Introduction
Globally, cancer is a significant public health
problem, with ~14.1 million new patients and 8.2 million
cancer-associated mortalities reported worldwide in 2012 (1). Currently, radiotherapy, chemotherapy and
surgery are common tumor treatments. However, the therapeutic
effects are unsatisfactory due to serious side effects, inability
of the treatments to accurately distinguish between normal and
tumor cells, resistance to chemoradiotherapy and the loss of effect
against later metastasis (2,3). Therefore, the development of specific
and highly efficient therapies is important. Mesenchymal stem cells
(MSCs) are multipotent progenitor cells with the capacity to
self-renew and differentiate becoming osteoplastic, adipogenic or
chondrogenic. MSCs are not only derived primarily from the bone
marrow (BM-MSCs) but also have been successfully derived from
numerous tissues, including adipose tissue (AD-MSCs) and the
umbilical cord (UC-MSCs) (4). These
MSCs share useful characteristics, such as immune modulation,
cytokine secretion and differentiation, which make them popular
candidates for use in the development of new tumor therapies.
The stroma of solid cancers contains multiple cell
types and non-cellular components, creating a complex signaling
network to maintain tumor development (5). Decades of research has proven that MSCs
can be recruited to the stroma of solid tumors, via direct contact
or paracrine signaling, to effect the cell growth, apoptosis and
metastasis of the surrounding tumor cells (6). Additionally, MSCs have been suggested to
act as a cellular delivery system, owing to their targeted
recruiting ability, which derives from the innate tropism of MSCs
to tumors. MSCs can deliver interferon (IFN)-β to inhibit the
proliferation of malignant tumor cells in in vitro
co-culture systems (7). Combination
treatment with AD-MSC-IFN-β and cisplatin synergistically reduces
tumor volume. Exogenous tumor necrosis factor-α (TNF-α) primed
human MSCs express high levels of membrane-bound TNF-related
apoptosis-inducing ligand (TRAIL) and induce apoptosis in MDA cells
(8). Our previous research has
indicated that the administration of MSCs inhibits tumor migration
in mice with Lewis lung cancer (9).
However, experimental data has also revealed the capability of MSCs
to promote tumor progression and metastasis. MSCs integrate into
the prostate tumor stroma, are converted into cancer-associated
fibroblasts (CAFs), and secrete chemokine (C-X-C motif) ligand 12
(CXCL12), which ultimately leads to an epithelial-to-mesenchymal
transition and distant metastasis of the tumor (10). Subsequent to being preactivated by the
inflammatory cytokines TNF-α and IFN-γ, BM-MSCs accelerate colon
cancer growth in vivo to a greater degree, by expressing
higher levels of vascular endothelial growth factor to promote
angiogenesis (11). Tsai et al
reported that immunodeficient mice injected with MSCs and human
colorectal cancer prominin-1 (CD133)−/cluster of
differentiation 166−/epithelial cell adhesion
molecule− cells exhibited enhanced tumor formation and
the expression of CD133 through interleukin-6/signal transducer and
activator of transcription 3; furthermore, MSC-derived CAFs
participated in this process (12).
Taken together, these findings suggest the
discrepant effects of MSCs on tumor progression; however, the
factors to which these effects are attributable remain unclear. The
heterogeneity of MSCs from different tissues may underlie the
discrepant effects. In addition, differences in surface receptors
and the different signal cascades they activate in different tumor
cells may induce different biological behaviors (13). The aim of the present study was to
determine the role of MSC sources and tumor cell receptors in the
interaction between MSCs and tumor progression.
Materials and methods
Human MSC preparation and cell
culture
UC-, AD- and BM-MSCs were isolated from human
umbilical cord, adipose tissues and bone marrow, respectively. The
samples were obtained with consent from donors at the Chinese
People's Liberation Army Hospital (Beijing, China) between March,
2013 and November, 2013. The cells were maintained in α-modified
minimum essential medium (α-MEM) (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C
in a humidified 5% CO2 atmosphere. Human lung
adenocarcinoma A549 cells and human breast adenocarcinoma
MDA-MB-231 cells were obtained from the American Type Culture
Collection (Manassas, VA, USA) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS at 37°C in a humidified 5% CO2
atmosphere. The study was approved by the Ethical Committee of the
Academy of Military Medical Sciences (Beijing, China). All samples
were collected with the informed consent of patients.
In vitro adipogenic or osteogenic
differentiation analysis
UC-, AD- and BM-MSCs were seeded in 24-well plates
at a density of 20,000 cells/well with adipogenic-differentiation
medium (Cyagen Biosciences, Santa Clara, CA, USA) or 5,000
cells/well with osteogenic-differentiation medium (Cyagen
Biosciences), and cultured with α-MEM supplemented with adipogenic-
or osteogenic-differentiation medium. Subsequent to 3 weeks of
culturing, the cells were fixed with 4% paraformaldehyde at room
temperature and stained with oil red O or alkaline phosphatase
(Cyagen Biosciences) to detect adipogenic or osteogenic
differentiation.
Immunophenotyping analysis
For detection of surface antigens, UC-, AD- and
BM-MSCs (1×106 cells for each sample) were trypsinized
with the use of 0.05% trypsin, centrifuged (112 × g for 5 min at
room temperature), and incubated for 30 min at 4°C with the
following monoclonal antibodies with 2% FBS:
Phycoerythrin-conjugated anti-human CD-45 (catalogue no., 560975; 5
µg per 106 cells), CD-73 (catalogue no., 550257; 5 µg
per 106 cells), CD-90 (catalogue no., 561970; 5 µg per
106 cells), and CD-105 (catalogue no., 562380; 5 µg per
106 cells), and fluoroisothiocyanate (FITC)-conjugated
anti-human CD-19 (catalogue no., 560994; 5 µg per 106
cells) and CD-34 (catalogue no., 555821; 5 µg per 106
cells) (Biolegend, Inc., San Diego, CA, USA) at room temperature
for 30 min. The cells were then harvested using a FACScan flow
cytometer and analyzed by FlowJo vX.0.7 (FC500; Beckman Coulter,
Inc., Brea, CA, USA).
Collection of conditioned medium
UC-, AD- and BM-MSCs were cultured in basic α-MEM
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS, until they reached 70% confluence. The culture medium was
then replaced with serum-free DMEM (Thermo Fisher Scientific,
Inc.), and the cells were incubated for an additional 24 h. The
complete supernatant was collected (112 × g for 5 min at room
temperature), filtered through 0.45 µm filters, and designated as
MSC-conditioned medium (MSC-CM) (6).
Cell proliferation analysis
A549 and MDA-MB-231 cells were seeded at a density
of 2×103 cells/well into 96-well plates and incubated
with MSC-CM (200 µl/well) at 37°C for 48 h. Tumor cells cultured in
serum-free DMEM served as the normal control. At the experimental
endpoint, 10 µl Cell Counting Kit-8 (CCK-8) solution (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was added to each
well, and the cells were incubated at 37°C for an additional 2 h.
The optical density (OD) value was determined at 450 nm on a
microplate reader (Model 680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Cell migration analysis
The present study loaded 24-well polycarbonate
inserts (8 µm; Corning Incorporated, Corning, NY, USA) in 24-well
plates for Transwell assays. UC-, AD- and BM-MSCs at
5×104 cells/well were incubated at 37°C in DMEM with 10%
FBS and added to the lower chamber (600 µl/well) until they adhered
to the well. Tumor cells at 4×104 cells/well were
incubated in 200 µl/well serum-free medium and plated into the
upper chamber. Subsequent to being cultured at 37°C for 7 h, the
cells remaining on the upper surface of the membrane were wiped
away with a cotton tip applicator. The cells on the lower surface
were fixed with 4% paraformaldehyde and stained with 1% crystal
violet. Images in 5 random fields were captured for quantification
using microscopy (Eclipse TS100; Nikon, Tokyo, Japan).
Western blot analysis
A549 and MDA-MB-231 cells were seeded at a density
of 5×105 cells/well into 25 cm2 flasks and
grown to 70% confluence. Subsequent to being starved in serum-free
DMEM for 24 h, the tumor cells were incubated with MSC-CM for 30
min at room temperature. Cell membrane proteins were used for Human
phospho-receptor-associated tyrosine kinase (RTK) array
(RayBiotech, Norcross, GA, USA), according to the manufacturer's
protocol. Membrane protein concentration was determined using a BCA
protein assay kit (Beijing Yuanpinghao Biological Technology Co.,
Ltd., Beijing, China). In total, 20 µg proteins were separated
using 10% SDS-PAGE and were transferred to a nitrocellulose
membrane by electroblotting. The membrane was blocked with 5%
non-fat milk in 0.1%, v/v Tris-buffered saline/Tween-20 and
incubated overnight at 4°C with the appropriate primary antibodies.
The antibodies used were as follows: P-IR (dilution, 1:1,000;
catalogue no., 3023) and P-Her3 (dilution, 1:1,000; catalogue no.,
2842; Cell Signaling Technology, Inc., Boston, MA, USA); and rabbit
monoclonal antibody against GAPDH (dilution, 1:3,000; catalogue
no., 21018; Santa Cruz Biotechnology, Inc., Santa Cruz
Biotechnology, Inc., CA, USA). The membrane was washed and
incubated with a secondary anti-rabbit IgG antibody conjugated with
horseradish peroxidase (dilution, 1:5,000; catalogue no., ab6734;
Abcam, Cambridge, UK) at room temperature for 2 h, and proteins
were detected using ECL Plus Western Blotting Detection Reagents
(EMD Millipore, Billerica, MA, USA). Bands were compared against
GAPDH and data was presented as relative density ratios (Quantity
One software; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using Student's
t-test or one-way analysis of variance and GraphPad Prism 5
software (GraphPad Software, San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
MSCs from disparate sources share the
same characteristics
MSCs were harvested, and the immunophenotypes of the
cells from the 3 sources was analyzed using flow cytometry. As
shown in Table I, the surface markers
of MSCs were not changed. Cells from all 3 sources expressed CD29,
CD44, CD73 and human leukocyte antigen (HLA)-A, B, C-FITC and did
not express CD14, CD31, CD34, CD45 and HLA-DR-FITC. The present
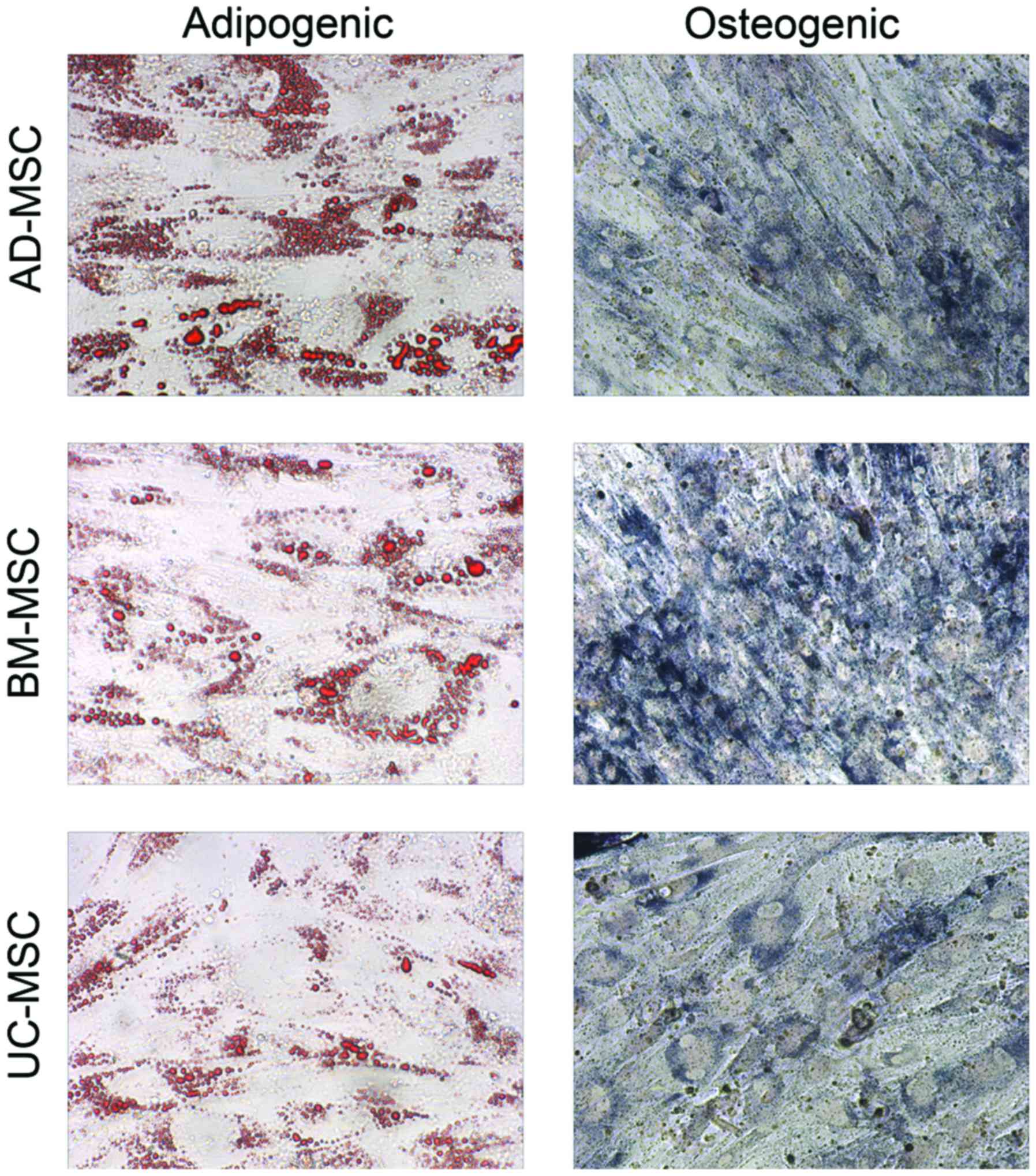
study next considered the differentiation of the MSCs into
osteogenic cells and adipogenic cells. It was found that MSCs were
successfully induced to undergo chondrogenic and adipogenic
differentiation, revealing they had the same differentiation
ability (Fig. 1).
 | Table I.Flow cytometry assay of the
immunophenotype of MSCs. |
Table I.
Flow cytometry assay of the
immunophenotype of MSCs.
| Variables | UC-MSC | AD-MSC | BM-MSC |
|---|
| CD14-PE | − | − | − |
| CD29-PE | +++ | +++ | +++ |
| CD31-PE | − | − | − |
| CD34-PE | − | − | − |
| CD44-PE | +++ | +++ | +++ |
| CD45-PE | − | − | − |
| CD73-PE | +++ | +++ | +++ |
| HLA-A,B,C-FITC | +++ | +++ | +++ |
| HLA-DR-FITC | − | − | − |
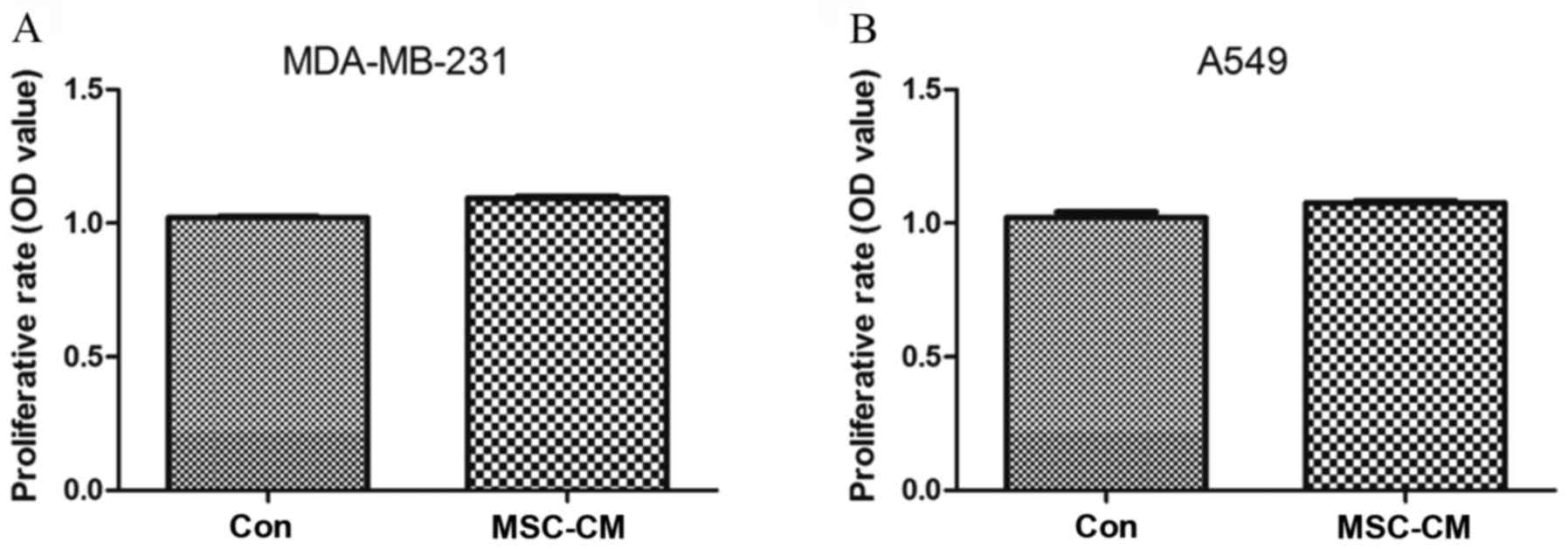
UC-MSC-CM has no effect on tumor cell
proliferation
Breast cancer MDA-MB-231 cells and lung cancer A549
cells were seeded at a density of 3,000 cells/well into 96-well
plates for 12 h and then replaced with UC-MSC-CM for 48 h. Cell
viability was determined using a CCK-8 assay, and the results were
expressed as the OD value to represent the relative proliferation
ability of the cells. The results showed that UC-MSC-CM had no
significant effect on the proliferation of MDA-MB-231 (P=0.1770)
and A549 cells (P=0.0766) (Fig.
2).
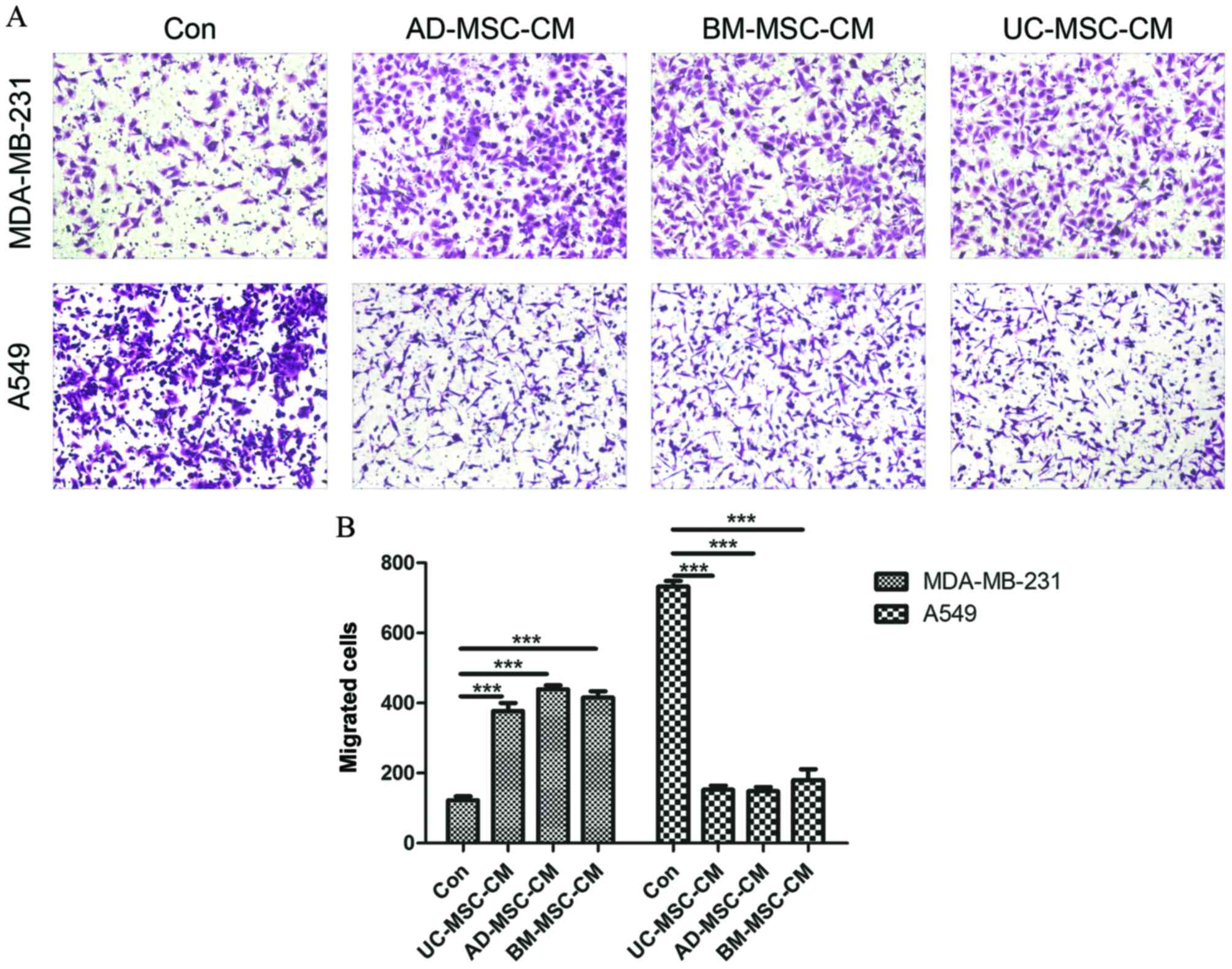
MSC-CM inhibits the migration of A549
cells but promotes the migration of MDA-MB-231 cells
The present study investigated the effect of MSC-CM
on the migration of A549 and MDA-MB-231 cells by using 24-well
polycarbonate Transwell inserts. As shown in Fig. 3A, compared with basic α-MEM medium,
MSC-CM significantly attenuated the migration of A549 cells
(UC-MSC, P<0.0001; AD-MSC, P<0.0001; BM-MSC, P<0.0001) but
enhanced the migration of MDA-MB-231 cells (UC-MSC, P<0.0001;
AD-MSC, P<0.0001; BM-MSC, P<0.0001). Differences in the
sources of MSCs had no effect on the cell migration ability. The
quantitative determination of cell migration is shown in Fig. 3B.
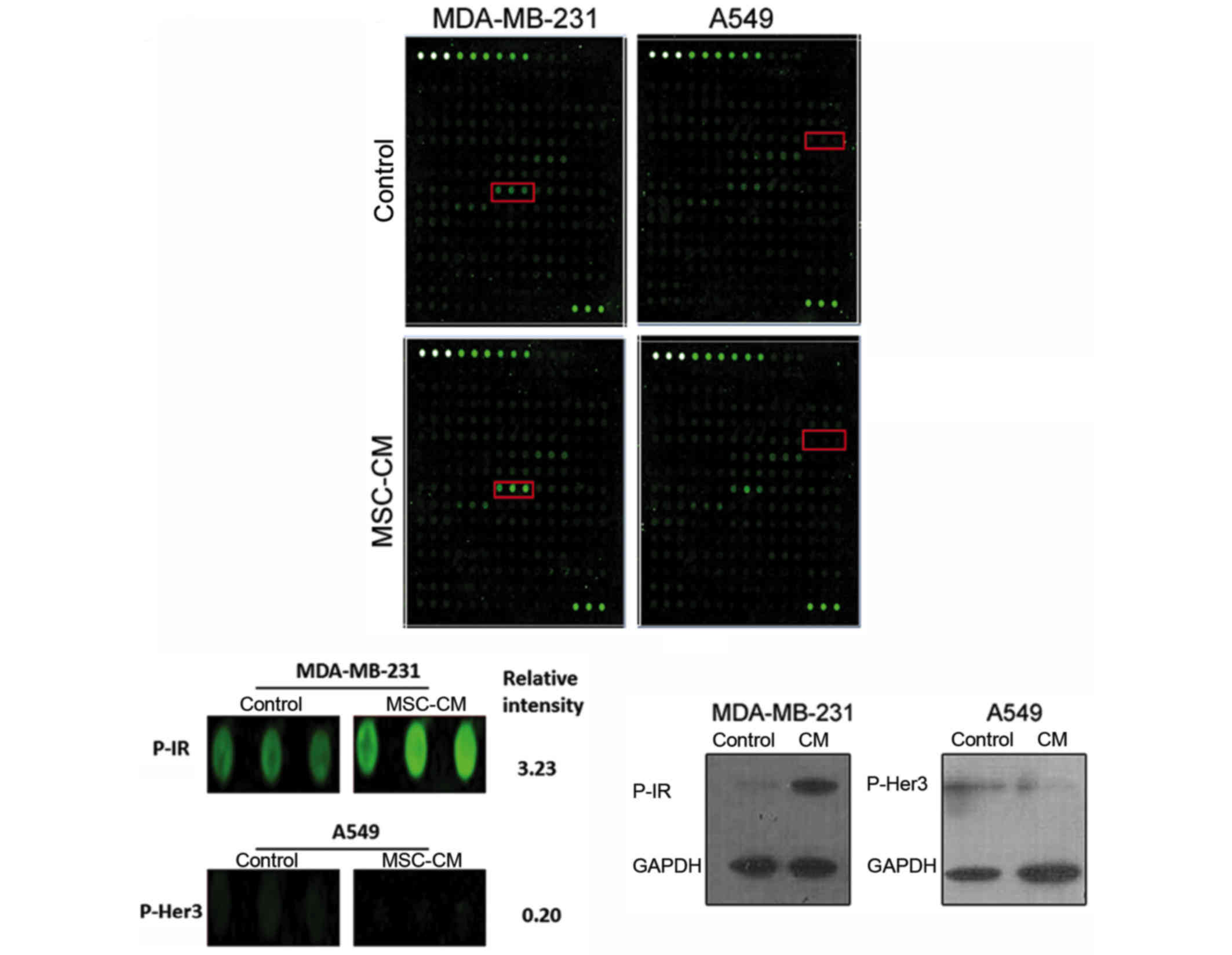
Levels of phosphorylated insulin
receptor and human epidermal growth factor receptor 3 differed
between the 2 cell lines
To investigate the cause of the difference in the
motility of A549 and MDA-MB-231 cells under the effect of MSC-CM, a
phospho-RTK array analysis of the membrane protein extracts of the
2 cell lines was performed. As shown in Fig. 4, the phosphorylation of insulin
receptors (IRs) in MDA-MB-231 cells was enhanced due to the MSC-CM
treatment, whereas in A549 cells, human epidermal growth factor
receptor 3 (Her3) phosphorylation was attenuated. Western blot
analysis was also performed, and consistent results were acquired,
confirming the findings of the phosphor-RTK array analysis
(Fig. 4C).
Discussion
In the present study, initial evidence has been
provided that MSC-CM has opposite effects on the motility of the
lung cancer A549 cell line and the breast cancer MDA-MB-231 cell
line. The present results showed a significant promotion of
MDA-MB-231 cell migration and a significant inhibition of A549 cell
migration. However, MSC-CM appeared to have no significant impact
on tumor cell proliferation in the present study. The data also
indicated that MSCs derived from different tissues, namely, the
umbilical cord, adipose tissue and bone marrow, did not have
discrepant effects on tumor cell migration. Phospho-RTK array
analysis indicated an upregulation of IR phosphorylation in
MSC-CM-treated MDA-MB-231 cells and a downregulation of Her3
phosphorylation in MSC-CM-treated A549 cells, suggesting that the
modulation of IR and Her3 phosphorylation may a major role in the
promotion and inhibition, respectively, of tumor cell migration by
MSC-CM.
By binding to IRs, insulin enhances the
proliferation and migration of MCF-7 via extracellular
signal-regulated kinase (ERK) signaling in vivo and in
vitro (14,15). Consistent with this, the present
results showed that the level of phosphorylated IRs was increased
in MSC-pretreated human breast cancer MDA-MB-231 cells, without
significant alteration in the level of phosphorylated insulin-like
growth factor 1 receptors (IGF-1Rs) and the subsequent c-Jun
N-terminal kinase (JNK) activation, thus enhancing cell
proliferative and migratory abilities. It was therefore deduced
that MSC-CM mainly stimulates IRs rather than IGF-1Rs to act as a
pro-tumor effector in a JNK-independent manner. However, this
conclusion should be explored in additional experiments.
Her3 is a transmembrane receptor and a member of the
erythroblastosis oncogene B family, which is associated with tumor
cell proliferation, motility and survival by the ligand-driven
activation of the RTK domains, stimulating downstream signaling
cascades (16). Previously,
accumulating evidence has indicated that Her3 is a potential
therapeutic target in cancers (17,18). Her3
was shown to be highly expressed in lung adenocarcinomas and
associated with decreased survival in patients (19). Sheng et al reported that the
loss of Her3 function slows ovarian tumor progression in
vitro and prolongs survival in mouse xenograft models of
ovarian cancer (17). Notably, in the
present experiments, inactivation and decreased phosphorylation of
Her3 in A549 cells was observed; these changes inhibited cell
migration, indicating an anti-tumor effect of MSC-CM on lung
cancer.
In oncology, receptor patterns in patients have been
the basis of promising, individualized, biological anti-tumor
therapies, such as the HER2-targeting monoclonal antibody
trastuzumab for breast cancer and lung cancer (20). In addition, MSC-based therapy is
becoming a new strategy, due to the abundant secreted cytokines,
which bind to their corresponding receptors and stimulate
anti-tumor signals (21). Zhu et
al (22) reported that
conditioned medium derived from BM-MSCs enhance human gastric
cancer cell growth via the activation of Ras homolog gene family,
member A-guanosine-5′-triphosphate and ERK1/2 signaling in
vivo and in vitro. Similarly, BM-MSCs promote the
invasion and tumorigenesis of colorectal cancer cells by secreting
soluble neuregulin1 to bind Her3 and activate the PI3K/AKT
signaling cascade in cancer cells (23). In the present study, MSCs were not
found to be suitable for all types of tumors. In breast cancer
cells induced by MSCs, IRs were phosphorylated, enhancing the
malignant traits of the cells; opposite effects were observed in
lung cancer cells, in which the phosphorylation of Her3 was
decreased. Additionally, more precise and specific therapeutic
strategies should be formulated based on the different receptors
expressed in tumors, particularly for MSC-based therapy.
In conclusion, the present research demonstrated
that MSC-CM promotes MDA-MB-231 cell migration and inhibits A549
cell migration by modulating the phosphorylation of IR and Her3,
respectively. Differences in the source of MSCs have no impact on
tumor progression. The present results suggest that the 2 cancer
cell lines behaved differentially owing to the effect of MSC-CM. A
better understanding of the conditions in which MSCs enhance tumor
progression is crucial to safely develop MSCs as a therapeutic tool
and to prevent tumor progression.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant nos. 81270894 and 81201760).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baskar R, Yap SP, Chua KL and Itahana K:
The diverse and complex roles of radiation on cancer treatment:
Therapeutic target and genome maintenance. Am J Cancer Res.
2:372–382. 2012.PubMed/NCBI
|
|
3
|
Collins KK, Liu Y, Schootman M, Aft R, Yan
Y, Dean G, Eilers M and Jeffe DB: Effects of breast cancer surgery
and surgical side effects on body image over time. Breast Cancer
Res Treat. 126:167–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rastegar F, Shenaq D, Huang J, Zhang W,
Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, et al: Mesenchymal
stem cells: Molecular characteristics and clinical applications.
World J Stem Cells. 2:67–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egeblad M, Nakasone ES and Werb Z: Tumors
as organs: Complex tissues that interface with the entire organism.
Dev Cell. 18:884–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kucerova L, Skolekova S, Matuskova M,
Bohac M and Kozovska Z: Altered features and increased
chemosensitivity of human breast cancer cells mediated by adipose
tissue-derived mesenchymal stromal cells. BMC Cancer. 13:5352013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahn J, Lee HW, Seo KW, Kang SK, Ra JC and
Youn HY: Anti-tumor effect of adipose tissue derived-mesenchymal
stem cells expressing interferon-β and treatment with cisplatin in
a xenograft mouse model for canine melanoma. PLoS One.
8:e748972013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RH, Yoon N, Reneau JC and Prockop DJ:
Preactivation of human MSCs with TNF-α enhances tumor-suppressive
activity. Cell stem cell. 11:825–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di GH, Jiang S, Li FQ, Sun JZ, Wu CT, Hu X
and Duan HF: Human umbilical cord mesenchymal stromal cells
mitigate chemotherapy-associated tissue injury in a pre-clinical
mouse model. Cytotherapy. 14:412–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra
A, Joseph J, Berry JE, McGee S, Lee E, Sun H, et al: Recruitment of
mesenchymal stem cells into prostate tumours promotes metastasis.
Nat Commun. 4:17952013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX,
Wang CY, Sun K, Jiang GC, Zhao X, Li R, et al: Effects of
inflammatory factors on mesenchymal stem cells and their role in
the promotion of tumor angiogenesis in colon cancer. J Biol Chem.
286:25007–25015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai KS, Yang SH, Lei YP, Tsai CC, Chen
HW, Hsu CY, Chen LL, Wang HW, Miller SA, Chiou SH, et al:
Mesenchymal stem cells promote formation of colorectal tumors in
mice. Gastroenterology. 141:1046–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini F III: Concise review: Dissecting a discrepancy in the
literature: Do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan F and Hong LQ: Insulin promotes
proliferation and migration of breast cancer cells through the
extracellular regulated kinase pathway. Asian Pac J Cancer Prev.
15:6349–6352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gallagher EJ, Alikhani N, Tobin-Hess A,
Blank J, Buffin NJ, Zelenko Z, Tennagels N, Werner U and LeRoith D:
Insulin receptor phosphorylation by endogenous insulin or the
insulin analog AspB10 promotes mammary tumor growth independent of
the IGF-I receptor. Diabetes. 62:3553–3560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morrison MM, Hutchinson K, Williams MM,
Stanford JC, Balko JM, Young C, Kuba MG, Sánchez V, Williams AJ,
Hicks DJ, et al: ErbB3 downregulation enhances luminal breast tumor
response to antiestrogens. J Clin Invest. 123:4329–4343. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheng Q, Liu X, Fleming E, Yuan K, Piao H,
Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, et al: An
activated ErbB3/NRG1 autocrine loop supports in vivo proliferation
in ovarian cancer cells. Cancer Cell. 17:298–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gala K and Chandarlapaty S: Molecular
pathways: HER3 targeted therapy. Clin Cancer Res. 20:1410–1416.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sithanandam G, Smith GT, Masuda A,
Takahashi T, Anderson LM and Fornwald LW: Cell cycle activation in
lung adenocarcinoma cells by the ErbB3/phosphatidylinositol
3-kinase/Akt pathway. Carcinogenesis. 24:1581–1592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma J, Lyu H, Huang J and Liu B: Targeting
of erbB3 receptor to overcome resistance in cancer treatment. Mol
Cancer. 13:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greco SJ and Rameshwar P: Mesenchymal stem
cells in drug/gene delivery: Implications for cell therapy. Ther
Deliv. 3:997–1004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan
Y, Mao F, Wu X and Xu WR: Mesenchymal stem cell-secreted soluble
signaling molecules potentiate tumor growth. Cell Cycle.
10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Boeck A, Pauwels P, Hensen K, Rummens
JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems
G, et al: Bone marrow-derived mesenchymal stem cells promote
colorectal cancer progression through paracrine neuregulin 1/HER3
signalling. Gut. 62:550–560. 2013. View Article : Google Scholar : PubMed/NCBI
|