted under a light microscope. Then,
we dissociated the primary sphere cells into single-cell
suspensions, which were cultured to allow the regeneration of
spheres.
SP assay
SP cell analysis and isolation were performed by
fluorescence-activated cell sorting (FACS) (Beckman Coulter, Inc.,
Brea, CA, USA). Before SP cell analysis, cells were pretreated with
different concentrations of LK-A for 48 h. Subsequently, the cells
were resuspended at a density of 1×106 cells/ml in RPMI
1640 supplemented with 2% FBS. Then, cells were incubated with 5
µg/ml Hoechst 33342 (Sigma-Aldrich; Merck Millipore) either alone
or with 100 µg/ml verapamil (Sigma-Aldrich; Merck Millipore) at
37°C in the dark for 90 min. Cells were washed, centrifuged at 1811
× g for 10 min at 4°C and resuspended in cold PBS. All cells
were kept at 4°C in the dark before FACS analysis using dual
wavelength analysis.
MTT cell viability assay
First, 2,000 cells were seeded into 96-well plates,
incubated overnight and then treated with various concentrations of
LK-A for 48 h. Then, 20 µl of MTT (5 mg/ml) was added to each well,
and the plate was incubated at 37°C for 4 h. Subsequently, the
supernatant was carefully removed, and 150 µl/well DMSO was added
to dissolve the formazan crystals. The absorbance of the soluble
product was measured with a microplate spectrophotometer at 490 nm
(µQuant™; Biotek Instruments, Inc., Winooski, VT, USA). This
experiment was performed in six replicates and repeated three
times. We calculated the percentage of cell viability for each
concentration of LK-A by using the following formula: Cell
viability (%) = A570 nm (sample) / A570 (control DMSO) × 100. The
half maximal inhibitory concentration (IC50) was
determined with GraphPad Prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA).
Drug interaction analysis
S18 and S26 cells were counted, plated in triplicate
at 2,000 cells per well (200 ml) in 96-well plates, and allowed to
grow overnight. For the experiment group, a concentration gradient
of cisplatin (DDP; Jiangsu Hansoh Pharmaceutical Group Co., Ltd.,
Lianyungang, China) was added to the wells. For the combination
groups, 1.0 µM LK-A was mixed with a concentration gradient of DDP
and then added to the wells. Cell viability was measured 48 h later
by adding an MTT solution. The observation value was detected at
490 nm. The results from the assays were analyzed for the
combination effect between LK-A and DDP according to the method
described by Jin (17). The following
formula was used: Q = Ea + b / [Ea + Eb - (Ea × Eb)], where Ea + b,
Ea and Eb are the average effects of the combination treatment,
LK-A only and DDP only, respectively (17). Q<0.85 indicates antagonism,
0.85≤Q<1.15 indicates additivity and Q≥1.15 indicates
synergism.
Radiation clonogenic cell survival
assay
The NPC cells were seeded into six-well plates with
400 cells per well. Twenty-four hours after plating, LK-A was added
at a concentration of 0.4 µM. After 24 -h exposure to LK-A, the
Sune2 cells and the radioresistant Sune2-IR cells were irradiated
at doses of 0, 1, 2 and 3 Gy. Immediately following irradiation,
the cells were incubated for 7 days at 37°C to allow colony
formation in a 5% CO2 environment. After 10 days, the
colonies were washed with PBS, fixed with pure ethanol and stained
with 0.5% crystal violet. Colonies containing >50 cells were
counted as clonogenic survivors. Each point on the survival curves
represents the mean surviving fraction (SF) from at least three
dishes. The equation SF=1-(1-e-D⁄D0)N was
applied to calculate several parameters, including the cellular
radiosensitivity (mean lethal dose, D0), the capacity for sublethal
damage repair (quasi-threshold dose, Dq) and the extrapolation
number (N). Those parameters were used to calculate the
sensitization enhancement ratio (SER) and the SF2 (the SF after
irradiation at a dose of 2 Gy).
Western blotting
Cells were seeded into 6-well plates and incubated
overnight. Then, the cells were treated with various concentrations
of LK-A for 48 h. We used a previously described western blotting
method (18). Briefly, equal amounts
of protein were separated by 9% SDS-PAGE and electrophoretically
transferred onto polyvinyl difluoridine membranes. Rabbit
anti-human c-myc antibody (1:1,000; Cell Signaling Technology,
Inc.) and mouse anti-human fibronectin antibody (1:2,000; BD
Biosciences) were used to detect the expression of c-myc and
fibronectin, and were incubated with the membranes for 12 h at 4°C.
Anti-rabbit immunoglobulin (Ig)G and anti-mouse IgG secondary
antibodies were also used, and α-tubulin was used as an internal
control. A chemiluminescence substrate and photographic X-ray
imaging was used for visualization.
Statistical analysis
Data are presented as the mean ± standard deviation.
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was
used to determine IC50, and SigmaPlot v10.0 (Systat
Software, Inc., San Jose, CA, USA) was used to construct cell
survival curves. Student's t-test was used to compare the means of
various groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
LK-A inhibits the self-renewal
capacity of NPC S18 and S26 cells
To investigate the effect of LK-A on the
self-renewal ability of NPC CSCs, we evaluated the sphere-forming
capacity after adding LK-A to S18 and S26 cells growing in
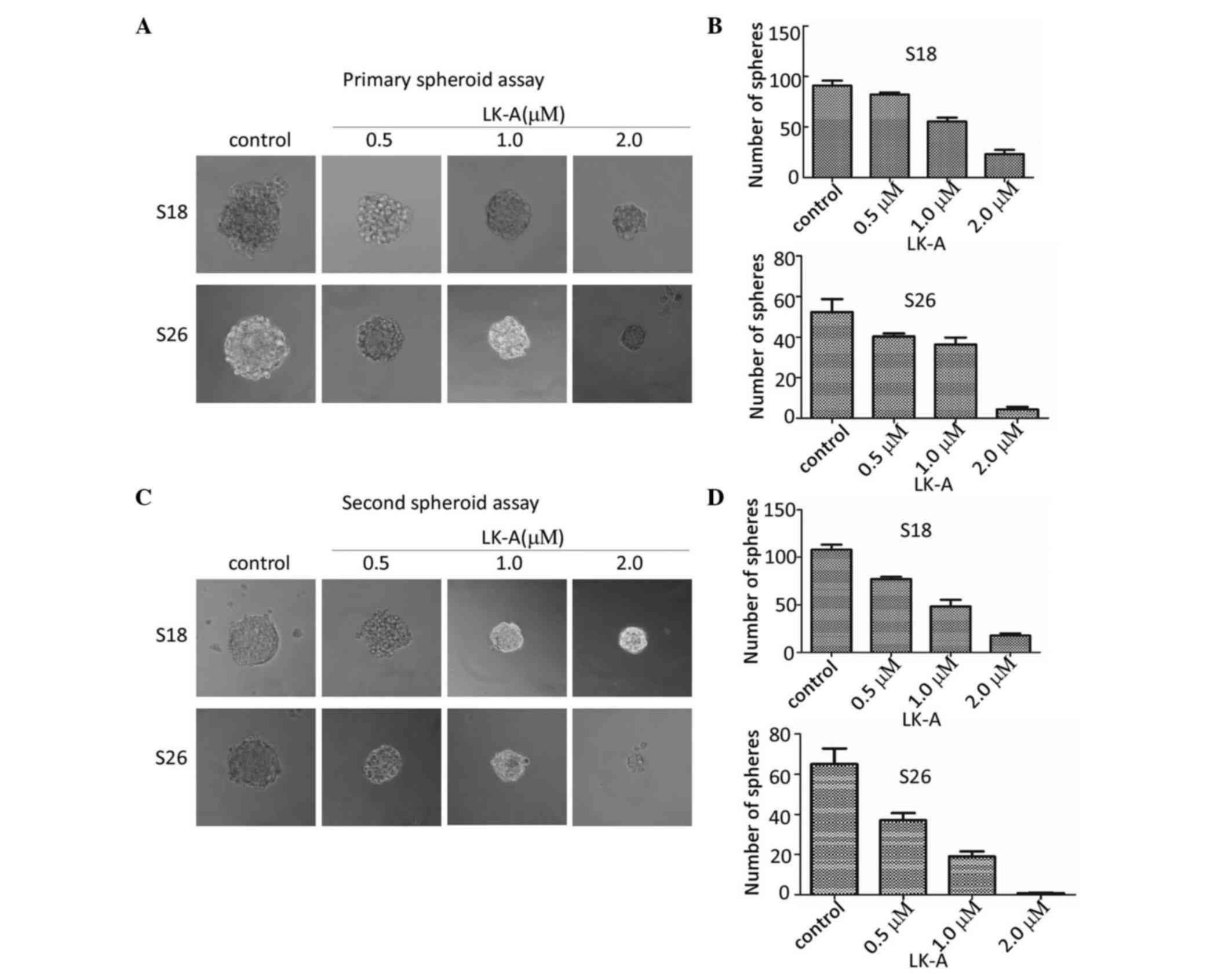
serum-free non-adherent medium. The result revealed that
interference by LK-A resulted in markedly diminished sphere size
and number of spheres in a dose-dependent manner in both S18 and
S26 cells (Fig. 1A and B).
To further investigate the effect of LK-A on
second-generation spheres, after spheres formation, we dissociated
the sphere cells into single cells, and we cultivated these single
cells in serum-free non-adherent culture with various
concentrations of LK-A again. The first-generation spheres were
capable of generating second-generation spheres, suggesting that
NPC sphere-generated cells have the capacity of self-renewal
(Fig. 1C). Meanwhile, we observed
that second-generation spheres formed more spheres than primary
spheres (Fig. 1B and D). These
second-generation spheres were observed to have decreased
sphere-forming efficiency under treatment with increasing
concentrations of LK-A, with smaller sphere size and lower number
of spheres (Fig. 1C and D). These
data indicated that LK-A inhibited the self-renewal capacity of NPC
cells in a dose-dependent manner.
LK-A decreases the percentage of SP
cells in the S18 cell line
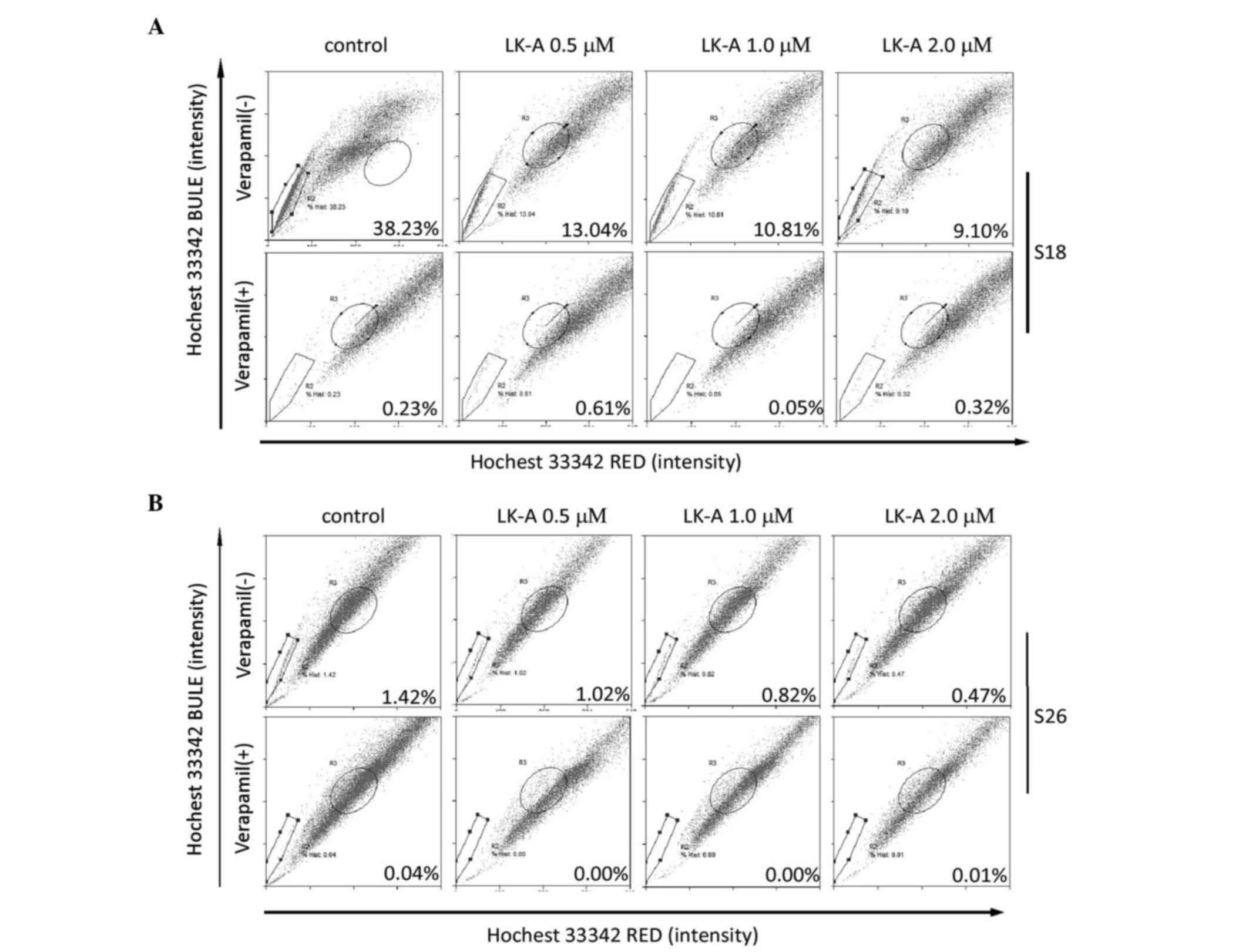
SP cells are one subpopulation of the cancer cells
that effluxes the DNA binding dye Hoechst 33342 out of the cell
membrane. Previous studies have shown that the SP cells have stem
cell characteristics and enrich the stem cell population in NPC
(19). To investigate whether LK-A
could impact the percentage of the SP cells in NPC cells, we
measured the SP cell percentage of S18 and S26 cells treated with
LK-A for 48 h. S18 and S26 are two cell lines originated from CNE2,
and S18 has a higher stemness characteristic with a high SP cell
proportion, while S26 has a lower stemness characteristic with a
lower SP cell proportion (13). As
shown in Fig. 2A and B, both in S18
and S26 cells, SP cells are blocked by verapamil at a final
concentration of 100 µg/ml. These results indicated that the
percentage of SP cells was ~26-fold higher in S18 cells than in S26
cells (38.23 vs. 1.47%). Upon treatment with LK-A, the percentages
of SP cells in S18 and S26 cells were all decreased (Fig. 2A and B). S18 cells showed a 2.93-fold
decrease in the SP cell proportion upon addition of 0.5 µM LK-A
(Fig. 2A). Taken together, these
results indicate that LK-A could decrease the percentage of the SP
cells in NPC cells.
LK-A potentiates the additivity effect
of DDP in NPC cells
CSCs are believed to be the cause of chemotherapy
resistance (6). Chemotherapy is not
effective for a large number of patients with NPC. As LK-A could
decrease the proportion of SP cells in NPC cells, we attempted to
determine whether LK-A could increase the chemotherapy sensitivity
to DDP of NPC cells. As shown in Fig.
3A-C, the dose-response curves of DDP with 1 µM LK-A were
obviously shifted to the left in NPC cells. The IC50
value for DDP in the presence of LK-A in S26, S18 and 5–8F cells
was significantly reduced (Table I).
According to the method by Jin (17),
the Q value was 1.04 in S18 cells, 0.86 in S26 cells and 1.02 in
5–8F cells at the concentration of 6.0 µM LK-A (Fig. 3D). As shown in Fig. 3E, at a higher concentration of LK-A
(8.0 µM), the Q value was 0.97 in S18 cells, 0.91 in S26 cells and
0.87 in 5–8F cells. To the best of our knowledge, Q<0.85
indicates that the combination of two drugs has an effect of
antagonism, while a Q value between 0.85 and 1.15 indicates an
additivity effect and Q>1.15 means synergism. Therefore, these
data suggested that LK-A has an additive effect to that of DDP in
the treatment of NPC cells.
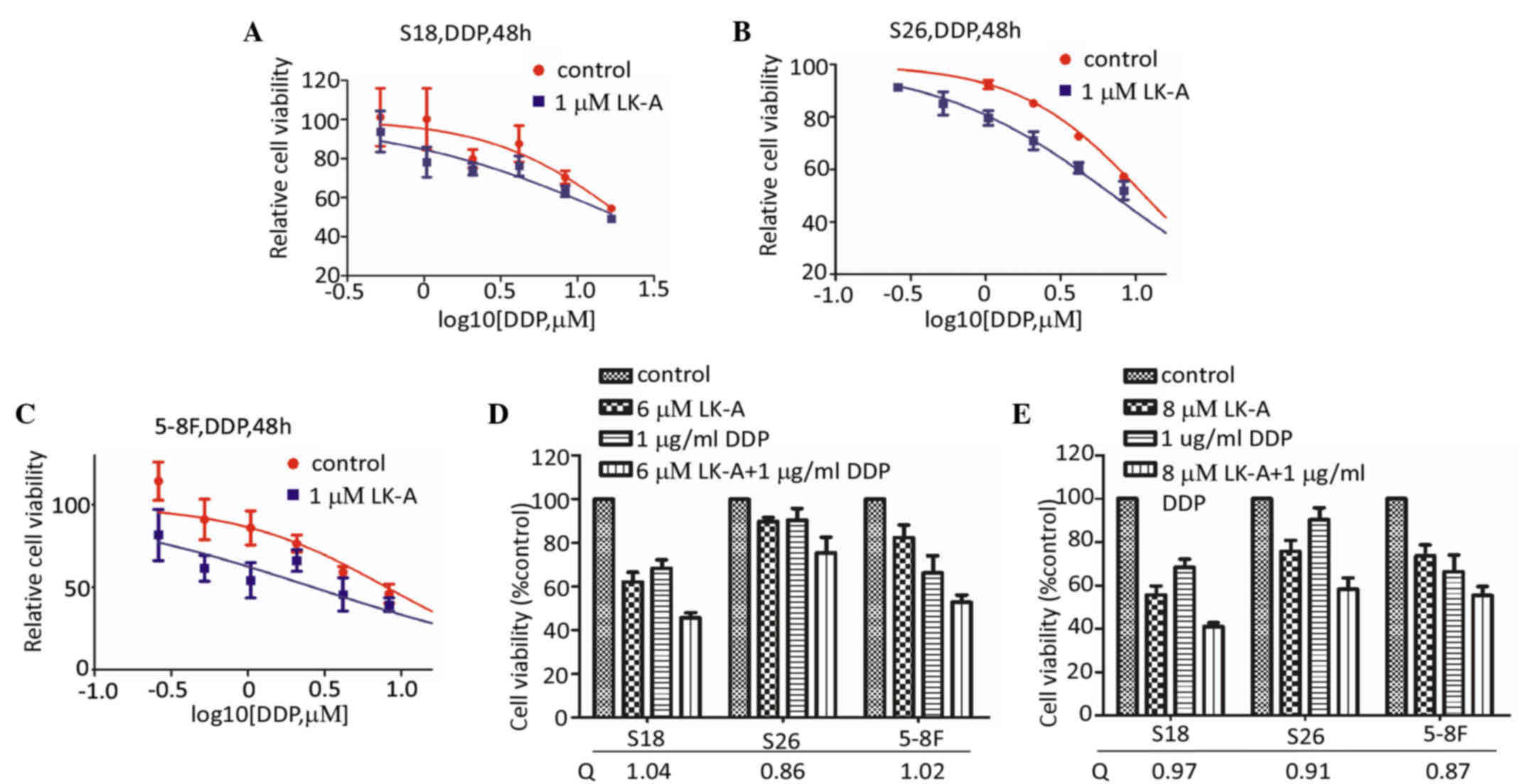 | Figure 3.Cytotoxic effect of cisplatin alone
or in combination with LK-A. (A-E) Shifting of dose-response curves
of cisplatin by LK-A. Three nasopharyngeal carcinoma cell lines,
(A) S18, (B) S26 and (C) 5–8F, were assayed by MTT assay. (D) LK-A
potentiated the effect of cisplatin in the three cell lines.
Q=Ea+b/(Ea+Eb-EaxEb), where Ea+b, Ea and Eb are the average effects
of the combination treatment, LK-A only and cisplatin only,
respectively. Q<0.85 indicates antagonism, 0.85≤Q<1.15
indicates additivity and Q≥1.15 indicates synergism. LK-A,
longikaurin A; DDP, cisplatin. |
 | Table I.IC50 value for DDP in the
presence or absence of LK-A in S26, S18 and 5–8F cells. |
Table I.
IC50 value for DDP in the
presence or absence of LK-A in S26, S18 and 5–8F cells.
| IC50
value for DDP in the cell lines (48 h) | S18 | S26 | 5-8F |
|---|
| Control | 10.10±1.00 µM | 11.45±1.06 µM | 8.00±0.90 µM |
| 1 µM LK-A | 7.00± 0.85 µM | 7.12±0.85 µM | 2.65±0.42 µM |
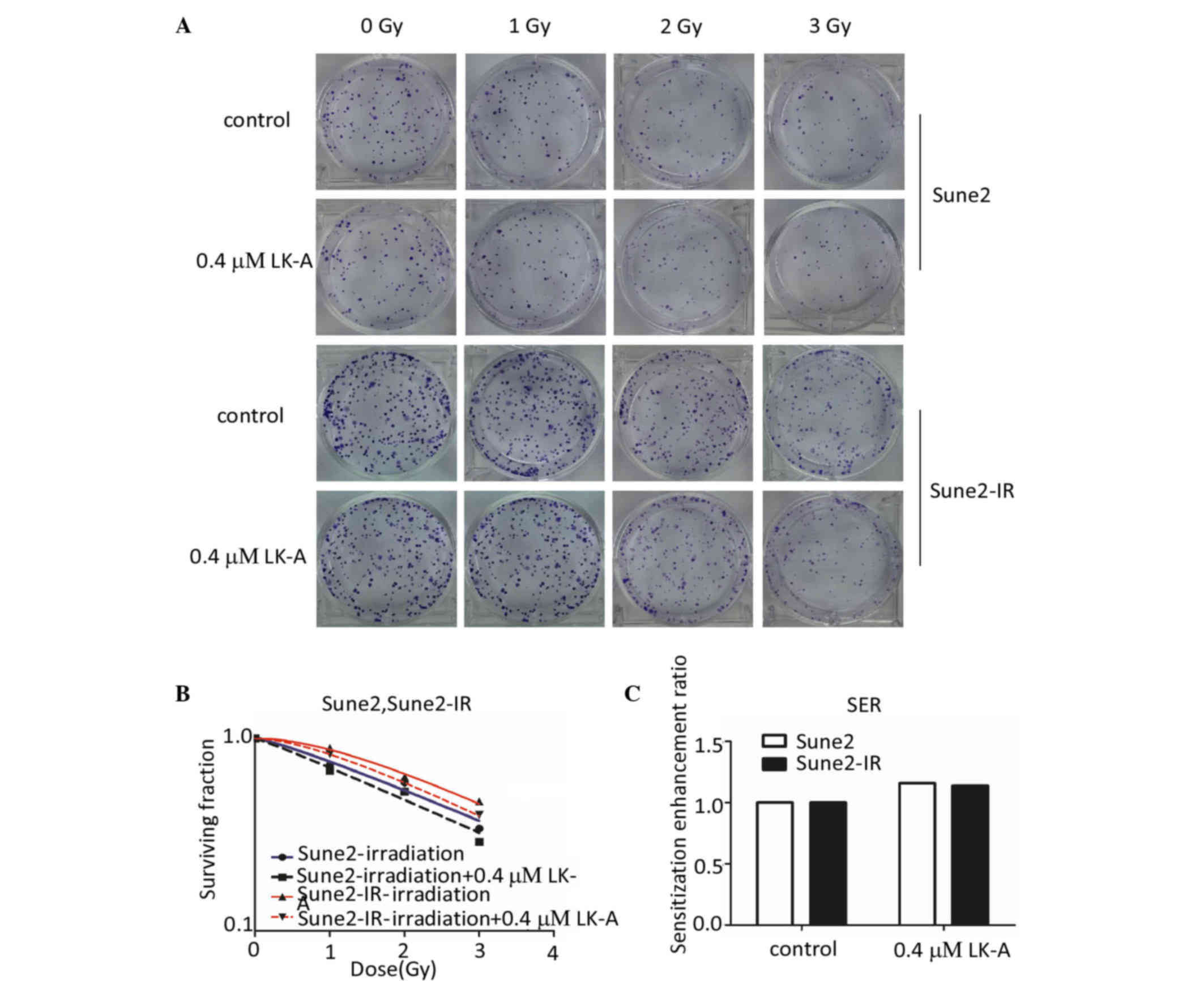
LK-A sensitizes NPC cells to radiation
and reverses radioresistance in NPC cells
Despite improvements in radiation technology, local
recurrence or metastasis occurs in a high proportion of NPC
patients due to radioresistance (20). According to the experiment results
described above, we aimed to investigate whether LK-A could
sensitize the radioresistance of NPC cells. The Sune2-IR
radioresistant cell line was obtained from Sune2 cells exposed to
ionizing radiation (cumulative dose is 28 Gy). Using the clonogenic
survival assay, we found a marked reduction in clone formation in
Sune2 and Sune2-IR cells after irradiation combined with the
intervention of LK-A (Fig. 4A). Each
point on the survival curve represents the mean SF from triplicate
experiments (Fig. 4B). There was a
significant difference in SF between parental and radioresistant
cells at 1, 2 and 3 Gy of radiation, indicating that the Sune2-IR
cells were more radiation resistant than the Sune2 cells.
Additionally, LK-A (0.4 µM) shifted the Sune2 and Sune2-IR cell
dose survival curves clearly to the left, which indicated the
increased radiosensitivity of Sune2 and Sune2-IR cells caused by
LK-A treatment (Fig. 4B). Sune2 and
Sune2-IR cells treated with LK-A (0.4 µM) had a SER of 1.16 and
1.14, respectively (Fig. 4C). The
survival curve parameters are listed in Table II. All parameters of the LK-A groups
were smaller than those of the control groups.
 | Table II.Clonogenic survival parameters of
Sune2 and Sune2-IR cells after LK-A exposure. |
Table II.
Clonogenic survival parameters of
Sune2 and Sune2-IR cells after LK-A exposure.
| Cell lines | Groups | D0 | Dq | SF2 | N | SER (Dq) |
|---|
| Sune2 | Control | 2.58 | 1.17 | 0.59 | 1.25 | 1.16 |
|
| 0.4 µM LK-A | 2.48 | 1.01 | 0.53 |
1.10 |
|
| Sune2-IR | Control | 2.22 | 1.51 | 0.63 | 2.04 | 1.14 |
|
| 0.4 µM LK-A | 2.15 | 1.33 | 0.58 | 1.76 |
|
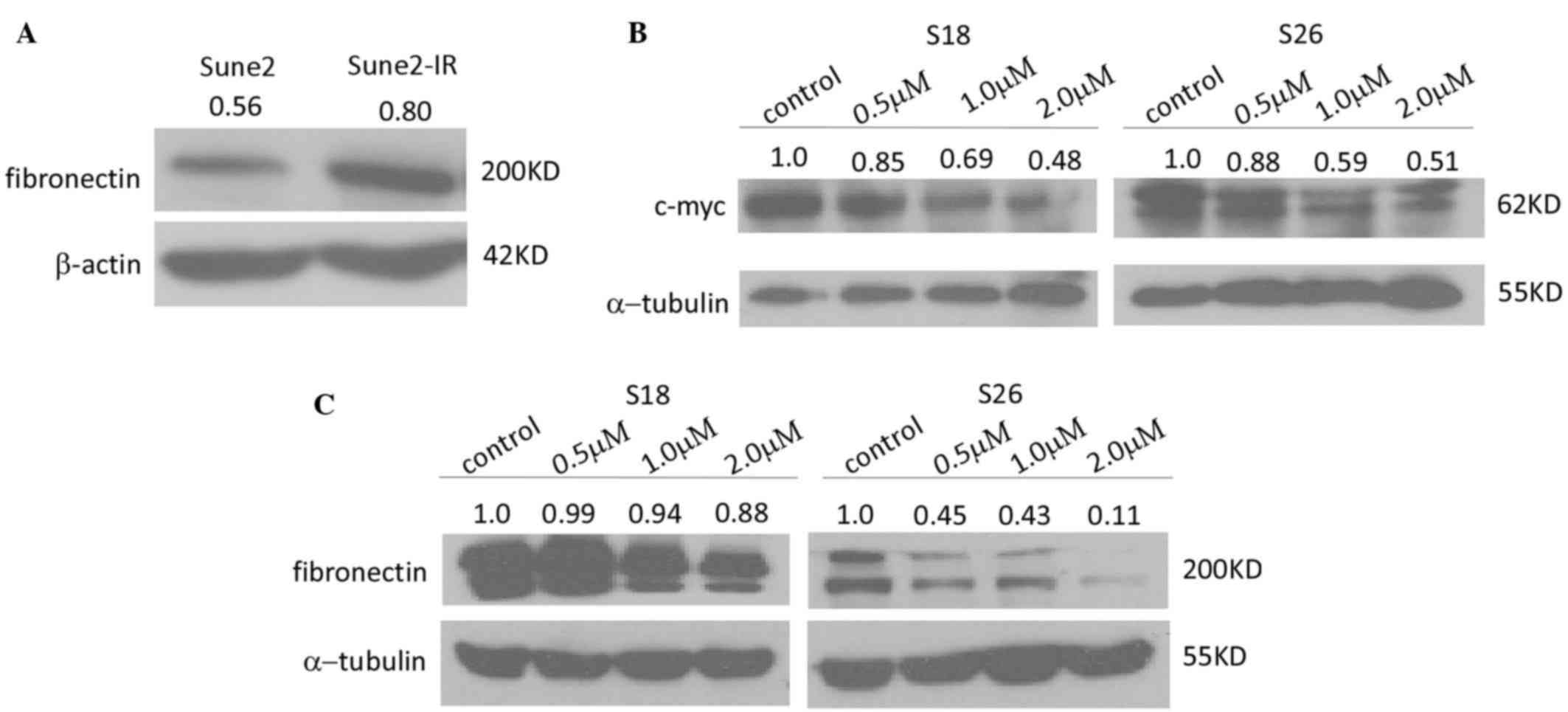
It was reported that silencing fibronectin extra
domain A (EDA) enhances radiosensitivity in NPC (21). Therefore, we performed western
blotting to detect the protein expression of fibronectin in the
Sune2 and Sune2-IR cell lines. Fig.
5A demonstrates that Sune2-IR cells expressed fibronectin at a
higher level than Sune2 cells. These results demonstrated that LK-A
could sensitize the radioresistance of NPC cells to
irradiation.
LK-A downregulates c-myc and
fibronectin
As it is well known that there are not reliable CSC
markers in NPC cells (22), we
investigated whether LK-A could influence the expression of stem
cell markers in NPC cells. The expression of Nanog, sex determining
region Y-box 2 and octamer-binding transcription factor 4 could not
be detected in the S18 or S26 cell lines. In addition, the
expression of the Bmi-1, ATP-binding cassette (ABC) G2 and
β-catenin were not downregulated by the interference of LK-A (data
not show). However, we could detect that LK-A decreased the
expression of c-myc in NPC cells (Fig.
5B). c-myc is sufficient to impart high self-renewal and
tumor-propagating capacities in breast cancer (23), and overexpression of c-myc increases
the stemness of NPC cells (24).
These data indicated that LK-A may influence the stemness of NPC
cells by downregulating the expression of c-myc.
As aforementioned, LK-A may sensitize Sune2 cells to
radiation and reversed the radioresistance of Sune2-IR cells
through influencing fibronectin protein expression. Fibronectin is
known to play important roles in angiogenesis, lymphangiogenesis
and metastasis in malignant tumors (25). Therefore, we wondered whether LK-A
treatment could downregulate the protein expression of fibronectin
in NPC cells. As shown in Fig. 5C,
LK-A treatment downregulated the protein expression of fibronectin
in a dose-dependent manner. These data indicated that LK-A may
impair radioresistance by downregulation of fibronectin.
Discussion
Currently, the main treatments of NPC are
radiotherapy and platinum-based chemotherapy (3). However, patients still relapse from
primary treatment with radiotherapy or chemo-irradiation, and some
NPC patients that are diagnosed at advanced stages fail to react to
the treatment and succumb to cancer progression (26). Therefore, CSCs, which should be
responsible for the malignant neoplasm recurrence and metastasis
(5), may be a novel therapy target
for NPC. In addition, natural products and their derivatives act as
new therapy drugs to target the NPC CSCs.
As a natural ent-kaurene diterpenoid, LK-A has been
reported to induce apoptosis in multiple myeloma H929 cells,
hepatocellular carcinoma cells and NPC cells (15,16,27).
However, there are limited studies on the role of LK-A in targeting
CSCs. Our recent study revealed that LK-A inhibited the colony
formation ability of NPC cells at a low concentration and exhibited
anti-tumor activity in CNE2 xenograft tumor models (15). This may be due to a key role of LK-A
in targeting the NPC CSCs. In the present study, in a
dose-dependent manner, LK-A reduced the nasosphere formation rate,
and decreased the number and volume of the primary spheres.
Moreover, the secondary nasosphere formation efficiency of S18 and
S26 cells was also impaired following treatment with LK-A (Fig. 1A-D). To the best of our knowledge, SP
cells have numerous stem cell properties, including unlimited
proliferation potential, self-renewal, differentiation, resistance
to chemotherapy and radiation, and a strong tumor formation ability
in vivo (19). According to
Fig. 2A, LK-A could decrease the
percentage of the SP cells in the S18 cells. Due to the low numbers
of SP cells in S26 cells, we found that LK-A could not increase the
percentage of SP cells (Fig. 2B).
Together, these data suggest that LK-A suppressed the stemness of
NPC cells in vitro.
In addition to serving as a potential therapeutic
for NPC CSCs, our results suggest that LK-A exerts additive effects
with DDP on NPC cells (Fig. 3A-E).
Radiotherapy is known as the main standard treatment of NPC.
Therefore, we wonder whether LK-A could enhance the efficacy of
radiation in NPC cells. The results demonstrated that LK-A
treatment not only could sensitize Sune2 cells to radiation, but
also reverse the radioresistance of Sune2-IR cells (Fig. 4A-C).
Previous studies indicated that silencing
fibronectin EDA increases radiosensitivity in NPC involving the
focal adhesion kinase/Akt/c-Jun N-terminal kinase pathway (20). According to Fig. 5A, LK-A may enhance the radiation
efficacy though downregulation of fibronectin. Moreover, to the
best of our knowledge, fibronectin includes three components: EDA,
EDB and connecting segment III, an important extracellular matrix
glycoprotein in the tumor microenvironment (28). It has been recently reported that
fibronectin can enhance the formation of multi-cellular spheroids
in ovarian cancer (29). Therefore,
as expected, the protein expression level of fibronectin decreased,
which was induced by LK-A, in NPC cells (Fig. 3B). However, the mechanism requires to
be further investigated.
Previous research about LK-A indicated that LK-A
induces G2/M phase arrest via downregulation of S-phase
kinase-associated protein 2 (Skp2) and apoptosis induction in
hepatocellular carcinoma cells (17).
Recent studies suggest that Skp2 regulates the NPC CSC maintenance
(30). However, Skp2 was not affected
in NPC cells treated with LK-A.
Furthermore, we explored the effects of LK-A on stem
cell markers. Despite the fact that LK-A treatment decreased the
percentage of SP in NPC cells, the expression of ABCG2 protein was
not affected (data not show). However, we found that LK-A reduced
the expression of c-myc in a dose-dependent manner (Fig. 5B and C). Meanwhile, c-myc is one
member of the myc oncoprotein family, whose role in the
pathogenesis of numerous human neoplastic diseases has received
wide empirical support. It is largely believed that c-myc plays an
important role in carcinogenesis and tumor progression due to its
influence on all basic cellular processes (31). In our study, both S18 and S26 cells
overexpressed c-myc. Following treatment with LK-A, the expression
of c-myc was clearly downregulated (Fig.
5B). The self-renewal ability of cells can be verified by
spheroid formation assay. Previous studies have indicated that
c-myc serves a key role in cooperative actions with p53 and
phosphatase and tensin homolog in the regulation of normal and
malignant stem/progenitor cell differentiation, self-renewal and
tumorigenic potential (32).
Therefore, LK-A may affect the self-renewal ability of NPC cells
through c-myc. Moreover, c-myc could promote radioresistance in
PKH26+ NPC cells, which are enriched for a stem cell-like
subpopulation and are resistant to radiotherapy (24). Therefore, LK-A enhances
radiosensitivity in NPC due to fibronectin and c-myc. However, the
mechanism by which LK-A influences the expression of c-myc requires
to be further investigated.
In summary, LK-A could target CSCs in NPC cells, and
it could enhance the efficacy of chemotherapy drugs and
radiotherapy in vitro. However, future studies are required
to clarify the mechanisms by which LK-A targets CSCs. Determination
of the feasibility of the clinical application of this compound is
also important for the treatment of NPC patients.
References
|
1
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou
ZX and Chen WQ: Nasopharyngeal carcinoma incidence and mortality in
China in 2010. Chin J Cancer. 33:381–387. 2014.PubMed/NCBI
|
|
3
|
Xu T, Tang J, Gu M, Liu L, Wei W and Yang
H: Recurrent nasopharyngeal carcinoma: A clinical dilemma and
challenge. Curr Oncol. 20:e406–e419. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C: Radiation Therapy for Head and
Neck Neoplasms. 3rd. Wiley-Liss; New York, NY: 1997
|
|
5
|
Zhang Z, Filho MS and Nör JE: The biology
of head and neck cancer stem cells. Oral Oncol. 48:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ke J, Wu X, Wu X, He X, Lian L, Zou Y, He
X, Wang H, Luo Y, Wang L and Lan P: A subpopulation of
CD24+ cells in colon cancer cell lines possess stem cell
characteristics. Neoplasma. 59:282–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: An enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Chen ZG, Du CZ, Wang HW, Yan L and
Gu J: Cancer stem cell marker CD133+ tumour cells and clinical
outcome in rectal cancer. Histopathology. 55:284–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng CC, Liang Y, Wu MS, Feng FT, Hu WR,
Chen LZ, Feng QS, Bei JX and Zeng YX: Nigericin selectively targets
cancer stem cells in nasopharyngeal carcinoma. Int J Biochem Cell
Biol. 45:1997–2006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu MS, Wang GF, Zhao ZQ, Liang Y, Wang HB,
Wu MY, Min P, Chen LZ, Feng QS, Bei JX, et al: Smac mimetics in
combination with TRAIL selectively target cancer stem cells in
nasopharyngeal carcinoma. Mol Cancer Ther. 12:1728–1737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou QF, Du JK, Zhang H, Wang HB, Hu ZD,
Chen SP, Du Y, Li MZ, Xie D, Zou J, et al: Anti-tumour activity of
longikaurin A (LK-A), a novel natural diterpenoid, in
nasopharyngeal carcinoma. J Transl Med. 11:2002013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao YJ, Bai HY, Li ZH, Zou J, Chen JW,
Zheng F, Zhang JX, Mai SJ, Zeng MS, Sun HD, et al: Longikaurin A, a
natural ent-kaurane, induces G2/M phase arrest via downregulation
of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in
hepatocellular carcinoma cells. Cell Death Dis. 5:e11372014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin ZJ: About the evaluation of drug
combination. Acta Pharmacol Sin. 25:146–147. 2004.PubMed/NCBI
|
|
18
|
Cao JY, Liu L, Chen SP, Zhang X, Mi YJ,
Liu ZG, Li MZ, Zhang H, Qian CN, Shao JY, et al: Prognostic
significance and therapeutic implications of centromere protein F
expression in human nasopharyngeal carcinoma. Mol Cancer.
9:2372010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Y, Zhu XD, Qu S, Li L, Su F, Li Y,
Huang ST and Li DR: Identification of genes involved in
radioresistance of nasopharyngeal carcinoma by integrating gene
ontology and protein-protein interaction networks. Int J Oncol.
40:85–92. 2012.PubMed/NCBI
|
|
21
|
Ou J, Pan F, Geng P, Wei X, Xie G, Deng J,
Pang X and Liang H: Silencing fibronectin extra domain A enhances
radiosensitivity in nasopharyngeal carcinomas involving an
FAK/Akt/JNK pathway. Int J Radiat Oncol Biol Phys. 82:e685–e691.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei P, Niu M, Pan S, Zhou Y, Shuai C, Wang
J, Peng S and Li G: Cancer stem-like cell: A novel target for
nasopharyngeal carcinoma therapy. Stem Cell Res Ther. 5:1–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair R, Roden DL, Teo WS, McFarland A,
Junankar S, Ye S, Nguyen A, Yang J, Nikolic I, Hui M, et al: c-Myc
and Her2 cooperate to drive a stem-like phenotype with poor
prognosis in breast cancer. Oncogene. 33:3992–4002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X,
Zhang QB and Yao KT: MYC regulation of CHK1 and CHK2 promotes
radioresistance in a stem cell-like population of nasopharyngeal
carcinoma cells. Cancer Res. 73:1219–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou JJ, Wu F and Liang HJ: Colorectal tumor
derived fibronectin alternatively spliced EDA domain exserts
lymphangiogenic effect on human lymphatic endothelial cells. Cancer
Biol Ther. 9:186–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu T, Tang J, Gu M, Liu L, Wei W and Yang
H: Recurrent nasopharyngeal carcinoma: A clinical dilemma and
challenge. Curr Oncol. 20:e406–e419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao S, Pu JX, Sun HD and Wu YL:
Longikaurin A induces apoptosis of multiple myeloma H929 cells.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 20:611–615. 2012.(In Chinese).
PubMed/NCBI
|
|
28
|
French-Constant C: Alternative splicing of
fibronectin-many different proteins but few different functions.
Exp Cell Res. 221:261–271. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie XY, Liu SL, Wang H, Lin L, Zhu HM,
Zhang JJ and Zheng Y: The influence of fibronectin on the formation
of multi-cellular spheroid of ovarian cancer. Sichuan Da Xue Xue
Bao Yi Xue Ban. 45:240–244. 2014.(In Chinese). PubMed/NCBI
|
|
30
|
Wang J, Huang Y, Guan Z, Zhang JL, Su HK,
Zhang W, Yue CF, Yan M, Guan S and Liu QQ: E3-ligase Skp2 predicts
poor prognosis and maintains cancer stem cell pool in
nasopharyngeal carcinoma. Oncotarget. 5:5591–5601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng H, Ying H, Yan H, Kimmelman AC,
Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al: Pten
and p53 converge on c-Myc to control differentiation, self-renewal
and transformation of normal and neoplastic stem cells in
glioblastoma. Cold Spring Harb Symp Quant Biol. 73:427–437. 2008.
View Article : Google Scholar : PubMed/NCBI
|