Introduction
Cervical cancer arises from viral infections and is
particularly associated with persistent human papillomavirus (HPV)
infection. As a result, the tumor cells themselves express
virus-specific antigens, including the oncoproteins E6 and E7,
which are involved in cellular malignant transformation (1).
Cluster of differentiation 8 (CD8+)
cytotoxic lymphocytes are the primary effector cells in
cell-mediated immunity, and the activation of these cells is
required for the recognition of the epitope-loaded major
histocompatibility complex I (MHC-I) of the target cell (2). These epitopes are typically produced by
proteasomal protein degradation and are subsequently carried by
transporters associated with antigen processing proteins (TAPs),
for entry into the endoplasmic reticulum (ER) and loading onto
MHC-I (3).
Only a single peptide is loaded onto the MHC-I for
every 104 degraded proteins (4). In tumors or virus-infected cells, MHC-I
expression may be affected by a reduction in antigen presentation,
leading to immune system evasion (5,6). In
previous years, various strategies, involving DNA and adenovirus
vaccines, have been designed to overcome this limitation and to
potentiate the antitumor immune response (7–9). A
promising strategy is based on targeted antigen delivery directly
to the ER, through the fusion of these proteins to an ER resident
protein or an ER signal peptide. Previous studies have shown that
the fusion of antigens with ER resident proteins, particularly
calreticulin (CRT), enhances the immune response compared with
antigens alone (10,11). However, CRT overexpression has
recently been associated with pancreatic cancer through the mitogen
activated protein kinase/extracellular signal-regulated kinase
signaling pathway (12) and breast
cancer through elevated humoral immunity to CRT (13).
In a previous study using mice, we demonstrated that
treatment with an adenovirus (Ad SP-E7-KDEL) expressing antigen E7
fused to a CRT signal peptide (SP: MLLPVPLLLGLLGLAAAL) and a
retention peptide (KDEL) exerted a protective antitumor effect
equal to that obtained following treatment with the same antigen
fused to CRT (14). In the present
study, an improved vaccine was constructed that includes the E6 and
E7 antigens, which provides a better antitumor effect according to
the literature (15–17). In addition, the two antigens were
mutant versions to eliminate the risk of cellular transformation
(18,19) and were codon-optimized for efficient
expression in mammalian cells (20).
These antigens were fused to the human CRT signal peptide and the
KDEL signal (SP-E6E7m-KDEL). The effect of this construct was
compared with that obtained by the fusion of these antigens to
human full-length CRT (hCRT-E6E7m); as a reference control, rabbit
CRT (rCRT-E7) was fused to wild-type E7 (10). These constructs were evaluated as DNA
vaccines via gene gun-mediated transfer.
Materials and methods
Mice
Female C57BL/6 mice (6–8 weeks old) were purchased
from Harlan (Mexico City, Mexico), housed under a 12 h light/12 h
dark cycle and provided with ad libitum access to food and
water. The experiments reported in the present study were conducted
according to the principles set forth in the Guide for the Care and
Use of Laboratory Animals of the U.S. National Institutes of
Health. The protocol was approved by the Ethics Committee of the
School of Medicine, Universidad Autonoma de Nuevo Leon (Monterrey,
Mexico) (protocol HT14-002).
Cell lines
HEK-293 human embryonic kidney (#CRL-1573) and TC-1
mouse lung tumor (#CRL-2785) cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). HEK-293 cells
were cultured in advanced Dulbecco's modified Eagle medium
supplemented with 4% heat-inactivated fetal calf serum, 2 mM
L-glutamine and 100 U/ml penicillin/streptomycin (all from Cellgro,
Mediatech Inc., Manassas, VA, USA). TC-1 cells were cultured in
RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf
serum, 1 mM sodium pyruvate (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin/streptomycin and G418 at 0.5
mg/ml (#A1720; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany).
All cells were maintained at 37°C in a 5% CO2
atmosphere.
DNA constructs
The sequences of the fusion proteins SP-E6E7m-KDEL
and hCRT-E6E7m were designed in silico and synthesized at
Eurofins Company (Huntsville, AL, USA). These genes were subcloned
using the NotI and BglII restriction sites in the
pShuttle-CMV vector and purified using an endotoxin-free kit
(Qiagen, Inc., Valencia, CA, USA). The plasmid pShuttle:rCRT-E7 was
donated by Dr Jorge Gomez-Gutierrez from the Division of Surgical
Oncology of the University of Louisville (Louisville, KY, USA)
(11). DNA cartridges (1 µg/shot) for
the Helios gene gun system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) were prepared according to the manufacturer's protocol and
stored in desiccant chambers at 4°C until further use.
Western blot analysis
A total of 3×105 HEK-293 cells were
seeded onto a 6-well plate. The next day, 4 µg of the corresponding
DNA was mixed with Turbofect (#R0531; Thermo Fisher Scientific,
Inc.) and the cells were transfected according to the
manufacturer's protocol. After 18 h, the cells were collected and
lysed with radioimmunoprecipitation assay (RIPA) buffer. The
supernatants were mixed with loading buffer, denatured at 100°C for
10 min, and centrifuged at 18,000 × g. The samples were
subjected to denaturing electrophoresis on 12% acrylamide gels and
subsequently transferred to nitrocellulose membranes. The membranes
were blocked for 1 h with 3% bovine serum albumin (#A1311;US
Biological, Salem, MA, USA) in Tris-buffered saline and Tween 20
(TBST) (135 mM NaCl, 2.7 mM KCl, 24.8 mM Tris-HCl, 0.05% Tween 20,
pH 7.4). Similarly, the antibodies were diluted with 0.3% BSA in
TBST. Subsequently, the membranes were incubated overnight with the
mouse monoclonal IgG2a raised against amino acids 35–56 of E7 HPV16
(#sc-65711; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
diluted 1:3,000. The membranes were washed with TBST and incubated
for 2 h with a horseradish peroxidase (HRP)-conjugated rabbit
anti-mouse antibody (#A9044; Sigma-Aldrich; Merck Millipore)
diluted 1:10,000. The signal detection was performed using Super
Signal West Pico Chemiluminescent Substrate (#34080; Pierce
Biotechnology; Thermo Fisher Scientific, Inc.). The same membrane
was washed with Restore Western Blot Stripping Buffer (#21063;
Thermo Fisher Scientific, Inc.) and incubated with the mouse
monoclonal IgG1 raised against HPV16/18 E6 protein
(#sc-460; Santa Cruz Biotechnology, Inc.) diluted 1:3,000.
Immunofluorescence
Glass coverslips were sterilized and placed into the
wells of a 24-well plate. Subsequently, 5×104 HEK-293
cells were seeded into the wells of the prepared plates and
transfected the next day with 1 µg of DNA using Turbofect (#R0531;
Fermentas; Thermo Fisher Scientific, Inc.). After 16 h, the cells
were fixed with methanol:acetone (1:1), blocked with 3% horse serum
(#16050122; Thermo Fisher Scientific, Inc) and incubated overnight
with mouse monoclonal IgG2a raised against amino acids 35–56 of E7
HPV16 and rabbit polyclonal raised against amino acids 1–70 of
human calnexin, diluted 1:250 each one (#sc-65711 and #sc-11397;
Santa Cruz Biotechnology, Inc.). The membranes were then washed
with TBST and incubated for 2 h with goat polyclonal anti-mouse IgG
(H+L) Alexa 594 and goat polyclonal anti-rabbit IgG (H+L) Alexa 488
(#A-11005 and #A-11034; Invitrogen; Thermo Fisher Scientific, Inc.)
each diluted 1:500. The glass coverslips were mounted with
Vectashield Antifade Mounting Medium with
4′,6-diamidino-2-phenylindole (DAPI) (#H-1200; Vector Laboratories,
Inc., Burlingame, CA, USA) and observed using an Apotome
fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY, USA).
Interferon (IFN)-γ quantification
Groups of 3 mice (6–7 weeks old) were immunized on
days 0 and 7 with 1 µg of DNA in the abdominal area using the gene
gun system. The mice were sacrificed 7 days subsequent to
immunization, and the spleens were collected. A pool of spleens for
each treatment was mashed on a cell strainer (#352350; BD Falcon,
Bedford, MA, USA), and the splenocytes were stimulated with 1 µg/ml
E7 immunodominant epitope (RAHYNIVTF; amino acids 49–57) or E6
(YDFAFRDL; amino acids 50–57) (both from GenWay Biotech, Inc., San
Diego, CA USA) for 48 h. The culture supernatants were collected,
and IFN-γ levels were determined using a mouse IFN-γ platinum
enzyme linked immunosorbent assay (ELISA) kit (#BMS606;
eBioscience, Inc., San Diego, CA, USA). The basal levels of IFN-γ
produced under non-antigen stimulation conditions were used to
normalize the results.
Antitumor therapeutic assay
Groups of 5 mice were used for each treatment. The
mice were injected with 1×104 TC-1 cells in the tail
vein, simulating a metastasis model. When this lung tumor cell line
is injected in the tail vein, the first capillary bed that cells
face is in the lungs, and therefore all cells are retained and grow
on lung epithelium. The gene gun system was used for immunization
in the abdominal area using 300 psi as output pressure. The mice
were immunized with 1 µg of the corresponding DNA construct on the
third and tenth days following tumor implantation and were
sacrificed on day 25. The lungs were rinsed in phosphate buffered
saline (PBS) and fixed in 4% paraformaldehyde (PFA) in PBS for
tumor foci counts.
Histological analysis of the tumor
foci
After counting the tumor foci, lungs were fixed in
4% PFA-PBS, dehydrated and embedded in paraffin. Lung orientation
was disposed, prioritizing areas with more tumor foci; 4 µm
sections were deparaffinized and hydrated for hematoxylin and eosin
(H&E) staining (H3136 and E6003; Sigma-Aldrich; Merck
Millipore), followed by dehydration and mounting using Entellan
mounting medium (#17960; Merck Millipore). The lung sections were
analyzed using a Primo Star microscope (Carl Zeiss, Inc.,
Thornwood, NY, USA), the images were obtained by bright-field
microscopy.
Statistical analysis
Analysis of variance (one-way ANOVA) followed by
post hoc Dunnett's tests was performed using Prism software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed at least twice.
Results
Design and characterization of DNA
constructs
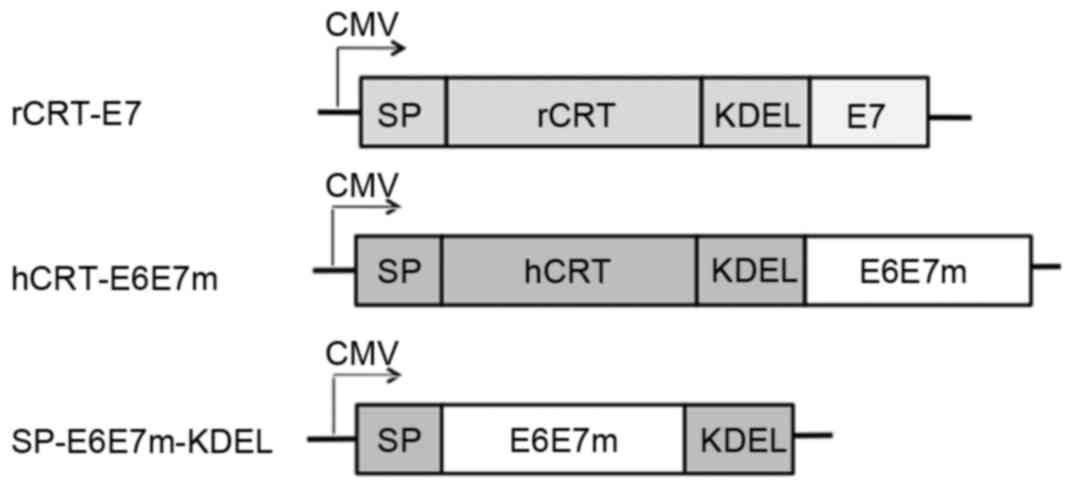
The construct SP-E6E7m-KDEL consists of the fusion
of full-length E6-E7 mutant antigens to the signal peptide of hCRT
at the N-terminus and the fusion of the KDEL signal to the
C-terminus for retention in the ER. The construct hCRT-E6E7m
consists of the fusion of full-length E6-E7 mutant antigens to the
N-terminus of hCRT. The resulting synthetic constructs were
deposited in the GenBank database under the accession numbers
KP898251 and KP898250, respectively. The DNA constructs are shown
in Fig. 1.
Protein extracts from transfected HEK-293 cells
transfected with the different constructs were analyzed through
western blotting (Fig. 2). Bands were
observed at 70 kDa for rCRT-E7, 80 kDa for hCRT-E6E7m and 25 kDa
for SP-E6E7m-KDEL, confirming the correct expression of the
recombinant proteins containing E6 and E7 from the different
constructs.
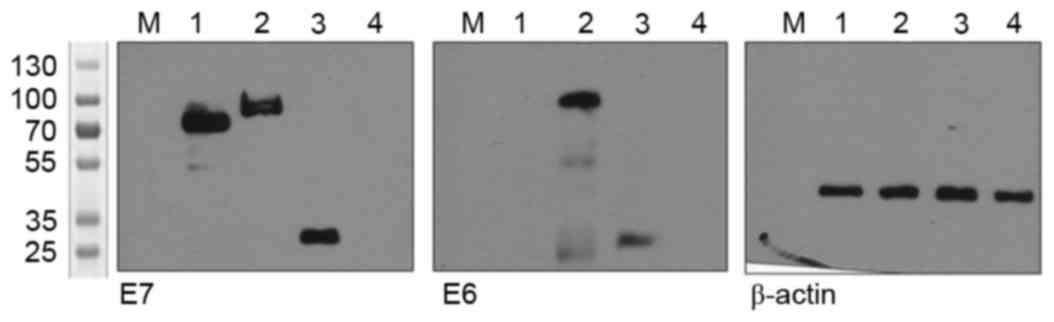 | Figure 2.Detection of the recombinant proteins
through western blotting. HEK-293 cells transfected with constructs
expressing rCRT-E7, hCRT-E6E7m, SP-E6E7m-KDEL or the empty vector.
After 24 h, the cell protein extracts were subjected to SDS-PAGE
and incubated with specific antibodies. Lane 1, rCRT-E7; Lane 2,
hCRT-E6E7m; Lane 3, SP-E6E7m-KDEL; Lane 4, empty vector. A
molecular-weight size marker was loaded on lane M. rCRT, rabbit
calreticulin; hCRT, human calreticulin, KDEL, lysine-aspartic
acid-glutamic acid-leucine peptide sequence; SP, signal
peptide. |
Fusion to hCRT or the
targeting/retention signal peptides targets the E6-E7 antigens to
the ER
To determine whether the recombinant proteins are
directed to the ER, HEK-293 cells were transfected with the DNA
constructs and prepared for immunofluorescence. Co-localization was
detected between the E7 signal (red) and the calnexin signal
(green), a well-characterized resident protein of the endoplasmic
reticulum (Fig. 3). This result
demonstrated that antigens fused to rCRT, hCRT or
targeting/retention signal peptides are directed to the ER.
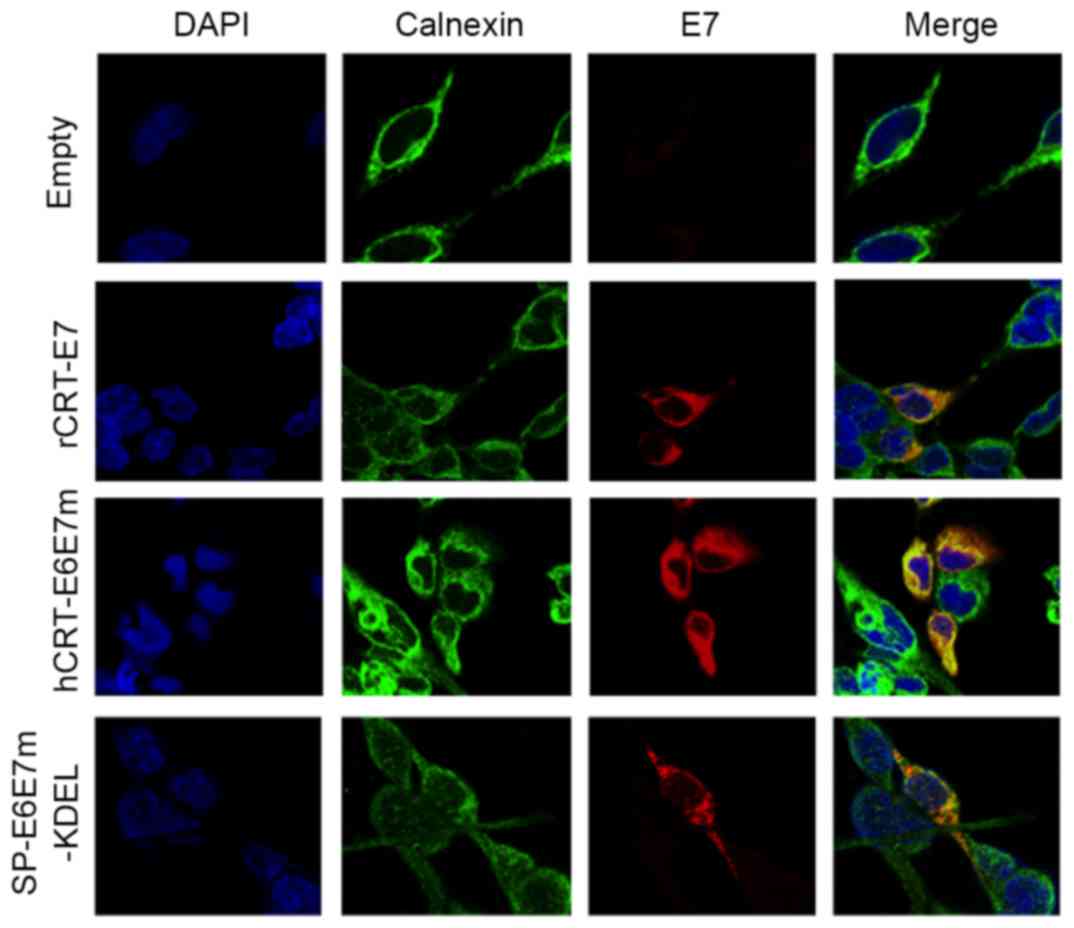 | Figure 3.Subcellular localization of
recombinant proteins. HEK-293 cells were transfected with
constructs expressing rCRT-E7, hCRT-E6E7m, SP-E6E7m-KDEL or the
empty vector. After 24 h, the cells were fixed and incubated with
specific primary antibodies and secondary antibodies conjugated
with fluorochromes. The samples were examined using a confocal
fluorescence microscope. Original magnification, ×630. rCRT, rabbit
calreticulin; hCRT, human calreticulin, KDEL, lysine-aspartic
acid-glutamic acid-leucine peptide sequence; SP, signal peptide;
DAPI, 4′,6-diamidino-2-phenylindole. |
Immunization with SP-E6E7m-KDEL
induces IFN-γ production
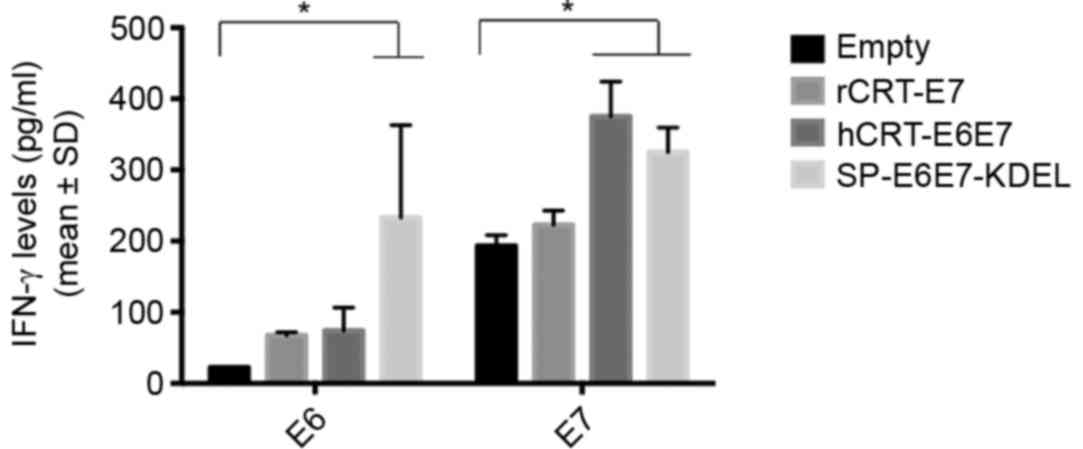
To determine whether hCRT-E6E7m and SP-E6E7m-KDEL
induce an antigen-specific IFN-γ-mediated response, the mice were
immunized with each DNA construct, as previously described. After 1
week, the spleens were harvested, and the splenocytes were isolated
and subsequently stimulated in vitro with the E7 or E6
epitopes for 48 h. The supernatant was collected and then analyzed
by ELISA. The results show that splenocytes from the hCRT-E6E7m
group had high IFN-γ production when stimulated with E7 (P=0.007);
however, most importantly, splenocytes from the SP-E6E7m-KDEL group
that were stimulated with E6 or E7 antigens had increased IFN-γ
production (P=0.021 and P=0.017, respectively). This suggests that
targeting these antigens to the ER promotes an antigen-specific
response mediated by IFN-γ (Fig.
4).
Treatment with SP-E6E7m-KDEL induces
the reduction of TC-1 tumor pulmonary nodules in C57BL/6 mice
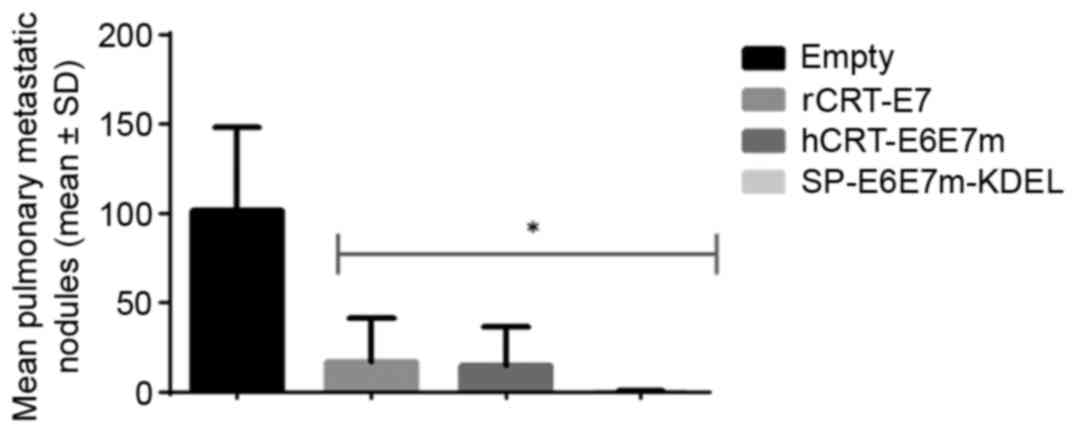
A therapeutic antitumor effect was demonstrated
in vivo. TC-1 tumor cells were implanted in mice C57BL/6
through intravenous (IV) injection. This IV implantation serves as
a metastasis model to disseminate the tumor cells through the
organism, although the implanted cells are predominately detected
on the lungs, forming multiple nodules on the epithelium. The mice
were subjected to the antitumor therapeutic assay as described
above, and were sacrificed at 25 days following tumor implantation
for the evaluation of therapeutic antitumor effects by counting the
number of tumor foci between treatments (Fig. 5). Treatments with rCRT-E7, hCRT-E6E7m,
and SP-E6E7m-KDEL resulted in equivalent therapeutic antitumor
effects, as the number of nodules drastically decreased compared
with the control (P<0.05), without a significant difference
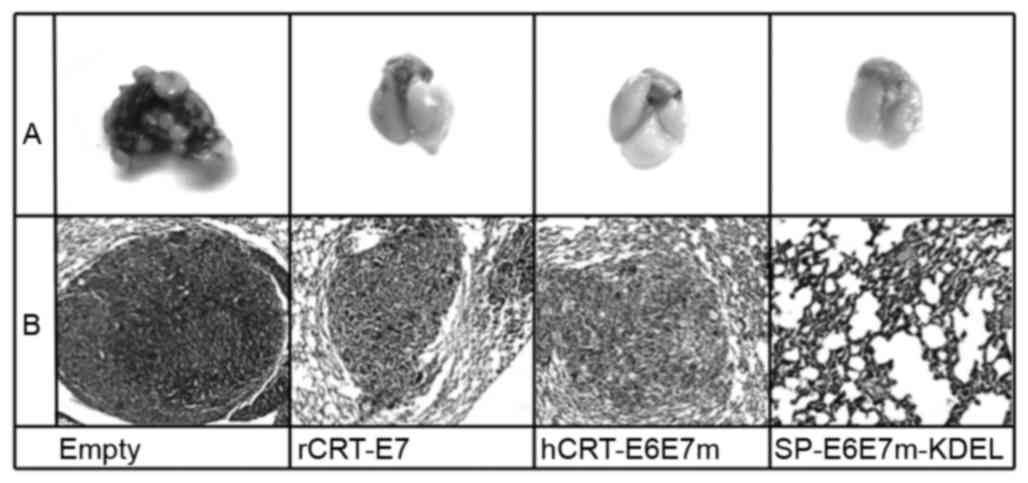
between each treatment (P>0.05). Histological sections from the
lungs with the highest number of tumor nodules were stained with
H&E to determine changes in the morphology of these tumor
nodules (Fig. 6). The present study
observed that the tumors from immunized mice showed certain
alterations in the integrity of the capsule, as certain remnants of
the extracellular matrix were detected in the tissues, suggesting
increased tumor regression (21).
Discussion
CRT is a chaperone protein typically present in the
ER, thereby possessing a signal peptide and the KDEL retention
sequence. As a chaperone protein, CRT plays multiple roles
associated with MHC-I molecule loading (22), calcium homeostasis (23) and cell migration (24,25).
Multiple studies have reported that the fusion of antigens to CRT
and other chaperones (26) increases
antigen-specific immune responses through cross-priming and
inflammation (27–29). Although immunization with full-length
CRT alone shows an antitumor effect, this protein is not as potent
as the fusion of CRT with antigens (29).
Clinical studies have reported alterations in CRT
expression in certain types of cancer, where overexpression has
been associated with increased invasiveness, metastasis and
decreased survival (30), likely
reflecting the role of CRT in calcium homeostasis. Calcium mediates
cell migration, and upregulation through transduction seriously
affects cell invasiveness (31,32).
The present study aimed to identify alternatives to
the use of full-length calreticulin, in order to eliminate the
overexpression of xenogeneic proteins to diminish any misbalance in
homeostasis while preserving the antitumor effect (14). In the present study, we designed an
improved simplified version of a DNA construct by fusing the mutant
E6 and E7 antigens from HPV16 to the hCRT signal peptide and the
KDEL retention sequence (SP-E6E7m-KDEL). The effects of fusion to
the full-length hCRT (hCRT-E6E7m) and fusion to rCRT-E7, which was
previously reported to promote antigen distribution to the ER and
to enhance the antitumor response specific to this antigen
(10), were compared.
In the present study, immunofluorescence analysis
revealed that fusing the E6-E7 antigens to the CRT signal peptide
and the KDEL retention sequence is sufficient to target these
antigens to the ER, and the intracellular distribution pattern is
similar to that obtained through fusion with the full-length CRT.
These results are consistent with previous studies showing that the
linkage of E6 and E7 to ER chaperoning proteins causes the
translocation of these proteins from the nucleus to the ER
(14,26,27,29),
resulting in an almost null signal in the nucleus.
After confirming the expression and functional
targeting, the present study evaluated the antitumor effects of the
constructs. Immunization by ballistic gene gun was used as a
user-friendly system with the capacity to stimulate skin dendritic
cells, one of the immune cell types that actively participate upon
the onset of a cellular immune response (33).
To simulate an advanced state of cancer to examine
these vaccines, the present study used an in vivo model
consisting of administration of TC-1 tumor cells through
intravenous injection to simulate a metastasis model (34). The results indicate that the
simplified version of the E6-E7 antigens fused only to the signal
peptide and the KDEL retention sequence from CRT significantly
reduced metastases nodules compared with the control, and this
antitumor effect is comparable with that obtained with the antigens
fused to full-length CRT, with no significant difference between
these treatments. The present study detected antigen-specific IFN-γ
production in the supernatants of cultured splenocytes, obtaining
high levels of production in splenocytes from the SP-E6E7m-KDEL
group. These results indicate that the antitumor effect can be
achieved using only signal peptides to deliver and retain the
antigens.
Although metastatic nodule reduction was observed
within treatments, it will be necessary to perform an evaluation on
advanced stages of cancer. Other studies of HPV vaccines have
previously reported that vaccine therapies are not sufficient and
require the support of chemotherapy (35,36).
In summary, the present study reports a strategy for
targeting antigens to the ER through fusion to the signal peptide
and KDEL sequence of CRT compared with full-length CRT. The
antitumor effects demonstrated using these constructs were as
effective as the reference constructs, providing an alternative for
vaccine design, as previously reported (14). The goal is to decrease the
overexpression of proteins that may affect cellular homeostasis
while also preserving the capacity of the antigen to generate an
antitumor response. To the best of our knowledge, the current study
is the first demonstration that a DNA vaccine encoding the antigens
E6 and E7 from HPV16 fused to a signal peptide and a KDEL sequence
to induce a potent therapeutic antitumor effect.
Acknowledgements
This study was supported through grants from the
Program to Support Research in Science and Technology (grant no.
CN1096-11) from the Universidad Autonoma de Nuevo Leon and National
Council for Science and Technology (CONACYT; grant no.
CB-10-158509). JJPT and RGM were recipients of scholarships from
CONACYT. The authors would like to thank Dr Juan Carlos
Segoviano-Ramirez from the Bioimaging Unit in the Center for
Research and Development in the Health Sciences, Autonomous
University of Leon for kindly providing assistance with confocal
imaging.
Glossary
Abbreviations
Abbreviations:
|
CRT
|
calreticulin
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
ER
|
endoplasmic reticulum
|
|
H&E
|
hematoxylin and eosin
|
|
hCRT
|
human calreticulin
|
|
HEK-293
|
human embryonic kidney 293 cells
|
|
HPV
|
human papillomavirus
|
|
IFN-γ
|
interferon γ
|
|
KDEL
|
lysine-aspartic acid-glutamic
acid-leucine peptide sequence
|
|
MHC-I
|
major histocompatibility complex I
|
|
rCRT
|
rabbit calreticulin
|
|
SP
|
signal peptide
|
|
TAPs
|
transporters associated with antigen
processing proteins
|
References
|
1
|
Tan S, de Vries EG, van der Zee AG and de
Jong S: Anticancer drugs aimed at E6 and E7 activity in
HPV-positive cervical cancer. Curr Cancer Drug Targets. 12:170–184.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Boer MA, Jordanova ES, van Poelgeest
MI, van den Akker BE, van der Burg SH, Kenter GG and Fleuren GJ:
Circulating human papillomavirus type 16 specific T-cells are
associated with HLA Class I expression on tumor cells, but not
related to the amount of viral oncogene transcripts. Int J Cancer.
121:2711–2715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bukur J, Jasinski S and Seliger B: The
role of classical and non-classical HLA class I antigens in human
tumors. Semin Cancer Biol. 22:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yewdell JW: Not such a dismal science: The
economics of protein synthesis, folding, degradation and antigen
processing. Trends Cell Biol. 11:294–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashrafi GH, Brown DR, Fife KH and Campo
MS: Down-regulation of MHC class I is a property common to
papillomavirus E5 proteins. Virus Res. 120:208–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hicklin DJ, Marincola FM and Ferrone S:
HLA class I antigen downregulation in human cancers: T-cell
immunotherapy revives an old story. Mol Med Today. 5:178–186. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatsis N and Ertl HC: Adenoviruses as
vaccine vectors. Mol Ther. 10:616–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neefjes J, Jongsma ML, Paul P and Bakke O:
Towards a systems understanding of MHC class I and MHC class II
antigen presentation. Nat Rev Immunol. 11:823–836. 2011.PubMed/NCBI
|
|
9
|
Yang B, Jeang J, Yang A, Wu TC and Hung
CF: DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother.
10:3153–3164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz
M, Boyd DA, He L, Chen PJ, Chen CH and Wu TC: Enhancement of
vaccinia vaccine potency by linkage of tumor antigen gene to gene
encoding calreticulin. Vaccine. 22:3993–4001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomez-Gutierrez JG, Elpek KG, de Oca-Luna
R Montes, Shirwan H, Sam Zhou H and McMasters KM: Vaccination with
an adenoviral vector expressing calreticulin-human papillomavirus
16 E7 fusion protein eradicates E7 expressing established tumors in
mice. Cancer Immunol Immunother. 56:997–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheng W, Chen C, Dong M, Zhou J, Liu Q,
Dong Q and Li F: Overexpression of calreticulin contributes to the
development and progression of pancreatic cancer. J Cell Physiol.
229:887–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erić-Nikolić A, Milovanović Z, Sánchez D,
Pekáriková A, Džodić R, Matić IZ, Tučková L, Jevrić M, Buta M,
Rašković S and Juranić Z: Overexpression of calreticulin in
malignant and benign breast tumors: Relationship with humoral
immunity. Oncology. 82:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loera-Arias MJ, Martínez-Pérez AG,
Barrera-Hernández A, Ibarra-Obregón ER, González-Saldívar G,
Martínez-Ortega JI, Rosas-Taraco A, Villanueva-Olivo A,
Esparza-González SC, Villatoro-Hernandez J, et al: Targeting and
retention of HPV16 E7 to the endoplasmic reticulum enhances immune
tumour protection. J Cell Mol Med. 14:890–894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng S, Tomson TT, Trimble C, He L, Hung
CF and Wu TC: A combination of DNA vaccines targeting human
papillomavirus type 16 E6 and E7 generates potent antitumor
effects. Gene Ther. 13:257–265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan J, Reichenbach DK, Corbitt N, Hokey
DA, Ramanathan MP, McKinney KA, Weiner DB and Sewell D: Induction
of antitumor immunity in vivo following delivery of a novel HPV-16
DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 27:431–440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou X, Qian X, Zhao Q, Lu Y and Xiong M:
Efficient expression of modified human papillomavirus 16 e6/e7
fusion protein and the antitumor efficacy in a mouse model. Biol
Pharm Bull. 27:303–307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi W, Bu P, Liu J, Polack A, Fisher S and
Qiao L: Human papillomavirus type 16 E7 DNA vaccine: Mutation in
the open reading frame of E7 enhances specific cytotoxic
T-lymphocyte induction and antitumor activity. J Virol.
73:7877–7881. 1999.PubMed/NCBI
|
|
19
|
Bahrami AA, Ghaemi A, Tabarraei A,
Sajadian A, Gorji A and Soleimanjahi H: DNA vaccine encoding HPV-16
E7 with mutation in L-Y-C-Y-E pRb-binding motif induces potent
anti-tumor responses in mice. J Virol Methods. 206:12–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung YK, Cheng SC, Sin FW and Xie Y:
Plasmid encoding papillomavirus Type 16 (HPV16) DNA constructed
with codon optimization improved the immunogenicity against HPV
infection. Vaccine. 23:629–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amine A, Rivera S, Opolon P, Dekkal M,
Biard DS, Bouamar H, Louache F, McKay MJ, Bourhis J, Deutsch E and
Vozenin-Brotons MC: Novel anti-metastatic action of cidofovir
mediated by inhibition of E6/E7, CXCR4 and Rho/ROCK signaling in
HPV+ tumor cells. PLoS One. 4:e50182009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Del Cid N, Jeffery E, Rizvi SM, Stamper E,
Peters LR, Brown WC, Provoda C and Raghavan M: Modes of
calreticulin recruitment to the major histocompatibility complex
class I assembly pathway. J Biol Chem. 285:4520–4535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gelebart P, Opas M and Michalak M:
Calreticulin, a Ca2+-binding chaperone of the endoplasmic
reticulum. Int J Biochem Cell Biol. 37:260–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy-Ullrich JE: The de-adhesive
activity of matricellular proteins: Is intermediate cell adhesion
an adaptive state? J Clin Invest. 107:785–790. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goicoechea S, Orr AW, Pallero MA, Eggleton
P and Murphy-Ullrich JE: Thrombospondin mediates focal adhesion
disassembly through interactions with cell surface calreticulin. J
Biol Chem. 275:36358–36368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JW, Hung CF, Juang J, He L, Kim TW,
Armstrong DK, Pai SI, Chen PJ, Lin CT, Boyd DA and Wu TC:
Comparison of HPV DNA vaccines employing intracellular targeting
strategies. Gene Ther. 11:1011–1018. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng S, Ji H, Trimble C, He L, Tsai YC,
Yeatermeyer J, Boyd DA, Hung CF and Wu TC: Development of a DNA
vaccine targeting human papillomavirus type 16 oncoprotein E6. J
Virol. 78:8468–8476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang TH, Chung JY, Monie A, Pai SI, Hung
CF and Wu TC: Enhancing DNA vaccine potency by co-administration of
xenogenic MHC class-I DNA. Gene Ther. 17:531–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng WF, Hung CF, Chai CY, Hsu KF, He L,
Ling M and Wu TC: Tumor-specific immunity and antiangiogenesis
generated by a DNA vaccine encoding calreticulin linked to a tumor
antigen. J Clin Invest. 108:669–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zamanian M, Veerakumarasivam A, Abdullah S
and Rosli R: Calreticulin and cancer. Pathol Oncol Res. 19:149–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiang WF, Hwang TZ, Hour TC, Wang LH,
Chiu CC, Chen HR, Wu YJ, Wang CC, Wang LF, Chien CY, et al:
Calreticulin, an endoplasmic reticulum-resident protein, is highly
expressed and essential for cell proliferation and migration in
oral squamous cell carcinoma. Oral Oncol. 49:534–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi F, Shang L, Pan BQ, Wang XM, Jiang YY,
Hao JJ, Zhang Y, Cai Y, Xu X, Zhan QM and Wang MR: Calreticulin
promotes migration and invasion of esophageal cancer cells by
upregulating neuropilin-1 expression via STAT5A. Clin Cancer Res.
20:6153–6162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peachman KK, Rao M and Alving CR:
Immunization with DNA through the skin. Methods. 31:232–242. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji H, Chang EY, Lin KY, Kurman RJ, Pardoll
DM and Wu TC: Antigen-specific immunotherapy for murine lung
metastatic tumors expressing human papillomavirus type 16 E7
oncoprotein. Int J Cancer. 78:41–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SY, Kang TH, Knoff J, Huang Z, Soong
RS, Alvarez RD, Hung CF and Wu TC: Intratumoral injection of
therapeutic HPV vaccinia vaccine following cisplatin enhances
HPV-specific antitumor effects. Cancer Immunol Immunother.
62:1175–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen S, Liao C, Lai Y, Fan Y, Lu G, Wang
H, Zhang X, Lin MC, Leng S and Kung HF: De-oncogenic HPV E6/E7
vaccine gets enhanced antigenicity and promotes tumoricidal synergy
with cisplatin. Acta Biochim Biophys Sin (Shanghai). 46:6–14. 2014.
View Article : Google Scholar : PubMed/NCBI
|