Introduction
Hemangioma is a common type of infantile congenital
benign tumor, which is characterized by abnormal proliferation of
vascular tissues. It usually appears in the first weeks of life and
grows most rapidly over the initial 6 months. Typically, growth is
complete and involution has commenced by 12 months. Hemangioma
proceeds through three phases including proliferation, regression
and involution. If not properly controlled, it is likely to cause
significant cosmetic injury or even threaten the patient's life
(1–3).
The cause of hemangioma is currently unknown. However, previous
studies have suggested that the expression levels of vascular
endothelial growth factor (VEGF) and basic fibroblast growth factor
(bFGF) are closely correlated with the proliferation of the
endothelial cells of a hemangioma (4–9). Takahashi
et al (10) demonstrated that
VEGF was highly expressed in the endothelial cells of proliferative
phase hemangioma, whereas it was undetectable during the regression
phase, indicating that VEGF is closely associated with the
incidence and involution of hemangioma. Bielenbery et al
(11) reported that high levels of
bFGF were expressed in proliferative phase hemangioma tissue,
whereas normal expression levels of bFGF were detected on the
surface of hemangioma in the regression phase. Currently, laser
therapy, hormonal therapy, radiotherapy, interferon treatment and
surgery are used to treat hemangioma. Laser therapy has been
increasingly adopted to manage infantile cutaneous hemangioma
because it is a noninvasive technique that yields no secondary
scarring or pigment alteration (12).
The clinical efficacy of laser therapy for treating hemangioma is
predominantly evaluated by a medical history and physical
examination. However, a simple and reliable detection method is
urgently required.
In the present study, the plasma levels of VEGF and
bFGF, which are related to vascular hyperplasia, were detected in
patients with hemangioma in varying phases to investigate the
effect of laser therapy on plasma VEGF and bFGF concentrations in
infants diagnosed with cutaneous hemangioma. The aim of this study
was to identify simple and objective biomarkers for evaluating the
clinical efficacy of laser therapy.
Patients and methods
Patient enrollment
A total of 109 patients, including 44 males and 65
females, diagnosed with superficial abdominal hemangioma at the
Department of Burn and Plastic Surgery of Shandong Provincial
Hospital Affiliated to Shandong University (Jinan, China) between
October 2009 and August 2012 were recruited in the present study.
Written informed consent was obtained from all patients, and
ethical approval was obtained from Shandong Provincial Hospital
Affiliated to Shandong University (Jinan, China). The inclusion
criteria were as follows: i) Those with complete clinical data; ii)
those who were able to comply with the doctors' prescription and
complete the entire treatment; and iii) those who were able to
complete the subsequent follow-up. Of the 109 cases, 74 were
assigned to the proliferation phase group, 20 to the regression
phase group and 15 to the involution phase group. Furthermore, 10
patients (5 male) without hemangioma were enrolled in the control
group, and 23 patients with hemangioma who were aged 40 days to 6
months, with lesion areas ranging from 4–30 cm2, were
randomly selected to observe the superficial abdominal hemangioma
and assigned to the dynamic observation group (Table I). All patients were admitted to our
hospital for the first time and had not previously received laser
therapy.
 | Table I.Demographic data of all
participants. |
Table I.
Demographic data of all
participants.
| Parameters | Proliferation phase
group (n=74) | Regression phase
(n=20) | Involution phase
group (n=15) | Control group
(n=10) | Dynamic observation
group (n=23) |
|---|
| Age (months) | 1.0–9.0 | 4.0–12.0 | 11.5–26.0 | 1.3–24.0 | 1.3–6.0 |
| Male/female | 27/47 | 8/12 | 9/6 | 5/5 | 8/15 |
| Lesion size
(cm2) | 3–36 | 3–20 | 0 | 0 | 4–30 |
Patients with hemangioma were staged according to
previously described criteria (6).
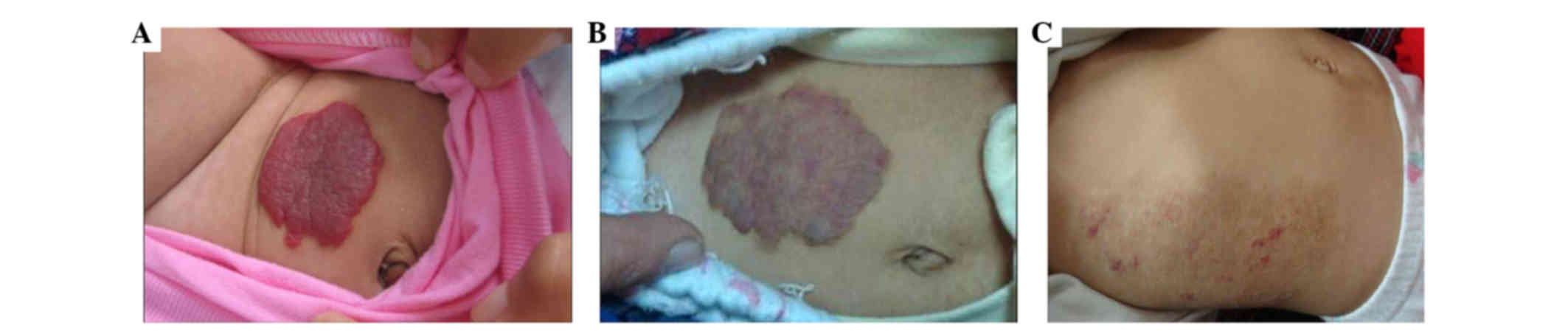
Briefly, hemangiomas were defined as in the proliferation phase if
they rapidly grew upon admission and were observed as having a
bright coloration and hard texture (Fig.
1A). Hemangiomas were classified as in the regression phase if
their growth was stable or gradually diminished, and if they became
white and the tumor texture was softened (Fig. 1B). Hemangiomas were defined as in the
involution phase if they were completely stable and no further
treatment was required (Fig. 1C).
Blood sample collection
Venous blood (2 ml) was collected from all patients
in sterile anticoagulation tubes and centrifuged at 3,500 ×
g for 10 min at 4°C. Subsequently, the plasma was collected,
distributed into sterile EP tubes and stored at −80°C until
use.
Measurement of VEGF and bFGF
concentrations
The plasma levels of VEGF and bFGF were determined
using ELISAs (Human VEGF ELISA kit; Abcam, Cambridge, MA, USA).
Laser therapy
Prior to laser treatment, the site, size, onset time
and severity of the hemangioma were evaluated. The lesional skin
was anesthetized using 2% lignocaine (Teva Pharmaceuticals, Petah
Tikva, Israel). Laser therapy was performed by a proficient
physician using an N-Lite Pulsed Dye Laser (ICN Photonics Ltd.,
Llanelli, UK) at a wavelength of 585 nm, pulse length of 0.45 ms,
spot diameter of 5 mm, at an energy density of 7.5 J/cm2
and a frequency of 1.5 Hz. Following 3 cycles of laser treatment,
the tumor site was disinfected with benzalkonium bromide at a
concentration of 0.1 mg/ml (Sigma-Aldrich; EMD Millipore,
Billerica, MA, USA) and intermittently cooled using an ice bag for
20–30 min. Subsequently, mupirocin ointment (Teva Pharmaceuticals)
was applied three times per day for 4 days. Each cycle of laser
therapy was delivered at a time interval of 1 month until the end
of the treatment.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Differences among multiple
groups were analyzed using one-way analysis of variance (ANOVA).
Differences between two groups were analyzed by Least Significance
Difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in the plasma levels of VEGF
and bFGF in patients with different phases of hemangioma
The plasma concentrations of VEGF in the different
groups are shown in Table II. ANOVA
revealed that the plasma concentration of VEGF was significantly
different among the proliferation phase, regression phase,
involution phase and control groups (F=27.824; P<0.001). LSD
demonstrated that the plasma level of VEGF in the proliferation
phase group significantly differed from those in the regression
phase, involution phase and control groups (all P=0<0.001).
Furthermore, it was demonstrated that there was no significant
difference in the plasma concentration of VEGF between any two
groups among the regression phase, involution phase and control
groups (all P>0.05).
 | Table II.Comparison of the plasma concentration
of VEGF among the different groups (means ± standard
deviation). |
Table II.
Comparison of the plasma concentration
of VEGF among the different groups (means ± standard
deviation).
| Parameters | Proliferation phase
group (n=45) | Regression phase
group (n=18) | Involution phase
group (n=11) | Control group
(n=10) |
|---|
| Age (months) | 3.88±2.16 | 9.17±2.68 | 19.18±5.80 | 7.83±6.32 |
| Male/female | 16/29 | 7/11 | 7/4 | 5/5 |
| Body weight (kg) | 7.05±1.54 | 9.92±2.87 | 11.32±1.66 | 8.05±2.05 |
| Lesion size
(cm2) | 6.67±6.94 | 7.44±11.52 | 0 | 0 |
| VEGF (pg/ml) |
268.64±256.16a | 42.89±37.90 | 25.55±21.76 | 26.50±23.50 |
The plasma concentrations of bFGF in different
patients are shown in Table III.
ANOVA revealed that the plasma concentration of bFGF significantly
differed among the proliferation phase, regression phase,
involution phase and control groups (F=38.738; P<0.001).
LSD demonstrated that the plasma level of bFGF in the proliferation
phase group significantly differed from those in the regression
phase, involution phase and control groups (all P<0.001). In
addition, it was shown that there was no significant difference in
the plasma concentration of bFGF between any two groups among the
regression phase, involution phase and control groups (all
P>0.05).
 | Table III.Comparison of the plasma
concentrations of bFGF among the different groups (means ± standard
deviation). |
Table III.
Comparison of the plasma
concentrations of bFGF among the different groups (means ± standard
deviation).
| Parameters | Proliferation phase
group (n=51) | Regression phase
group (n=20) | Involution phase
group (n=15) | Control group
(n=10) |
|---|
| Age (months) | 3.84±2.06 | 9.20±2.54 | 18.15±5.86 | 7.83±6.32 |
| Male/female | 19/32 | 8/12 | 9/6 | 5/5 |
| Body weight (kg) | 6.95±1.52 | 10.20±3.04 | 11.23±1.69 | 8.05±2.05 |
| Lesion size
(cm2) | 6.37±6.60 | 6.90±11.03 | 0 | 0 |
| bFGF (pg/ml) |
490.75±255.21a | 129.10±130.41 | 12.93±6.55 | 7.40±4.88 |
Changes in the plasma levels of VEGF
and bFGF in the dynamic observation group before and after laser
therapy
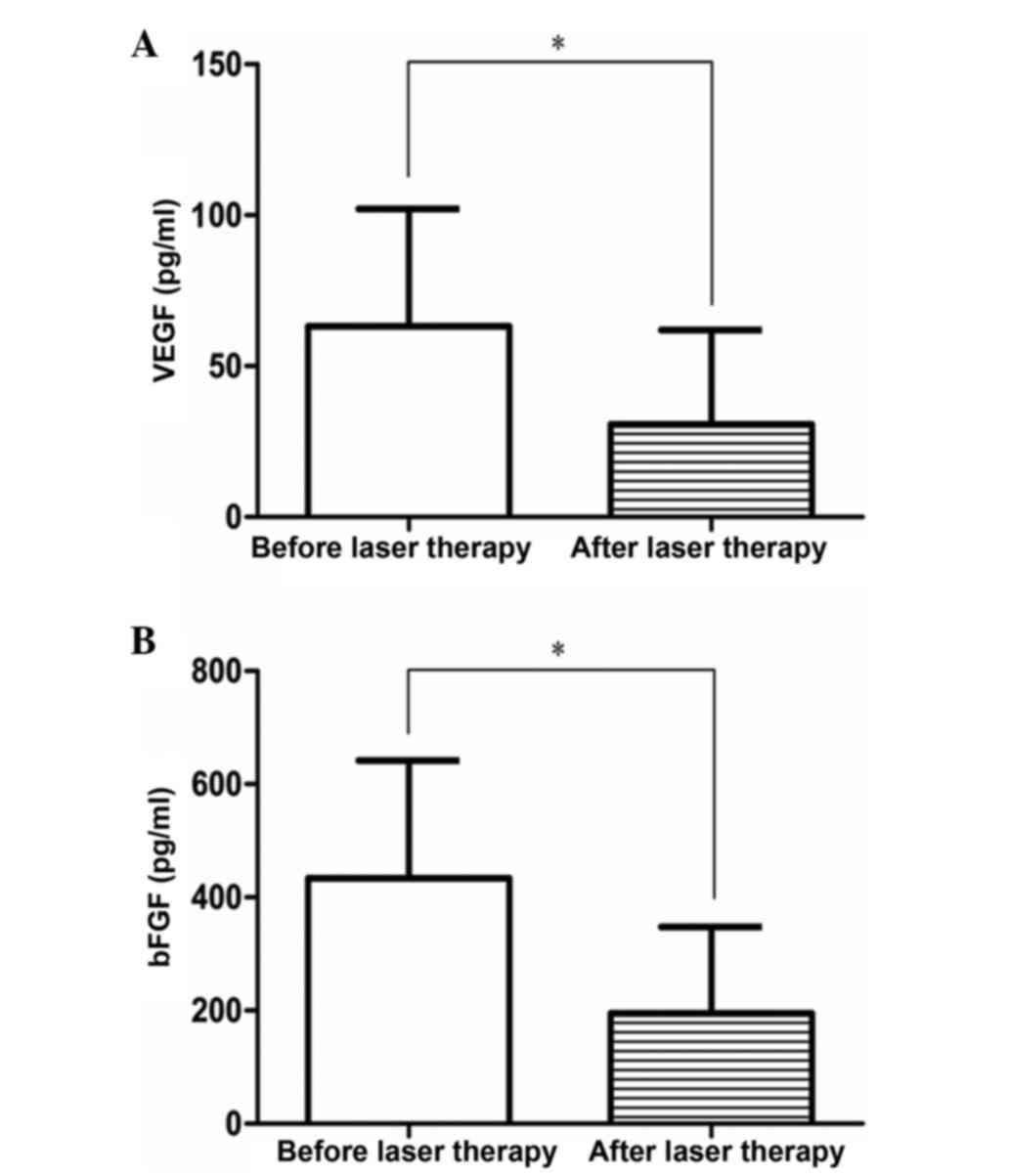
The plasma concentrations of VEGF and bFGF in the
dynamic observation group before and after laser therapy at the
same time points are shown in Table
IV and Fig. 2. Statistical
analysis revealed that the plasma concentrations of VEGF and bFGF
in the dynamic observation group at the same time points differed
significantly prior to and following laser therapy (VEGF: t=7.478,
P=0.000; bFGF: t=7.785, P<0.001).
 | Table IV.Comparison of plasma levels of VEGF
and bFGF in the dynamic observation group before and after laser
therapy (means ± standard deviation). |
Table IV.
Comparison of plasma levels of VEGF
and bFGF in the dynamic observation group before and after laser
therapy (means ± standard deviation).
| Parameters | Before laser
therapy | After laser
therapy |
|---|
| Age (months) | 3.79±1.86 | 6.31±1.76 |
| Male/female | 8/15 | 8/15 |
| Body weight (kg) | 7.67±2.39 | 9.13±2.39 |
| Lesion size
(cm2) | 9.46±10.99 | 9.46±10.99 |
| VEGF (pg/ml) |
63.22±38.84a | 30.74±31.22 |
| bFGF (pg/ml) |
433.83±207.71a | 195.09±152.62 |
Discussion
In 1982, Mulliken and Glowacki (6) proposed a biological classification of
vascular birthmarks on the basis of clinical manifestations,
histopathological features and natural history, which was
subsequently adopted by The International Society for the Study of
Vascular Anomalies in 1996 (7). Since
then, congenital vascular diseases have been further classified
into hemangiomas and vascular abnormalities (8). Hemangioma, which results from abnormal
proliferation of vascular endothelial cells, is one of the most
common types of congenital benign tumors during infancy, and
rapidly grows and proliferates prior to the infant reaching 1
year-of-age (9). Previous studies
have demonstrated that VEGF, bFGF and their receptors are closely
associated with the proliferation of infantile cutaneous hemangioma
(10). Takahashi et al
detected upregulated expression of recombinant VEGF-16539 in
transgenic rabbit models, and a histological examination revealed
hemangioma in the rabbit liver. Immunohistochemical analysis
demonstrated that human VEGF-165 was expressed in hepatocytes
rather than in rabbit plasma, suggesting that upregulated
expression of VEGF-165 results in the incidence of hemangioma in
transgenic rabbit models (13).
Takahashi et al (10) analyzed
hemangioma clinical specimens using immunohistochemical staining
and found that the expression of VEGF was significantly upregulated
in proliferative phase hemangioma, whereas it was markedly
downregulated in hemangiomas in the regression and involution
phases. Bielenberg et al (11)
utilized in situ hybridization and immunohistochemical
analysis to measure the expression of VEGF and bFGF in hemangioma
samples at various phases, and demonstrated that both VEGF and bFGF
were highly expressed, suggesting that VEGF and bFGF are closely
correlated with the incidence of hemangioma.
The majority of hemangiomas disappear by themselves
and do not require any treatment. However, hemangiomas should be
treated if they affect important structures, such as the eyes,
nose, ears or windpipe, if they keep growing at a high speed, and
if they become ulcerated and painful. Radiotherapy, hormonal
therapy, interferon treatment, surgery and laser therapy are
commonly employed for the treatment of hemangiomas (12). In plastic surgery, laser therapy has
been frequently applied to treat superficial vascular illnesses
including infantile cutaneous hemangioma and portwine stains
(14). No complications, including
hyperplastic or atrophic scars, have been reported after laser
therapy. Over the past few decades, laser therapy has been widely
applied to the treatment of hemangiomas at our hospital, resulting
in the accumulation of clinical experience and clinical data.
Furthermore, a favorable clinical efficacy and high degree of
satisfaction have been obtained. In the present study, 109 infants
diagnosed with hemangioma and undergoing laser therapy at our
institution between October 2009 and August 2012 were enrolled,
including 74 patients with hemangioma in the proliferation phase,
20 in the regression phase and 15 in the involution phase. The
potential effect of laser therapy on the plasma concentrations of
VEGF and bFGF in infants was evaluated to explore a simple and
reliable parameter to assess the clinical efficacy of laser therapy
in the management of hemangioma.
The exact pathogenesis of hemangioma remains largely
unknown. However, several studies have suggested the importance of
angiogenic factors in the incidence and proliferation of
hemangioma, which is likely caused by a disrupted balance in the
stimulating and inhibitory factors of angiogenesis (15–17). There
is evidence that the expression levels of VEGF and bFGF in the
peripheral blood of infants with proliferative phase hemangioma
were significantly upregulated compared with those in patients with
hemangiomas at the regression phase and normal controls, whereas
they did not significantly differ between regression phase infants
and healthy counterparts (16). In
the present study, the plasma concentrations of VEGF and bFGF in
the proliferation phase group were significantly higher compared
with those in the regression phase, involution phase and control
groups, while no significant difference was observed among the
regression phase, involution phase and control groups. After
several cycles of laser therapy, the plasma concentrations of VEGF
and bFGF were declined to within the normal ranges (<40 and
<300 pg/ml, respectively), from the proliferation phase to the
regression phase. For each hemangioma patient, the plasma levels of
VEGF and bFGF were significantly reduced after laser therapy,
suggesting that the laser therapy was able to lower the plasma
concentrations of VEGF and bFGF.
To date, the majority of studies have focused on the
histopathological evaluation of hemangioma specimens of different
phases, employing quantitative polymerase chain reaction and
immunohistochemical staining (17,18).
Conversely, the present study collected peripheral blood samples
from infants diagnosed with hemangioma, which was a safe,
noninvasive, sensitive and convenient method for assessing the
different phases, and may be widely applied in clinical practice
following validation by subsequent large-scale clinical trials.
Furthermore, the present study observed that individuals exhibited
different levels of sensitivity to laser therapy. The majority of
the infants were highly sensitive to laser therapy; the plasma
concentrations of VEGF and bFGF were rapidly decreased following
laser therapy and favorable clinical outcomes were obtained.
However, a minority of infants exhibited low sensitivity to laser
treatment; the plasma concentrations of VEGF and bFGF were
maintained at relatively high levels following laser therapy.
Therefore, the combined use of laser treatment with hormonal
therapy or injection of medicines, such as propranolol, vincristine
and interferon, may be considered (16).
In the present study, the plasma concentrations of
VEGF and bFGF rapidly declined following laser therapy. Although
the exact mechanism is unknown, it may result from the high-energy
laser selectively promoting the thermal coagulation of hemoglobin,
eventually leading to vascular occlusion. Hemoglobin absorbs the
laser energy, forms thrombi and causes injuries to endothelial
cells (18), which is directly caused
by laser therapy or indirectly by vascular occlusion. The
complications of thrombosis and vascular occlusion after laser
therapy block topical blood circulation to the hemangioma and lead
to hypoxia, thereby causing the swelling, degeneration and necrosis
of vascular endothelial cells. Upon the incidence of vascular
endothelial cell injury, the expression levels of VEGF and bFGF
were decreased and the proliferation of hemangioma was reduced
accordingly. Therefore, the authors of the present study
hypothesized that laser therapy may cause endothelial cell injury
and subsequently affect the expression of VEGF and bFGF in
endothelial cells, thereby promoting the progression of the
proliferation phase into the regression phase. However, the
underlying mechanism requires further elucidation. Furthermore, due
to the difficulty in collecting and preserving tumor samples, the
sample size was relatively small. Therefore, subsequent studies
with a larger sample size should be performed.
In conclusion, the present study demonstrated that,
after several cycles of laser therapy, the cutaneous hemangiomas of
infants with proliferative phase hemangioma were alleviated to the
regression phase. Simultaneously, the high plasma concentrations of
VEGF and bFGF in the proliferation phase were reduced to within
their normal ranges during the regression phase, which was
consistent with the clinical characteristics. Therefore, the plasma
levels of VEGF and bFGF may be used to evaluate the clinical
efficacy of laser therapy.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Shandong Province (funding no.
ZR2013HM058).
References
|
1
|
Zhao FY, Gao Y, Wu MJ, Luo QF, Liu Y and
Xu ZQ: Diagnosis and therapy on hemangiomas and vascular
malformation in view of the new classification. Beijing Da Xue Xue
Bao. 41:21–27. 2009.(In Chinese). PubMed/NCBI
|
|
2
|
Smolinski KN and Yan AC: Hemangiomas of
infancy: Clinical and biological characteristics. Clin Pediatr
(Phila). 44:747–766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackson IT: Hemangiomas. Eur J Plast Surg.
31:275–280. 2008. View Article : Google Scholar
|
|
4
|
Hohenleutner U, Landthaler M, Hamm H and
Sebastian G: Hemangiomas of infancy and childhood. J Dtsch Dermatol
Ges. 5:334–338. 2007.(In English, German). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang QL and Feng XL: Advances of research
on infantile hemangioma associated factors. Zhongguo Meirong Yixue.
20:880–882. 2011.(In Chinese).
|
|
6
|
Mulliken JB and Glowacki J: Hemangiomas
and vascular malformations in infants and children: A
classification based on endothelial characteristics. Plast Reconstr
Surg. 69:412–422. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu QH, Lin XX and Wang W: Association
between VEGF and its receptors and proliferative hemangiomas. Zhong
Hua Zheng Xing Wai Ke Za Zhi. 18:2372002.(In Chinese).
|
|
8
|
Su T, Zhao YF and Zhang WF: Proliferative
hemangioma and bFGF. Guo Wai Yi Xue. 32:113–114. 2005.(In
Chinese).
|
|
9
|
Przewratil P, Sitkiewicz A and
Andrzejewska E: Local serum levels of vascular endothelial growth
factor in infantile hemangioma: Intriguing mechanism of endothelial
growth. Cytokine. 42:141–147. 2010. View Article : Google Scholar
|
|
10
|
Takahashi K, Mulliken JB, Kozakewich HP,
Rogers RA, Folkman J and Ezekowitz RA: Cellular markers that
distinguish the phases of hemangioma during infancy and childhood.
J Clin Invest. 93:2357–2364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bielenberg DR, Bucana CD, Sanchez R,
Mulliken JB, Folkman J and Fidler IJ: Progressive growth of
infantile cutaneous hemangiomas is directly correlated with
hyperplasia and angiogenesis of adjacent epidermis and inversely
correlated with expression of the endogenous angiogenesis
inhibitor, IFN-beta. Int J Oncol. 14:401–408. 1999.PubMed/NCBI
|
|
12
|
Huang Y, Wang JF and Lv LQ: The
development of laser treatment for infant hemangioma. Zhong Guo Yi
Xue Wen Zhai-Pi Fu Ke Xue. 27:349–351. 2010.
|
|
13
|
Arndt S, Poser I, Schubert T, Moser M and
Bosserhoff AK: Cloning and functional characterization of a new Ski
homolog, Fussel-18, specifically expressed in neuronal tissues. Lab
Invest. 85:1330–1341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohmori S and Huang CK: Recent progress in
the treatment of portwine staining by argon laser: Some
observations on the prognostic value of relative
spectro-reflectance (RSR) and the histological classification of
the lesions. Br J Plast Surg. 34:249–257. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
North PE, Waner M, Mizeracki A and Mihm MC
Jr: GLUT1: A newly discovered immunohistochemical marker for
juvenile hemangiomas. Hum Pathology. 31:11–22. 2000. View Article : Google Scholar
|
|
16
|
Chang J, Most D, Bresnick S, Mehrara B,
Steinbrech DS, Reinisch J, Longaker MT and Turk AE: Proliferative
hemangiomas: Analysis of cytokine gene expression and angiogenesis.
Plast Reconstr Surg. 103:1–9; discussion 10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folkman J: Seminars in medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ritter MR, Butschek RA, Friedlander M and
Friedlander SF: Pathogenesis of infantile haemangioma: New
molecular and cellular insights. Expert Rev Mol Med. 9:1–19. 2007.
View Article : Google Scholar : PubMed/NCBI
|