Introduction
Human glioma is located within the brain or spinal
cord, and represents the most frequently occurring type of
malignant central nervous system tumor (1). The annual incidence rate of gliomas has
increased, and accounts for 1.9% of total tumor incidences in the
world (2). Gliomas are characterized
by rapid growth, high infiltration and difficulty in surgical
resection, and the majority of patients with glioma are diagnosed
at stage IV (3). Treatment strategies
for human gliomas primarily consist of surgery, followed by
radiotherapy and chemotherapy. However, this malignancy often has a
high degree of malignancy, recurrence rate, multi-drug resistance
and invasiveness, leading to poor efficacy and final clinical
outcomes (3). The 5-year survival
rate for patients with gliomas is poor. Particularly, the median
survival of patients who have glioblastoma multiforme (GBM) ranges
between 9 and 12 months (4). Previous
studies on molecular biology of tumors have indicated that human
gliomas may be complex diseases caused by interactions among
various genes, a series of multiple oncogene activation and
tumor-suppressor inactivation (5,6).
Therefore, it is of great clinical significance to understand the
molecular pathogenesis of gliomas in order to identify novel drug
targets to improve treatment efficacy and enhance patients'
survival.
Liver kinase B1 (LKB1), also termed serine/threonine
protein kinase 11, is located on chromosome 19p13.3 and encodes a
~48 kDa serine/threonine protein kinase (7). LKB1 was originally observed to be
mutated in Peutz-Jeghers syndrome, a rare cancer susceptibility
syndrome featured by predisposition to gastrointestinal polyposis,
mucocutaneous melanin pigmentation and multi-organ cancer
susceptibility (8). Functionally,
LKB1 serves roles in multiple cellular processes, including cell
structure control, cell cycle regulation, apoptosis and cellular
metabolism (9). As a multifunctional
protein, LKB1 acts as a key metabolic enzyme in the 5′ adenosine
monophosphate-activated protein kinase (AMPK) pathway, and its
inactivation often leads to the activation of the mammalian target
of rapamycin pathway, which is important for controlling cellular
energy metabolism, cell survival and growth under metabolic stress
such as nutrient deficiency (10).
Growing evidence shows that LKB1 acts as a tumor-suppressor gene by
activating AMPK or AMPK-related kinases, which in turn regulate
cell cycle, cell apoptosis, cell polarity and metabolism (11). In human gliomas, Godlewski et
al (12) identified microRNA
(miR)-451 as a regulator of the LKB1/AMPK pathway, which may be a
fundamental mechanism contributing to cellular adaptation in
response to altered energy availability. Jiang et al
(13) reported that probucol, which
exerts antitumor activities at various stages of tumor initiation,
promotion and progression, suppressed human glioma cell
proliferation in vitro via reactive oxygen species
production and LKB1-AMPK activation. Although these previous
findings suggested that the LKB1-AMPK pathway facilitates glioma
cell growth, the clinical significance of LKB1 expression in large
numbers of glioma patients remains unclear.
In the present study, western blotting and
quantitative polymerase chain reaction (qPCR) were performed to
examine the expression of LKB1 at the protein and messenger RNA
(mRNA) levels in 30 pairs of freshly prepared glioma and
non-neoplastic brain tissues. Subsequently, immunohistochemistry
was used to validate the expression pattern of LKB1 protein in 180
patients with glioma. The associations between LKB1 immunoreactive
scores and various clinicopathological characteristics were then
statistically analyzed. Furthermore, the prognostic value of LKB1
expression in glioma patients was additionally evaluated using
Kaplan-Meier survival curves and Cox proportional hazards
regression models.
Materials and methods
Patients and tissue samples
The current study was approved by the Research
Ethics Committee of The First Affiliated Hospital of Medical
College, Shantou University (Shantou, China). All patients enrolled
in the study provided written informed consent. All specimens were
handled and made anonymous according to ethical and legal standards
of The First Affiliated Hospital of Medical College, Shantou
University, and were obtained under sterile conditions during
surgery.
For western blotting and qPCR, 30 pairs of freshly
prepared glioma and non-neoplastic brain tissues were obtained from
the Department of Neurosurgery, The First Affiliated Hospital of
Medical College, Shantou University (Shantou, China) between
January 2010 and December 2014. The mean patient age was 50.6 years
(range, 12–88 years), and 20 (66.67%) of them were male, while 10
(33.33%) were female. According to the clinicopathological criteria
provided by the World Health Organization (WHO) (14): 3 patients were WHO grade I as
pilocytic astrocytomas; 10 patients were grade II, including 5
patients with fibrillary astrocytoma, 3 patients with protoplasmic
astrocytoma and 2 patients with oligodendroglioma; 12 patients were
WHO grade III, including 8 patients with anaplastic astrocytoma and
4 patients with anaplastic oligodendroglioma; and 5 patients were
WHO grade IV, all GBM.
For immunohistochemistry, 180 patients-derived
paraffin-embedded glioma tissues were obtained from the Department
of Neurosurgery, The First Affiliated Hospital of Medical College,
Shantou University between January 2005 and December 2014. The mean
patient age was 50.8 years (range, 10–86 years). Of these patients,
110 (61.11%) were male and 70 (38.89%) were female. According to
the clinicopathological criteria provided by WHO (14), 15 patients were WHO grade I as
pilocytic astrocytomas; 55 patients were WHO grade II, including 25
patients with fibrillary astrocytoma, 20 patients with protoplasmic
astrocytoma and 10 patients with oligodendroglioma; 85 patients
were WHO garde III, including 50 patients with anaplastic
astrocytoma and 35 patients with anaplastic oligodendroglioma; and
25 patients were WHO grade IV, all GBM. Follow-up data were
completed for all 180 patients, with a median follow-up time of 32
months (range, 2–118 months). The clinicopathological
characteristics are summarized in Table
I. All patients did not undergo any other treatments prior to
surgery.
 | Table I.Association of LKB1 expression with
clinicopathological characteristics in human glioma. |
Table I.
Association of LKB1 expression with
clinicopathological characteristics in human glioma.
| Clinicopathological
characteristics | Patients, n (%) | LKB1-low, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
<50 | 88 (48.89) | 45 (51.14) | NS |
| ≥50 | 92 (51.11) | 47 (51.09) |
|
| Gender |
|
|
|
| Male | 110 (61.11) | 56 (50.91) | NS |
|
Female | 70 (38.89) | 36 (51.43) |
|
| Tumor size, cm |
|
|
|
|
<5 | 108 (60.00) | 40 (37.04) | 0.020 |
| ≥5 | 72 (40.00) | 52 (72.22) |
|
| Tumor location |
|
|
|
|
Supratentorial | 160 (88.89) | 82 (51.25) | NS |
|
Subtentorial | 20 (11.11) | 10 (50.00) |
|
| Karnofsky performance
scale |
|
|
|
|
<90 | 100 (60.00) | 72 (72.00) | 0.010 |
| ≥90 | 80 (40.00) | 20 (25.00) |
|
| WHO grade |
|
|
|
| I | 15 (12.00) | 0 (0.00) | 0.006 |
| II | 55 (36.00) | 15 (27.27) |
|
| III | 85 (42.00) | 52 (61.18) |
|
| IV | 25 (10.00) | 25 (100.00) |
|
| Tumor recurrence |
|
|
|
|
Absent | 118 (65.56) | 62 (52.54) | NS |
|
Present | 62 (34.44) | 30 (48.39) |
|
qPCR
qPCR was performed to detect the expression of LKB1
mRNA in 30 pairs of freshly prepared glioma and non-neoplastic
brain tissues. Total RNA was extracted from 30 pairs of freshly
prepared glioma and non-neoplastic brain tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. The RNA was
pretreated with DNase, and single-stranded complementary DNA was
synthesized using the SuperScript First-Strand Synthesis System or
RT-PCR (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Relative expression of LKB1 mRNA was
determined using a SYBR Green PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and an ABI 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.), and
normalized using GAPDH. The primer sequences used in the present
study were as follows: LKB1 forward, 5′-AGGGATGCTTGAGTACGAACC-3′
and reverse, 5′-GTCCTCCAAGTACGGCACC-3′; GAPDH forward,
5′-TGAACGGGAAGCTCACTGG-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
PCR amplifications for each gene were repeated three times. The
fold-change of each gene was calculated using the 2−ΔΔCq
method (15,16).
Western blot analysis
Western blot analysis was performed to detect the
expression of LKB1 protein in 30 pairs of freshly prepared glioma
and non-neoplastic brain tissues. Tissue samples were ground with
liquid nitrogen and lysed at 48°C for 30 min in lysis buffer (50 mM
Tris, pH 7.4, 100 mM NaCl2, 1 mM MgCl2, 2.5
mM Na3VO4, 1 mM phenylmethylsulfonyl
fluoride, 2.5 mM EDTA, 0.5 % Triton X-100, 0.5% NP-40, and 5 mg/ml
each of aprotinin, pepstatin A and leupeptin), and centrifuged at
10,000 × g for 30 min to collect the supernatant. Protein
concentration was determined using the Bradford method. An
equivalent amount of protein from each sample was separated by 10%
SDS-PAGE and then transferred to a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA), followed by incubation in
blocking buffer (PBS containing 5% nonfat milk) for 2 h at room
temperature. Subsequently, the membrane was incubated with a goat
polyclonal antibody against LKB1 (dilution, 1:200; catalogue no.,
sc-5638; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight
at 4°C. Next, the membrane was washed twice with PBS for 5 min and
incubated with secondary horseradish peroxidase-conjugated rabbit
anti-goat immunoglobulin G (IgG) antibody (dilution, 1:1,000;
catalogue no., sc-2949; Santa Cruz Biotechnology, Inc.) for 2 h at
room temperature. GAPDH was detected using a specific goat
polyclonal antibody (dilution, 1:500; catalogue no., sc-20358;
Santa Cruz Biotechnology, Inc.) as a loading control. Proteins were
visualized using an enhanced chemiluminescence reagent (Santa Cruz
Biotechnology, Inc.). Relative expression of LKB1 protein was
normalized to GAPDH. The experiments were repeated three times.
Immunohistochemistry
Immunohistochemistry was performed to detect the
expression pattern and subcellular localization of LKB1 protein in
180 pairs of paraffin-embedded glioma and non-neoplastic brain
tissues. Tissue sections were dewaxed in xylene, rehydrated using
graded alcohol and soaked in 0.3% hydrogen peroxide to block
endogenous peroxidase activity. The sections were then rinsed in
PBS, pH 7.2, and 10% goat serum (Gibco; Thermo Fisher Scientific,
Inc.) was applied for 1.5 h at room temperature to block
nonspecific reactions. The aforementioned goat polyclonal antibody
against LKB1 was incubated with the tissue sections overnight at
4°C. Negative control slides were processed in parallel using a
nonspecific IgG (dilution, 1:1,000; catalogue no., sc-2949; Santa
Cruz Biotechnology, Inc.) at the same concentration as the primary
antibody. Subsequent to being rinsed in PBS, the peroxidase
reaction was visualized by incubating the sections with a solution
containing 0.1% phosphate buffer, 0.02% 3,3′-diaminobenzidine
tetrahydrochloride and 3% H2O2. Finally, the
sections were counterstained with hematoxylin, dehydrated and
mounted in mounting resin.
The immunohistochemical results were evaluated by
two independent pathologists blinded to the patients'
clinicopathological characteristics. The immunoreactive scoring
(IRS) of LKB1 expression was calculated by combining the intensity
and the percentage of positive cells. The percentage of positive
cells was as follows: 0–5% scored 0; 6–35% scored 1; 36–70% scored
2; and >70% scored 3. The staining intensity was as follows: No
staining scored 0; weakly staining scored 1; moderately staining
scored 2; and strongly staining scored 3. The final IRS was
designated as low or high expression using the percentage of
positive cell score × staining intensity score.
Statistical analysis
SPSS software version 11.0 for Windows (SPSS, Inc.,
Chicago, IL, USA) was used for the statistical analyses. All data
obtained from experiments were expressed as the mean ± standard
deviation. The differences of LKB1 mRNA and protein expression
between glioma and non-neoplastic brain tissues were evaluated by
Student's paired t-test. Spearman's rank correlation analysis was
used to analyze the correlation between the level of LKB1 mRNA and
protein expression. The associations between LKB1 expression and
various clinicopathological features were analyzed by the
χ2 test. Kaplan-Meier survival plots were constructed to
analyze survival data. Univariate and multivariate analyses were
performed using Cox's proportional hazards model. P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulation of LKB1 mRNA and
protein in glioma tissues
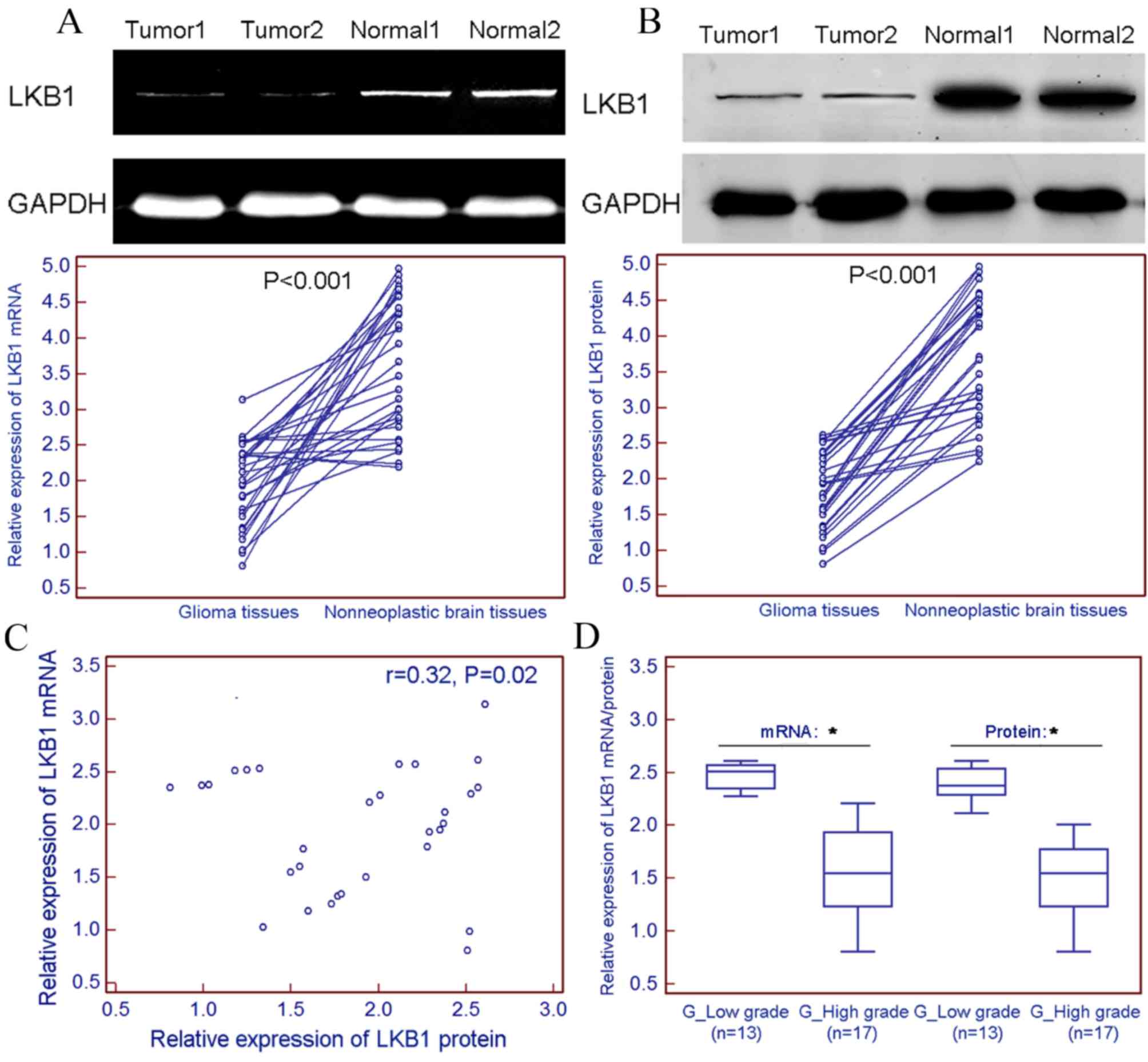
LKB1 expression was markedly decreased at the mRNA
(tumor vs. normal, 1.96±0.59 vs. 3.66±0.92; P<0.001; Fig. 1A) and protein (tumor vs. normal,
1.89±0.54 vs. 3.71±0.87; P<0.001; Fig.
1B) levels in 30 freshly prepared glioma tissues, compared with
non-neoplastic brain tissues. Spearman's rank correlation analysis
revealed that the expression levels of LKB1 mRNA in glioma tissues
were closely correlated with those of LKB1 protein in gliomas
(r=0.32; P=0.02; Fig. 1C). Notably,
the present results indicated a relatively lower expression level
of LKB1 mRNA and protein in freshly prepared high-grade glioma
tissue samples (WHO grades III–IV) compared with those in the
low-grade glioma samples (WHO grades I–II; Fig. 1D).
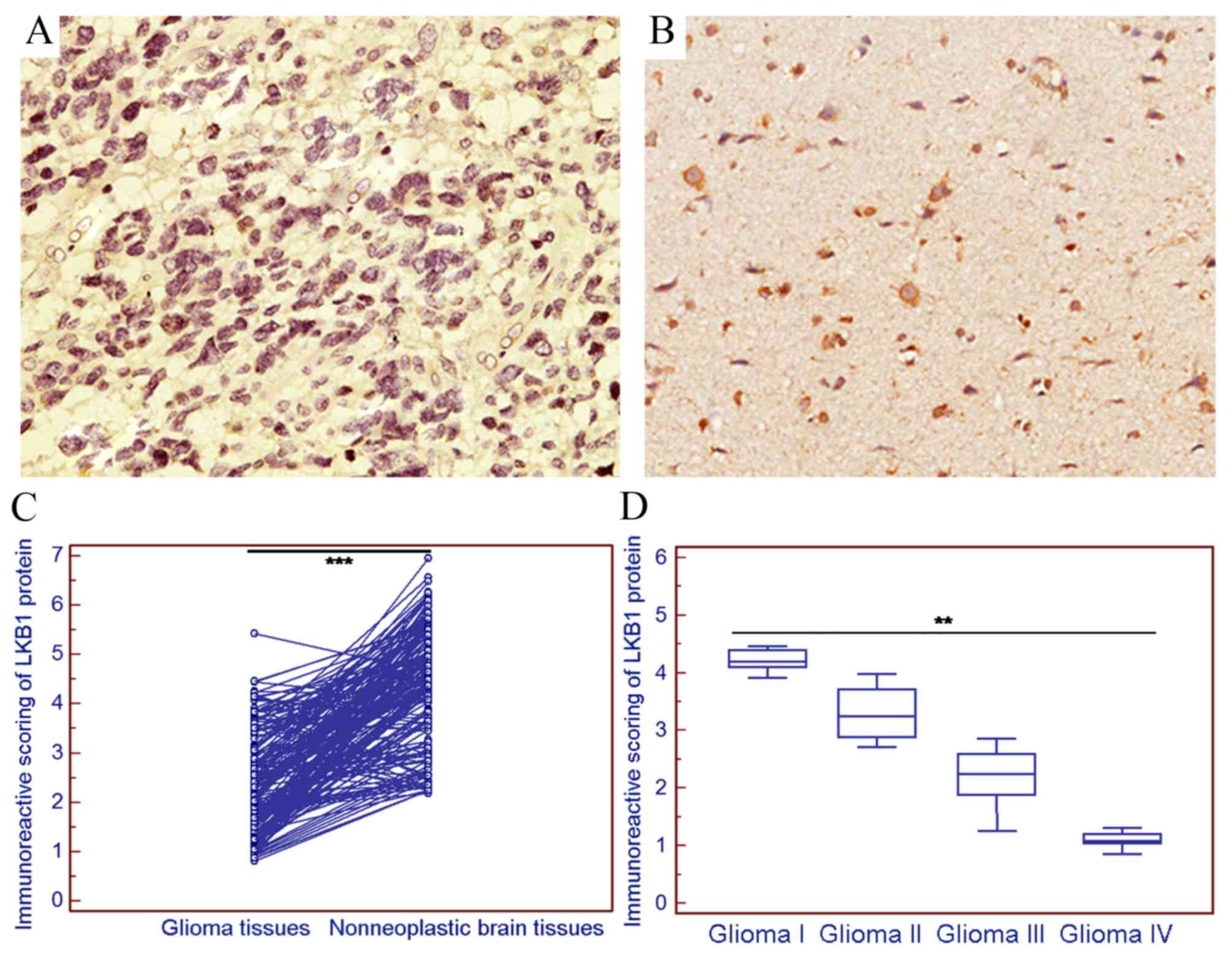
The positive expression of LKB1 protein was examined
in 36/180 (20.00%) gliomas in the present cohort (Fig. 2A), and markedly strong LKB1 protein
expression was observed in 132/180 (73.33%) non-neoplastic brain
tissue samples (Fig. 2B).
Statistically, the IRS of LKB1 protein expression in gliomas was
significantly decreased compared with that in the corresponding
non-neoplastic brain tissues (tumor vs. normal, 2.38±1.00 vs.
4.38±1.19; P<0.001; Fig. 2C).
Notably, the expression of LKB1 in high-grade glioma was decreased
compared with that in low-grade glioma (P<0.01; Fig. 2D).
Downregulation of LKB1 protein is
associated with aggressive tumor progression of human gliomas
To explore the clinical significance of LKB1
downregulation in human gliomas, the associations between LKB1 and
various clinicopathological parameters of glioma patients was
statistically analyzed. The median value (2.31) of the IRS of LKB1
protein expression in glioma tissues was used as a cut-off point to
divide all 180 patients with glioma into low LKB1 expression group
(LKB1-low; n=98) and high LKB1 expression group (LKB1-high; n=82).
Table I summarizes the associations
between LKB1 expression and various clinicopathological parameters
of glioma patients. As a result, low LKB1 expression was
significantly associated with large tumor size (P=0.02; Table I), advanced WHO grade (P=0.006;
Table I) and low Karnofsky
performance scale (P=0.01; Table I).
No significant associations of LKB1 with patients' age, gender,
tumor location or recurrence were observed (Table I).
Downregulation of LKB1 protein
predicts poor prognosis in patients with gliomas
To additionally evaluate the prognostic implication
of LKB1 downregulation in human gliomas, univariate and
multivariate analyses using the Cox's proportional hazards model
were constructed, including patients' age, gender, tumor size,
tumor location, WHO grade, Karnofsky performance scale, tumor
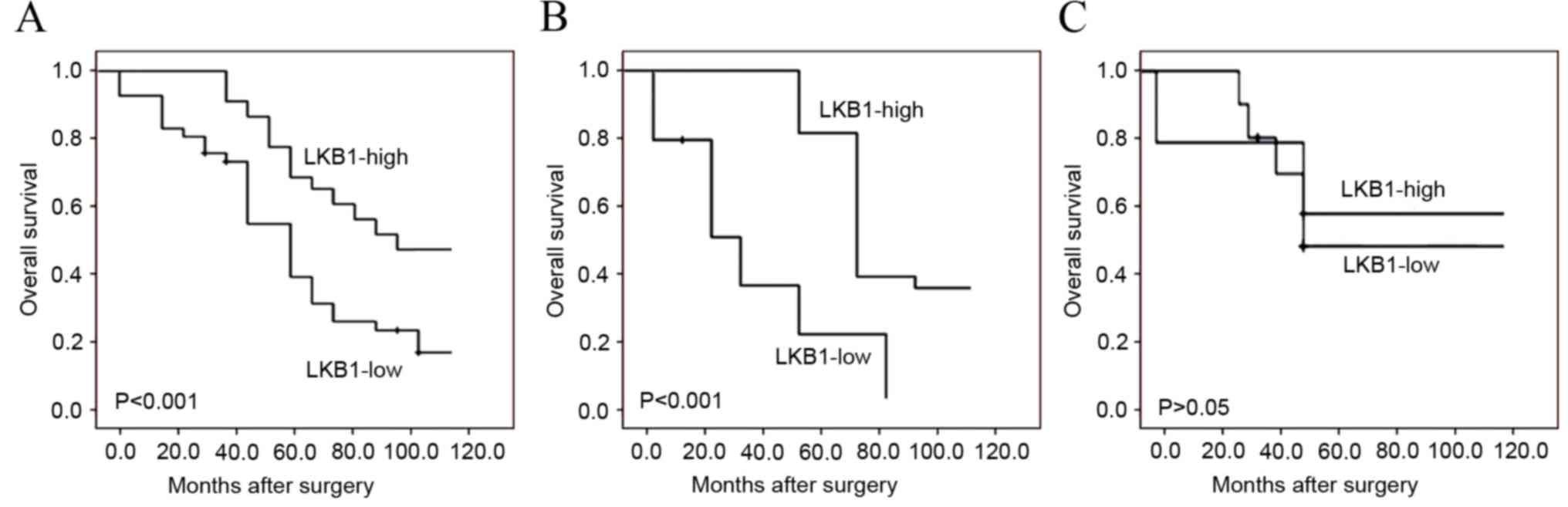
recurrence and LKB1 expression. Kaplan-Meier survival plots in
Fig. 3A revealed that the overall
survival of glioma patients with low LKB1 expression was clearly
shorter compared with that of patients with high LKB1 expression
(P<0.001). Notably, the subgroup analysis based on WHO
classification demonstrated that low LKB1 expression indicated a
poorer survival in high-grade glioma patients (P<0.001; Fig. 3B), but not in low-grade glioma
patients (Fig. 3C). Additionally, the
results of univariate and multivariate analyses revealed that LKB1
expression (P<0.001), WHO grade (P<0.001) and Karnofsky
performance scale (P=0.01) were independent prognostic factors for
overall survival of glioma patients (Table II).
 | Table II.Association of various candidate
prognostic factors with overall survival of 180 glioma patients, as
evaluated by Cox regression analysis. |
Table II.
Association of various candidate
prognostic factors with overall survival of 180 glioma patients, as
evaluated by Cox regression analysis.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Groups | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years | <50 vs. ≥50 | 1.082
(0.568–1.966) | NS | – | – |
| Gender | Male vs. female | 1.021
(0.553–1.922) | NS | – | – |
| Tumor size, cm | <5 vs. ≥5 | 1.168
(0.689–2.139) | NS | – | – |
| Tumor location | Supratentorial vs.
subtentorial | 1.026
(0.581–1.938) | NS | – | – |
|
|
| Karnofsky
performance scale | <90 vs. ≥90 | 2.689
(1.291–5.693) | 0.010 | 2.043
(0.896–4.338) | 0.030 |
| WHO grade | I–II vs.
III–IV | 3.625
(1.673–7.932) | <0.001 | 3.267
(1.392–6.581) | 0.001 |
| Tumor
recurrence | Absent vs.
present | 2.018
(0.819–3.983) | NS | – | – |
| LKB1
expression | Absent vs.
present | 3.348
(1.397–7.026) | <0.001 | 3.022
(1.002–6.016) | 0.001 |
Discussion
Human gliomas, as a group of highly aggressive,
angiogenic and incurable malignancies, exhibit poor clinical
outcomes (3). Thus, there is an
urgent requirement to screen novel and efficient molecular markers
for diagnosis and prognosis in patients with gliomas, and to
develop new therapeutic strategies. In the current study, decreased
expression of LKB1 mRNA and protein levels in glioma tissues were
observed, compared with those in adjacent normal brain tissues.
Subsequently, immunohistochemistry was performed to observe the
expression of LKB1 protein in glioma tissues of different stage
based on complete follow-up data and in the corresponding
non-neoplastic brain tissue specimens. The present data
demonstrated that the mean IRS of LKB1 protein expression in glioma
tissues, particularly in high-grade glioma tissues, was
significantly increased compared with that in the corresponding
non-neoplastic brain tissues. These observations strongly suggest
that the evaluation of LKB1 expression using immunohistochemistry
discriminated between glioma and non-neoplastic brain tissues. In
addition, LKB1 downregulation was significantly associated with
larger tumor size, higher WHO grade and lower Karnofsky performance
scale. Notably, the results revealed that LKB1 downregulation
appeared to be an independent prognostic factor for overall
survival in glioma patients, indicating that the detection of LKB1
may be valuable for the production of individual therapies and for
the identification of patients who may or may not benefit from
close monitoring subsequent to surgery. Additional research is
required to validate these findings.
LKB1, a serine/threonine kinase, has been indicated
to function as a tumor suppressor in various human cancers via
regulating cancer cell growth, metabolism, survival and polarity
(17). Mutations of LKB1 protein,
such as K78I, D17GN, W308C and L67P, result in the loss of its
kinase activity, and have been identified in different cancers,
including malignant melanoma, head and neck squamous cell
carcinoma, breast cancer, lung cancer, hepatocellular carcinoma,
pancreatic and biliary carcinoma, colorectal cancer, cervical
adenocarcinoma and testicular cancer (18–22). In
addition, LKB1 expression has been documented to be regulated
through epigenetic modification, such as hypermethylation in gene
promoter CpG islands and global demethylation of the genome,
transcriptional regulation and post-translational modification,
such as phosphorylation, prenylation and ubiquitination (20). Aberrant expression of LKB1 gene and
protein has been observed in multiple malignancies. For example,
Huang et al (23) reported
that LKB1 expression was decreased in hepatocellular carcinoma
samples, and noticed that loss of LKB1 expression was significantly
associated with aggressive clinical features and poor disease-free
and overall survival. He et al (24) indicated that LKB1 loss at
transcriptional level promoted tumor malignancy and poor patient
outcomes in colorectal cancer. Yang et al (25) reported that the protein expression
levels of LKB1 were significantly reduced in six pancreatic ductal
adenocarcinoma cell lines and downregulated in 31.3% of pancreatic
ductal adenocarcinoma lesions, compared with those in matched
non-tumorous tissues. The authors also confirmed that decreased
LKB1 expression predicted poor prognosis in pancreatic ductal
adenocarcinoma. Shen et al (26) demonstrated that low LKB1 protein
expression correlated with higher histological grade, larger tumor
size, progesterone receptor status, presence of lymph node
metastasis, higher relapse rate and poorer overall survival time.
Jiang et al (27) revealed
that the expression levels of LKB1 mRNA and protein were
significantly reduced in non-small cell lung cancer tissues
compared with those in the matched surrounding normal lung tissues,
and determined that reduced expression of LKB1 was associated with
poor survival of non-small cell lung cancer patients. Similar
results were also obtained by the present analyses regarding the
expression pattern and clinical implication of LKB1 in human
gliomas.
In conclusion, the current data indicated that
downregulation of LKB1 was closely associated with the malignant
degree of human gliomas, displaying lower expression at a higher
grade. Notably, LKB1 may serve as a potential prognostic biomarker
for glioma patients following surgery.
References
|
1
|
Shapiro WR and Shapiro JR: Biology and
treatment of malignant glioma. Oncology (Williston Park).
12:233–240. 1998.PubMed/NCBI
|
|
2
|
Preusser M, Haberler C and Hainfellner JA:
Malignant glioma: Neuropathology and neurobiology. Wien Med
Wochenschr. 156:332–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang B, Li M, Wu Z, Li X, Li Y, Shi X and
Cheng W: Associations between SOX2 and miR-200b expression with the
clinicopathological characteristics and prognosis of patients with
glioma. Exp Ther Med. 10:88–96. 2015.PubMed/NCBI
|
|
4
|
Wong ML, Kaye AH and Hovens CM: Targeting
malignant glioma survival signalling to improve clinical outcomes.
J Clin Neurosci. 14:301–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi J, Yang H, Wang X and Tu Y: The
progress in molecular biomarkers of gliomas. Cancer Transl Med.
2:125–129. 2016. View Article : Google Scholar
|
|
6
|
Rizzo D, Ruggiero A, Martini M, Rizzo V,
Maurizi P and Riccardi R: Molecular biology in pediatric high-grade
glioma: Impact on prognosis and treatment. Biomed Res Int.
2015:2151352015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun R, Li J, Wang B, Guo Y, Ma L, Quan X,
Chu Z and Li T: Liver kinase B1 promoter CpG island methylation is
related to lung cancer and smoking. Int J Clin Exp Med.
8:14070–14074. 2015.PubMed/NCBI
|
|
8
|
Rhodes LV, Tate CR, Hoang VT, Burks HE,
Gilliam D, Martin EC, Elliott S, Miller DB, Buechlein A, Rusch D,
et al: Regulation of triple-negative breast cancer cell metastasis
by the tumor-suppressor liver kinase B1. Oncogenesis. 4:e1682015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng DY, Song H and Liu LB:
Resveratrol-downregulated phosphorylated liver kinase B1 is
involved in senescence of acute myeloid leukemia stem cells. J
Huazhong Univ Sci Technolog Med Sci. 35:485–489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao F, Xu J, Fu C, Cha JY, Gadalla MM, Xu
R, Barrow JC and Snyder SH: Inositol pyrophosphates promote tumor
growth and metastasis by antagonizing liver kinase B1. Proc Natl
Acad Sci USA. 112:1773–1778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia C, Ye F, Hu X, Li Z, Jiang B, Fu Y,
Cheng X, Shao Z and Zhuang Z: Liver kinase B1 enhances
chemoresistance to gemcitabine in breast cancer MDA-MB-231 cells.
Oncol Lett. 8:2086–2092. 2014.PubMed/NCBI
|
|
12
|
Godlewski J, Bronisz A, Nowicki MO,
Chiocca EA and Lawler S: microRNA-451: A conditional switch
controlling glioma cell proliferation and migration. Cell Cycle.
9:2742–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang YS, Lei JA, Feng F, Liang QM and
Wang FR: Probucol suppresses human glioma cell proliferation in
vitro via ROS production and LKB1-AMPK activation. Acta Pharmacol
Sin. 35:1556–1565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vigneswaran K, Neill S and Hadjipanayis
CG: Beyond the world health organization grading of infiltrating
gliomas: Advances in the molecular genetics of glioma
classification. Ann Transl Med. 3:952015.PubMed/NCBI
|
|
15
|
Ballester M, Castelló A, Ibáñez E, Sánchez
A and Folch JM: Real-time quantitative PCR-based system for
determining transgene copy number in transgenic animals.
Biotechniques. 37:610–613. 2004.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Sun P, Sun B and Wang C: Low LKB1
expression results in unfavorable prognosis in prostate cancer
patients. Med Sci Monit. 21:3722–3727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Momcilovic M, McMickle R, Abt E, Seki A,
Simko SA, Magyar C, Stout DB, Fishbein MC, Walser TC, Dubinett SM
and Shackelford DB: Heightening energetic stress selectively
targets LKB1-deficient non-small cell lung cancers. Cancer Res.
75:4910–4922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gan RY and Li HB: Recent progress on liver
kinase B1 (LKB1): Expression, regulation, downstream signaling and
cancer suppressive function. Int J Mol Sci. 15:16698–16718. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Momcilovic M and Shackelford DB: Targeting
LKB1 in cancer-exposing and exploiting vulnerabilities. Br J
Cancer. 113:574–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou W, Zhang J and Marcus AI: LKB1 Tumor
Suppressor: Therapeutic opportunities knock when LKB1 is
inactivated. Genes Dis. 1:64–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Monteverde T, Muthalagu N, Port J and
Murphy DJ: Evidence of cancer-promoting roles for AMPK and related
kinases. FEBS J. 282:4658–4651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang YH, Chen ZK, Huang KT, Li P, He B,
Guo X, Zhong JQ, Zhang QY, Shi HQ, Song QT, et al: Decreased
expression of LKB1 correlates with poor prognosis in hepatocellular
carcinoma patients undergoing hepatectomy. Asian Pac J Cancer Prev.
14:1985–1988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He TY, Tsai LH, Huang CC, Chou MC and Lee
H: LKB1 loss at transcriptional level promotes tumor malignancy and
poor patient outcomes in colorectal cancer. Ann Surg Oncol.
21:(Suppl 4) S703-S710. 2014. View Article : Google Scholar
|
|
25
|
Yang JY, Jiang SH, Liu DJ, Yang XM, Huo
YM, Li J, Hua R, Zhang ZG and Sun YW: Decreased LKB1 predicts poor
prognosis in Pancreatic Ductal Adenocarcinoma. Sci Rep.
5:105752015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Z, Wen XF, Lan F, Shen ZZ and Shao
ZM: The tumor suppressor gene LKB1 is associated with prognosis in
human breast carcinoma. Clin Cancer Res. 8:2085–2090.
2002.PubMed/NCBI
|
|
27
|
Jiang L, Liang X, Liu M, Wang W, Ma J, Guo
Q, Han L, Yang C and Nan K: Reduced expression of liver kinase B1
and Beclin1 is associated with the poor survival of patients with
non-small cell lung cancer. Oncol Rep. 32:1931–1958.
2014.PubMed/NCBI
|