Introduction
Colon cancer is the only cancer that occurs in men
and women with approximately equal frequencies (1). The actual incidence rate of colon cancer
was ~71,830 men and 65,000 women in the US population during 2014.
That is 1 in 21 (4.7%) for men and 1 in 23 (4.4%) for women
(2). Furthermore, colon cancer is the
second most common cause of cancer-associated mortality in human
populations (1). Due to this, it is
essential to improve and discover novel colon cancer therapies by
understanding the molecular mechanisms that underlie the cancer. In
addition, the mortality rate due to colorectal cancer has changed
over the past 50 years (3,4). Colorectal cancer mortality rates
decreased by ~2% per year during the 1990s, and by ~3% per year
over the past decade (2). The reason
for this reduction in colorectal cancer mortality rates is due to
the use of standard screening and treatments in all populations
(2). Transforming growth factor
(TGF)β signaling has the ability to inhibit the proliferation of
colonic epithelium cells (5,6). TGFβ signaling is mediated by the Smad
family of intracellular proteins (7),
namely Smad2, Smad3 and Smad4, which transduce the appropriate
signals. Cyclooxygenase-2 (COX-2) is overexpressed in the majority
of human colon cancer cases (8,9). COX-2
converts arachidonic acid to prostaglandins and associated
eicosanoids, and induces inflammation and cell proliferation
(8,10). Due to the significance of Smad3 and
COX-2 in colorectal cancer, the present study was designed to
investigate the role of COX-2 in Smad3 mutant mice, which are known
to develop colon cancer.
Materials and methods
Generation of Smad3 (−/−) mutant
mice
The use of animals in the present study was approved
by the Institutional Animal Care and Ethical Committee, which was
formed for the purpose of the present study. Homozygous Smad3 (−/−)
mutant mice were generated from the inbred and hybrid Smad3 mouse
strains by intercrossing the appropriate heterozygotes obtained
from the Department of Colorectal Cancer Surgery (Zhejiang Cancer
Hospital, Zhejiang, China) (4).
Normal mice obtaiend from the Department of Colorectal Cancer
Surgery (Zhejiang Cancer Hospital) were used as a control. Animals
were housed in filter-top metal cages and maintained in a 12/12 h
light/dark cycle, as previously described (11). Mice were house at 20–22°C, in a
humidity of 50–60%. Water and food were available ad libitum
and the mice were handled according to international ethical
regulations (12). Female mice aged
22 weeks were used in the present experiments, with an average
weight of 10–14 g. The animals were observed twice a day and
subjected to weighing once a week. In the present study, 12
homozygous Smad3 (−/−) and 6 control mice were used.
Whole mount in situ hybridization
Homozygous Smad3 (−/−) E11.5 embryos were dissected
out of 24-week-old female mutant mice as previously described
(13), fixed for 30 min at room
temperature in 4% paraformaldehyde in phosphate-buffered saline
(PBS), washed twice with PBS, and incubated in X-gal staining
solution (Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) to
determine the colonic expression pattern (14).
Immunohistochemistry
A total of 6 normal (3 samples) and Smad3 (−/−)
mutant mouse (3 samples) samples were collected, formalin-fixed and
paraffin-embedded using standard protocol (15). The 22-week-old mice were anesthetized
with pentobarbital sodium (60 mg/kg, intraperitoneally;
Sigma-Aldrich; EMD Millipore) prior to surgery. Tissue sections (7
µm) were deparaffinized (with xylene; Sigma-Aldrich; EMD Millipore)
and hydrated (with ethanol; Sigma-Aldrich; EMD Millipore). Antigens
were retrieved by Tri-sodium citrate treatment (pH 6.0;
Sigma-Aldrich; EMD Millipore). Endogenous peroxides and nonspecific
immune staining were blocked by hydrogen peroxide and 10% fetal
bovine serum (Sigma-Aldrich; EMD Millipore), respectively. The
sections were subsequently incubated overnight at 4°C with primary
anti-COX-2 antibody (polyclonal goat; dilution, 1:100; catalog no.,
SAB2500267; Sigma-Aldrich; EMD Millipore). Following incubation
with primary antibody, tissue sections were washed with PBS and
incubated with secondary antibodies conjugated to horseradish
peroxidase (polyclonal rabbit anti-goat; dilution, 1:10,000;
catalog no., ab6741; Abcam, Cambridge, MA, USA) for 1 h at room
temperature. The washed slides were developed with
3,3′-diaminobenzidine substrate. The prepared slides were
counterstained with Mayer's hematoxylin (Sigma-Aldrich; EMD
Millipore), mounted with DPX and observed under a Nikon Ti-S
fluorescence microscope at ×4 and ×20 magnification.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
For RT-PCR analysis, RNA was isolated from the
normal and Smad3 (−/−) mutant mice and stored at −70°C according to
the manufacturer's protocol of the RNeasy Mini kit (Qiagen, Inc.,
Valencia, CA, USA) followed by treatment with DNase1 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The quality and
quantity of the RNA was analysed using a NanoDrop 2000 machine
(Thermo Fisher Scientific, Inc.). Reverse transcription (RT) was
performed using 0.5 µg total RNA as a template. A ThermoScript™
RT-PCR System for First-Strand cDNA Synthesis and
Platinum® Taq DNA Polymerase (both purchased from Thermo
Fisher Scientific, Inc.) was used for the RT-PCR experiments
according to the manufacturer's protocol. PCR was performed on 1/20
of the RT product using the following primers (Sigma-Aldrich; EMD
Millipore): Smad3 forward, 5′-TTCACAGACCCATCAAACTCGGA-3′ and
reverse, 5′-CACTATCACTTAGGCACTCAGCA-3′; P53 forward,
5′-ACAGGACCCTGTCACCGAGACC-3′ and reverse,
5′-GACCTCCGTCATGTGCTGTGAC-3′; COX-2 forward,
5′-CCCTTGGGTGTCAAAGGTAA-3′ and reverse, 5′-GCCCTCGCTTATGATCTGTC-3′;
and β-actin forward, 5′-CTAAGGCCAACCGTGAAAAGA −3′ and reverse,
5′-CAGTAATCTCCTTCTGCATCC-3′. The cycles were run according to the
manufacturer's protocol of the ThermoScript RT-PCR System for
First-Strand cDNA Synthesis, and the PCR products were resolved on
2% agarose gels with ethidium bromide. The gels were observed and
documented using a gel documentation unit (Gel Doc™ EZ System;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). All experiments
were repeated three times.
Results
Smad3 mutant mice develop colorectal
tumors
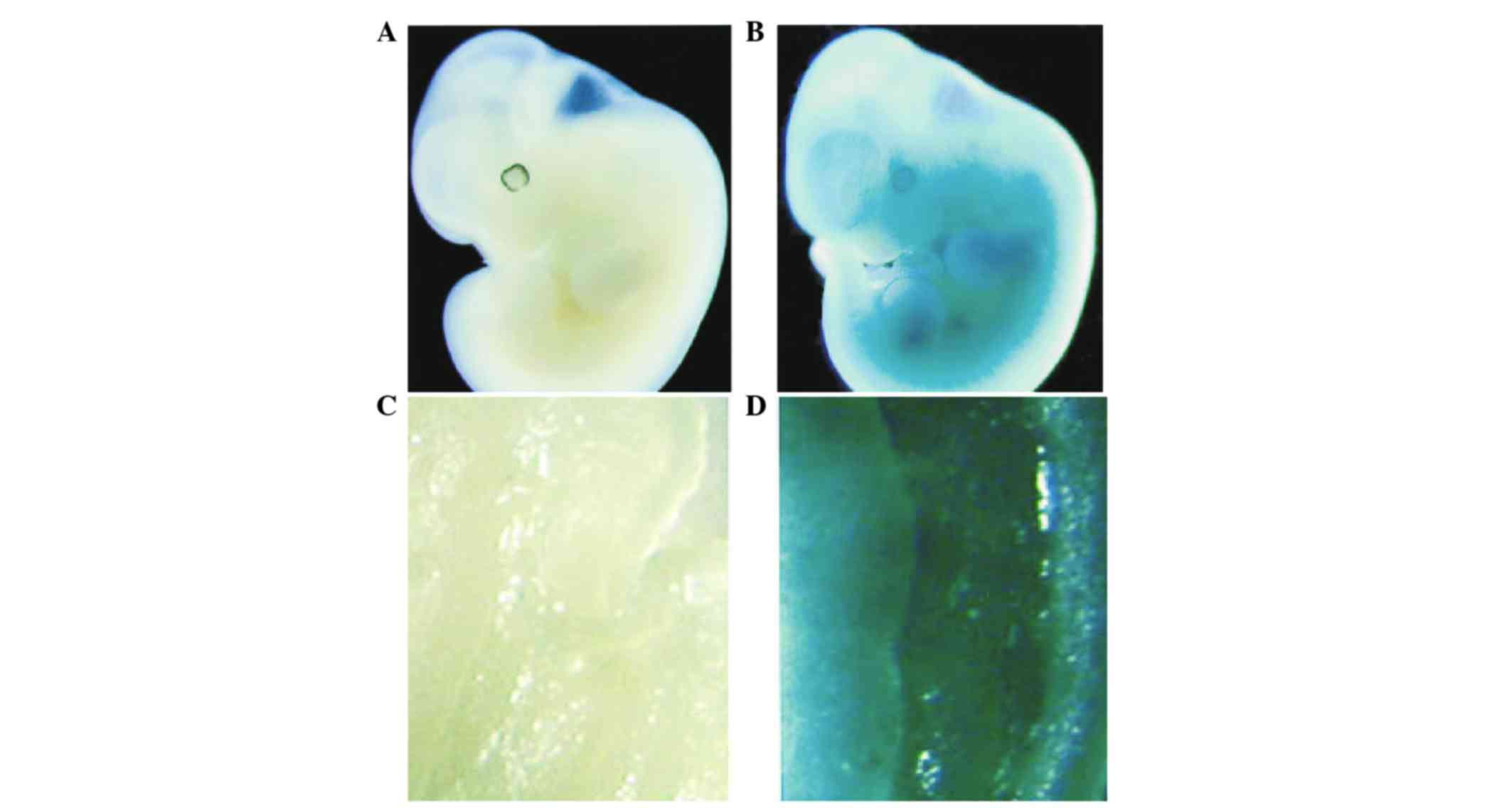
Homozygous mutant Smad3 mice were generated from the
inbred and hybrid Smad3 mouse strains by intercrossing the
appropriate heterozygotes. Based upon this protocol, 12 homozygous
inbred 129/Sv mutant Smad3 mice were generated. The expression
pattern of Smad3 in normal and homozygous mutant Smad3 (−/−) mice
was identified by whole-mount in situ hybridization of
embryos at day 11.5. The data is presented in Fig. 1. Between 20 and 22 weeks, 100% (10/10)
of inbred Smad3 mutants began to exhibit signs of colon cancer,
including rectal prolapse, and dilated large and small bowels,
leading to apparent distress and indicating evidence of tumor
formation. This appeared to indicate that homozygous mutant Smad3
mice develop colon cancer.
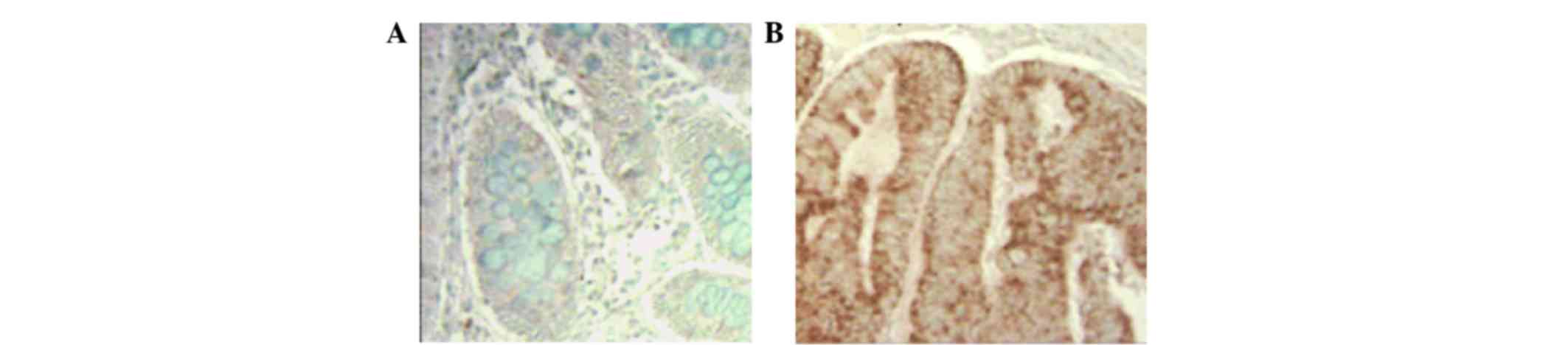
Overexpression of COX-2 in Smad3
mutant mice
Samples were prepared from normal and homozygous
mutant Smad3 mice at the 22nd week for immunohistochemistry with
COX-2 antibody as described in the Materials and Methods. The
results of immunohistochemistry are presented in Fig. 2. Fig. 2B
illustrates the overexpression pattern of COX-2 in homozygous
mutant Smad3 mice. Positive staining in Smad3 mice was increased
compared to the control, confirming the presence of overexpression
of COX-2 in Smad3 mutant mice.
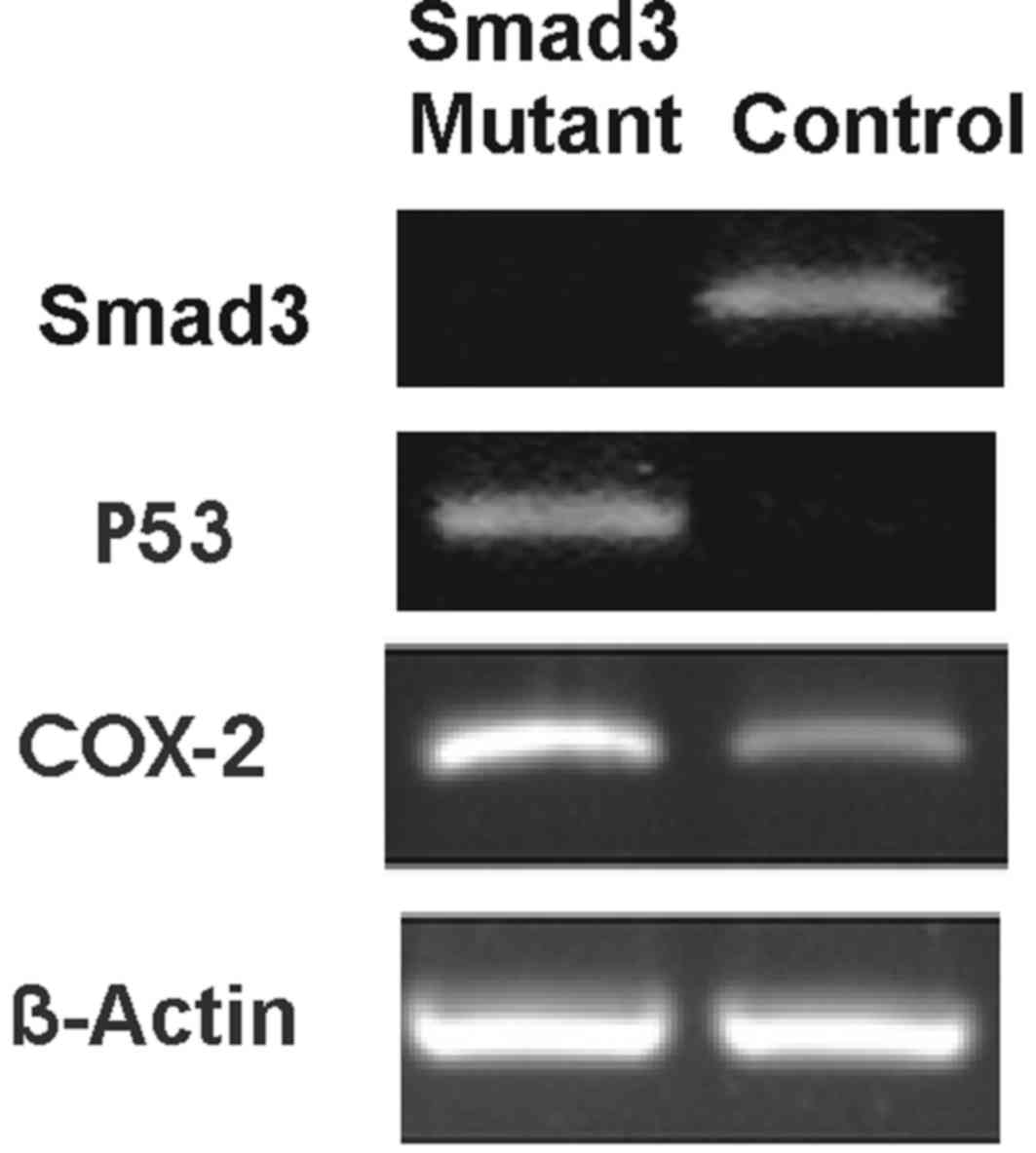
RT-PCR analysis
RNA was isolated from the normal and homozygous
mutant Smad3 mice at the 22nd week for RT-PCR analysis as described
in the Materials and Methods. The overexpression pattern of COX-2
in Smad3 mutant mice was investigated. Agarose gels produced
following RT-PCR are presented in Fig.
3, which show the positive expression of P53 and COX-2 in
homozygous mutant Smad3 mice.
Discussion
Dissection of the molecular pathway underlying colon
cancer is key to understanding the specific involvement of various
proteins. Studying molecular alterations and clinical outcomes is
important in cancer research (16–22).
Homozygous mutant Smad3 mice were generated and began to develop
colon cancer from the 20th to 22nd week. Based on the results of
the present study, it was concluded that Smad3 was mutated and not
expressed in the Smad3 (−/−) mutant mice. In addition, Smad3
expression was identified in various parts of the normal embryo,
suggesting its importance. Smad3 is expressed in numerous
developing tissues, with the highest levels observed in mesenchymal
derivatives. In addition, Smad3 is expressed in the adult colon,
with the highest levels observed in the muscularis propria and the
submucosa, and lower levels in the epithelium. Thus, Smad3 is a
widely expressed TGFβ signaling intermediate (23).
Immunohistochemistry with COX-2 antibody revealed
overexpression in the homozygous Smad3 mutant mouse. By comparing
immunohistochemical staining in the control and Smad3 samples,
COX-2 overexpression was clearly observed, and low levels of
expression of COX-2 were observed in the control sample. In
addition, following the development of colon cancer in homozygous
mutant Smad3 mice, COX-2 was overexpressed.
In order to validate the immunohistochemistry data,
RT-PCR was performed. The results of RT-PCR led to the conclusion
that COX-2 is overexpressed in homozygous mutant Smad3 mice. In
addition, P53 was upregulated in the homozygous mutant Smad3 mice
compared with the control. It has been previously reported that P53
is upregulated in cancer cells (24).
In the present study, P53 was upregulated in the homozygous mutant
Smad3 mice, appearing to demonstrate that these mice will go on to
develop colon cancer. Furthermore, overexpression of COX-2 was also
observed, therefore a link between P53 and COX-2 overexpression was
inferred. Due to the results of the immunohistochemistry and RT-PCR
analyses, it was concluded and validated that Smad3 mutant mice
develop colon cancer with overexpression of COX-2.
The results of the present study indicate that a
link may exist between Smad3 mutant mice, colon cancer and COX-2.
In addition, an overexpression pattern of COX-2 in Smad3 mutant
mice, which developed colon cancer, was identified. There is the
potential that COX-2 may be a good marker for the diagnosis of
colon cancer during early stages.
Acknowledgements
The present study was financially supported by
Zhejiang Medicines Health Science and Technology Program (grant
no., 2013KYB046) and the National Natural Science Foundation of
China (grant no., 81372210). The authors would like to thank the
Institutional Review Ethical Board Approval Committee for the
successful completion of this project.
References
|
1
|
Potter JD, Slattery ML, Bostick RM and
Gapstur SM: Colon cancer: a review of the epidemiology. Epidemiol
Rev. 15:499–545. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomlinson I, Ilyas M and Novelli M:
Molecular genetics of colon cancer. Cancer Metastasis Rev.
16:67–79. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Y, Richardson JA, Parada LF and Graff
JM: Smad3 mutant mice develop metastatic colorectal cancer. Cell.
94:703–714. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oshima M, Oshima H and Taketo MM: TGF-beta
receptor type II deficiency results in defects of yolk sac
hematopoiesis and vasculogenesis. Dev Biol. 179:297–302. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Sun Y, Constantinescu SN, Karam E,
Weinberg RA and Lodish HF: Transforming growth factor beta-induced
phosphorylation of Smad3 is required for growth inhibition and
transcriptional induction in epithelial cells. Proc Natl Acad Sci
USA. 94:10669–10674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Graff JM, Bansal A and Melton DA: Xenopus
Mad proteins transduce distinct subsets of signals for the TGF beta
superfamily. Cell. 85:479–487. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown JR and DuBois RN: COX-2: A molecular
target for colorectal cancer prevention. J Clin Oncol.
23:2840–2855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: A
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buchanan FG and DuBois RN: Connecting
COX-2 and Wnt in cancer. Cancer Cell. 9:6–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Humphreys BD, Valerius MT, Kobayashi A,
Mugford JW, Soeung S, Duffield JS, McMahon AP and Bonventre JV:
Intrinsic epithelial cells repair the kidney after injury. Cell
Stem Cell. 2:284–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington DC: 2011, PubMed/NCBI
|
|
13
|
Shea K and Geijsen N: Dissection of 6.5
dpc mouse embryos. J Vis Exp. 2:e16010.3791/160. 2007.
|
|
14
|
Hogan B, Costantini F and Lacy E:
Manipulating the Mouse Embryo: A Laboratory Manual. 1st. Cold
Spring Harbor Laboratory Press; NY: pp. 55–60. 1986
|
|
15
|
Zhong X, Wang H and Jian X: Expression of
matrix metalloproteinases-8 and −9 and their tissue inhibitor in
the condyles of diabetic rats with mandibular advancement. Exp Ther
Med. 8:1357–1364. 2014.PubMed/NCBI
|
|
16
|
Ogino S, Meyerhardt JA, Cantor M,
Brahmandam M, Clark JW, Namgyal C, Kawasaki T, Kinsella K,
Michelini AL, Enzinger PC, et al: Molecular alterations in tumors
and response to combination chemotherapy with gefitinib for
advanced colorectal cancer. Clin Cancer Res. 11:6650–6656. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Heer P, Gosens MJ, de Bruin EC,
Dekker-Ensink NG, Putter H, Marijnen CA, van den Brule AJ, van
Krieken JH, Rutten HJ, Kuppen PJ and van de Velde CJ: Dutch
Colorectal Cancer Group: Cyclooxygenase 2 expression in rectal
cancer is of prognostic significance in patients receiving
preoperative radiotherapy. Clin Cancer Res. 13:2955–2960. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zlobec I, Terracciano LM and Lugli A:
Local recurrence in mismatch repair-proficient colon cancer
predicted by an infiltrative tumor border and lack of CD8+
tumor-infiltrating lymphocytes. Clin Cancer Res. 14:3792–3797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henry LR, Lee HO, Lee JS, Klein-Szanto A,
Watts P, Ross EA, Chen WT and Cheng JD: Clinical implications of
fibroblast activation protein in patients with colon cancer. Clin
Cancer Res. 13:1736–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ginty F, Adak S, Can A, Gerdes M, Larsen
M, Cline H, Filkins R, Pang Z, Li Q and Montalto MC: The relative
distribution of membranous and cytoplasmic met is a prognostic
indicator in stage I and II colon cancer. Clin Cancer Res.
14:3814–3822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benson AB III: New approaches to assessing
and treating early-stage colon and rectal cancers: Cooperative
group strategies for assessing optimal approaches in early-stage
disease. Clin Cancer Res. 13:6913s–6920s. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris M, Platell C and Iacopetta B:
Tumor-infiltrating lymphocytes and perforation in colon cancer
predict positive response to 5-fluorouracil chemotherapy. Clin
Cancer Res. 14:1413–1417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Richardson JA, Parada LF and Graff
JM: Smad3 mutant mice develop metastatic colorectal cancer. Cell.
94:703–714. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He ZY, Shi CB, Wen H, Li FL, Wang BL and
Wang J: Upregulation of p53 expression in patients with colorectal
cancer by administration of curcumin. Cancer Invest. 29:208–213.
2011. View Article : Google Scholar : PubMed/NCBI
|