Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common malignancies in the world, and has a high mortality
rate (1). Invasive growth via the
lymphatic route is a typical feature of OSCC (2) and lymph node involvement status has been
identified as a reliable prognostic indicator in OSCC patients
(3,4).
Therefore, a number of studies are underway in order to investigate
the molecular mechanisms involved in regulating OSCC invasiveness
(5).
Galectin-7 is a member of the β-galactoside-binding
protein family. It is predominantly expressed in epithelial cells
within healthy tissue and plays an important role in epithelial
development and homeostasis (6,7).
Galectin-7 expression may be altered in epithelial cancer,
therefore it may serve an important role in cancer progression
(8). The exact role of galectin-7 may
vary in different types of cancers; it may play distinct and even
opposing roles in tumor development. For example, in human gastric
cancer specimens, galectin-7 is underexpressed due to epigenetic
modifications and this suppresses the proliferation and invasion of
gastric cancer cells (9). By
contrast, in high-grade breast cancer galectin-7 is overexpressed,
facilitating the spontaneous metastasis of breast cancer cells in
preclinical mouse models (10). The
tumor-promoting role of galectin-7 has also been noted in ovarian
cancer cells (11) and cervical
cancer cells (12).
Previous studies have demonstrated that galectin-7
increases the expression of matrix metalloproteinases (MMPs),
especially MMP-9, thus modulating the invasiveness of cancer cells
(11,12). Additionally, OSCC tissues exhibit
increased MMP-2 and MMP-9 activity compared with adjacent healthy
tissues (13). It has previously been
demonstrated that MMPs serve a critical role in the invasion and
metastasis of oral cancer (14).
Alves et al (15) reported
that galectin-7 is highly expressed in OSCC and its expression is
significantly correlated with the histological grade of disease.
These findings suggest that galectin-7 may contribute to OSCC
invasiveness by modulating the expression of MMP-2 and MMP-9. The
present study investigated the effects of manipulating galectin-7
expression on the biological phenotypes of human OSCC cells and
evaluated the involvement of MMP-2 and MMP-9 on the action of
galectin-7.
Materials and methods
Cell culture and treatment
The human OSCC cell lines SCC-4 and SCC-9 were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). All cells were maintained at 37°C in 5%
CO2 in Dulbecco's Modified Eagle's Medium supplemented
with 10% fetal bovine serum (FBS), 1 mmol/l L-glutamine, and 100
U/ml penicillin, 100 µg/ml streptomycin (all from Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). For inhibitor
experiments, cells were pretreated with the c-Jun N-terminal kinase
(JNK) inhibitor SP600125 (10 µM; Calbiochem; EMD Millipore,
Billerica, MA, USA), extracellular signal-related kinase (ERK)
inhibitor PD98059 (10 µM; Calbiochem; EMD Millipore), or 0.1%
dimethyl sulfoxide (DMSO) used as vehicle control 1 h before
transfection of galectin-7-expressing plasmid.
Plasmids, small interfering RNA
(siRNA), and transfection
A galectin-7-expressing plasmid (pCEP4-GAL7) was
purchased from Addgene (Cambridge, MA, USA) and an empty vector
(pCEP4) was also purchased (Invitrogen; Thermo Fisher Scientific,
Inc.) Galectin-7 siRNA, MMP-2 siRNA, MMP-9 siRNA, and negative
control siRNA were obtained from Santa Cruz Biotechnology (Dallas,
TX, USA). For overexpression or knockdown of galectin-7, cells were
seeded onto 6-well plates (4×105 cells/well) and
transfected with 1 µg pCEP4-GAL7, 1 µg pCEP4 and 50 nM galectin-7
siRNA, or 50 nM control siRNA using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Cells were incubated for 24 h, and
subsequently collected for further experiments. To validate the
involvement of MMP-2 and MMP-9, cells were co-transfected with 1 µg
pCEP4-GAL7 and 50 nM MMP-2 siRNA, MMP-9 siRNA, or control siRNA,
and tested for invasive ability following incubation for 24 h.
Cell proliferation assay
Cell proliferation was measured using the MTT assay.
Transfected cells were detached and re-seeded onto 96-well plates
(2×103/well). Following incubation for 1, 3, and 5 days,
0.5 mg/ml MTT (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
was added to the culture and incubated for additional 4 h at 37°C.
Formazan crystals were dissolved in DMSO. Absorbance was measured
at 570 nm using a multi-plate reader.
Apoptosis detection assay
Apoptosis analysis was performed using the Annexin
V-FITC Apoptosis Detection kit (Nanjing KeyGen Biotech Co.,
Nanjing, China), according to the manufacturer's instructions. In
brief, cells were incubated with a staining solution containing
fluorescein isothiocyanate (FITC)-conjugated annexin-V and
propidium iodide (PI) for 10 min at 4°C in the dark. The percentage
of apoptotic cells was determined using a FACScan flow cytometer
with the CellQuest software (BD Biosciences, San Jose, CA,
USA).
Wound-healing assay
Cells were seeded onto 6-well plates and allowed to
grow to ~95% confluence. A wound was made in the monolayer using a
100-µl pipette tip. The culture was washed to remove cellular
debris and incubated for 24 h at 37°C. Cells were imaged using a
phase contrast microscope at different time points. The extent of
wound closure was quantified by measuring its area before
migration, and 24 h after migration. Results were expressed as
percentage of wound closure.
Transwell invasion assay
Invasion assays were performed using Transwell
chambers, which were coated with Matrigel (BD Biosciences) 24 h
prior to use. The cells were subsequently harvested and resuspended
in serum-free medium containing 1% bovine serum albumin
(Sigma-Aldrich; Merck Millipore). The cell suspension was added to
the upper chamber and the lower chamber was filled with culture
medium containing 10% FBS. After incubation for 24 h at 37°C, cells
on the upper surface of the chamber were removed using a cotton
swab. Invaded cells on the lower surface were fixed in 4%
formaldehyde, stained with 0.5% crystal violet, and counted under a
microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using the TRIzol
reagent following the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc.). Reverse transcription was
performed using the PrimeScript First Strand cDNA Synthesis kit
(Takara Biotechnology Co., Dalian, China). RT-qPCR was performed on
an ABI 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with SYBR-Green detection mix (Takara
Biotechnology Co.). The following primers were used in the current
study: Galectin-7 forward 5′-TTGCTCCTTGCTGTTGAAGACCAC-3′, and
reverse 5′-AGGTTCCATGTAAACCTGCTGTGC-3′ (16); glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) forward, 5′-TGACTTCAACAGCGACACCCA-3′; and
reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′. PCR conditions were as
follows: 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec,
64°C for 30 sec, and 72°C for 30 sec. The relative galectin-7 mRNA
level was calculated using the 2−ΔΔCq method (17) following normalization against the
level of GAPDH.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (phosphate buffer solution, 1% NP40, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulphate) containing a protease
inhibitor cocktail (Cell Signaling Technology, Inc., Danvers, MA,
USA) on ice for 30 min. After centrifugation at 15,000 × g for 20
min, the supernatant was collected and protein concentrations were
measured using a protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Cell lysates were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. Membranes were probed with the following
antibodies at 1:300 dilution: Rabbit anti-galectin-7 monoclonal
antibody (cat. no. ab108623), rabbit anti-MMP-2 polyclonal antibody
(cat. no. ab97779), mouse anti-MMP-9 monoclonal antibody (cat. no.
ab119906), rabbit anti-GAPDH monoclonal antibody (cat. no.
ab181602; all from Abcam, Cambridge, MA, USA), rabbit
anti-phospho-ERK1/2 polyclonal antibody (cat. no. 9101), rabbit
anti-ERK1/2 polyclonal antibody (cat. no. 9102), rabbit
anti-phospho-JNK monoclonal antibody (cat. no. 4668), rabbit
anti-JNK polyclonal antibody (cat. no. 9252), rabbit
anti-phospho-p38 monoclonal antibody (cat. no. 4511) and rabbit
anti-p38 monoclonal antibody (cat. no. 8690; all from Cell
Signaling Technology, Inc.). Horseradish peroxidase-conjugated
secondary antibodies (cat. nos. sc-2004 and sc-2005; Santa Cruz
Biotechnology, Inc.) were diluted at 1:2,000 prior to use. Proteins
were visualized using an enhanced chemiluminescence kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). The blots were quantified
by densitometry with the Quantity One software (Bio-Rad
Laboratories).
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical differences were examined using one-way analysis of
variance (ANOVA) followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
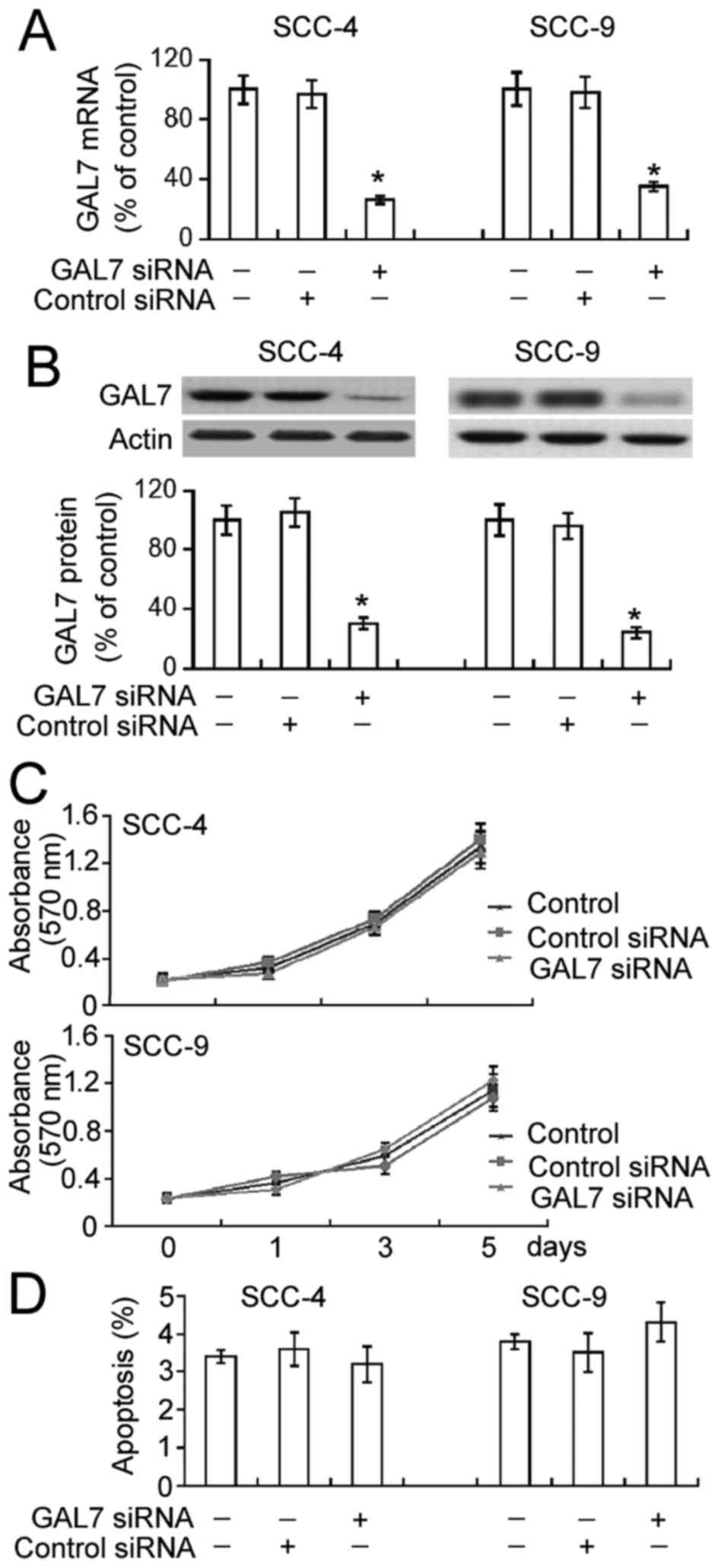
Galectin-7 silencing has no impact on
cell proliferation or apoptosis in OSCC cells
To analyze the role of galectin-7 in the
proliferation of OSCC cells, galectin-7-specific siRNA was
transiently transfected into SCC-4 and SCC-9 cell lines. The
delivery of galectin-7 siRNA significantly decreased mRNA and
protein levels of endogenous galectin-7 in both SCC-4 and SCC-9
cells (Fig. 1A and B; P<0.05). The
results of the MTT assay demonstrated that this downregulation of
galectin-7 did not significantly affect SCC-4 and SCC-9 cell
proliferation compared with non-transfected cells over a 5-day
period (Fig. 1C). Annexin-V/PI
staining analysis identified comparable percentages of apoptotic
cells in non-transfected and galectin-7 siRNA-transfected cells
(Fig. 1D).
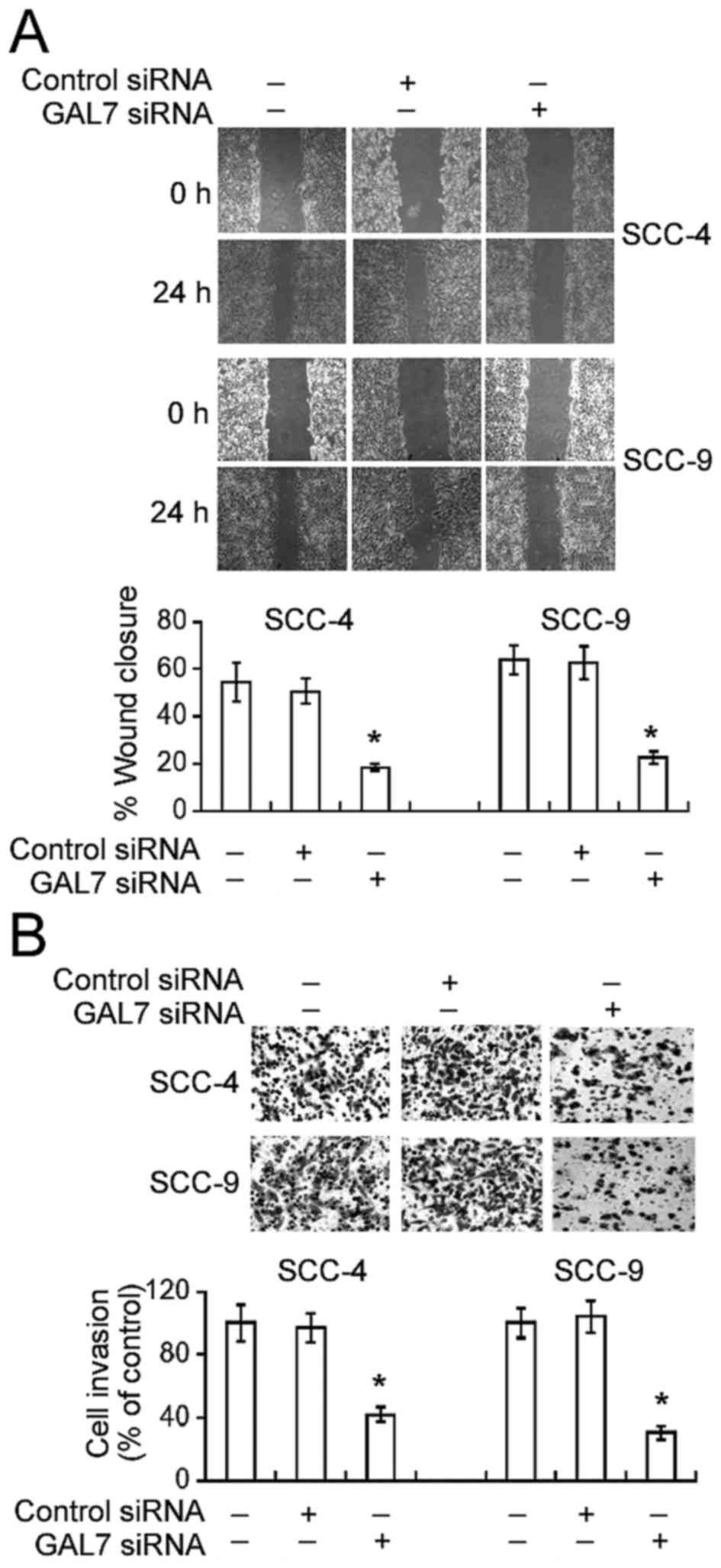
Galectin-7 knockdown attenuates the
migration and invasion of OSCC cells
The effect of galectin-7 downregulation on the
invasive properties of OSCC cells was then analyzed. Galectin-7
silencing caused a significant decline in cell motility during
in vitro wound-healing assays. Compared to non-transfected
SCC-4 cells, the percentage wound closure was significantly lower
in galectin-7-silenced SCC-4 cells 24 h following incubation
(18.5±3.2% vs. 54.4±6.4%, P<0.05; Fig.
2A). Similarly, galectin-7 siRNA transfection resulted in a
significant reduction in the motility of SCC-9 cells (P<0.05).
Matrigel invasion assays demonstrated that galectin-7 knockdown
significantly reduced the numbers of invaded cells by >60%,
compared with non-transfected cells (P<0.05; Fig. 2B).
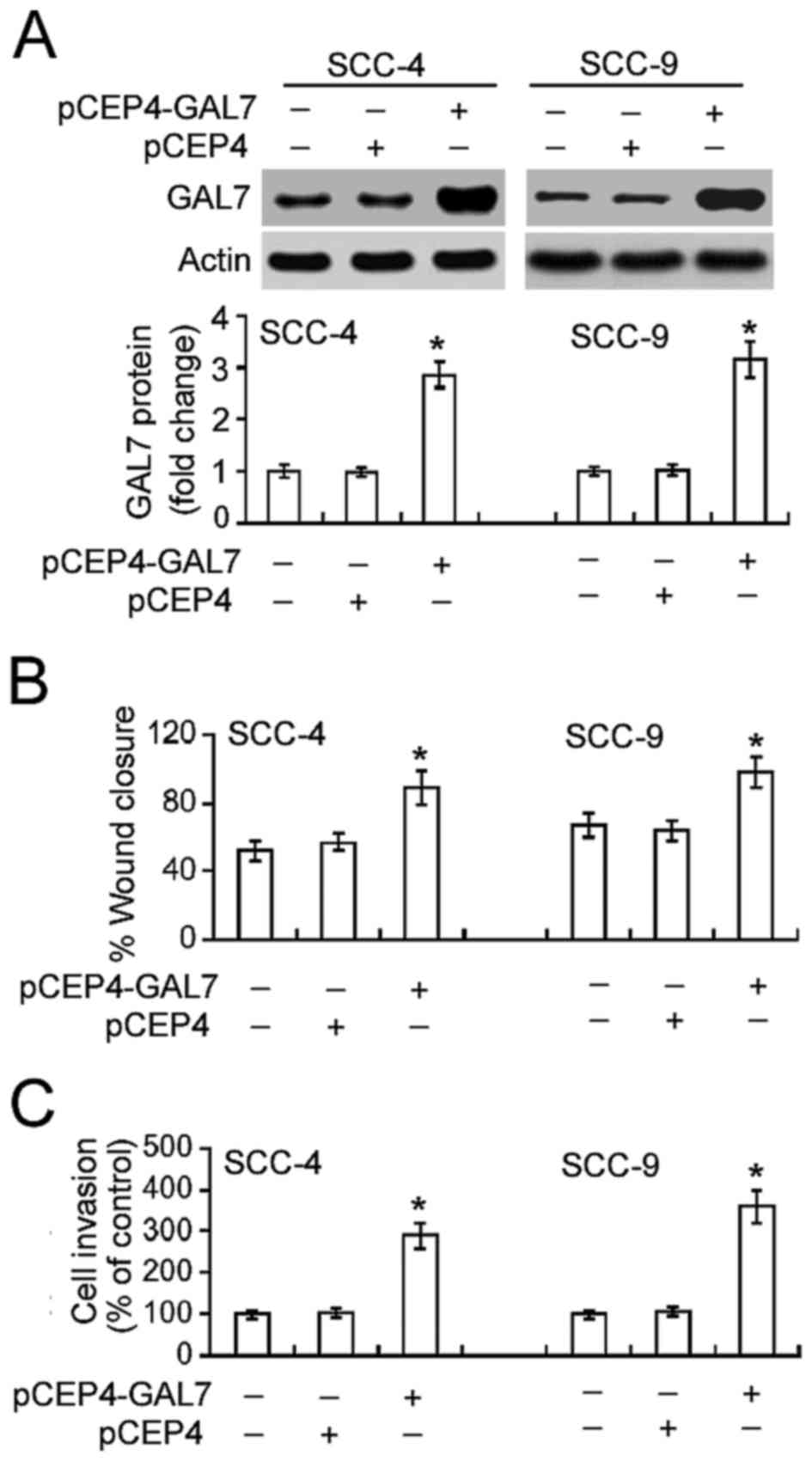
Overexpression of galectin-7
accelerates the migration and invasion of OSCC cells
Further tests confirmed the effect of increased
galectin-7 on the migration and invasion of OSCC cells.
Transfection of the plasmid pCEP4-GAL7 into SCC-4 and SCC-9 cells
led to a significant increase in galectin-7 expression compared
with non-transfected cells (Fig. 3A).
This increase in galectin-7 expression in turn significantly
increased OSCC cell migration and invasion (P<0.05; Figs. 3B and C).
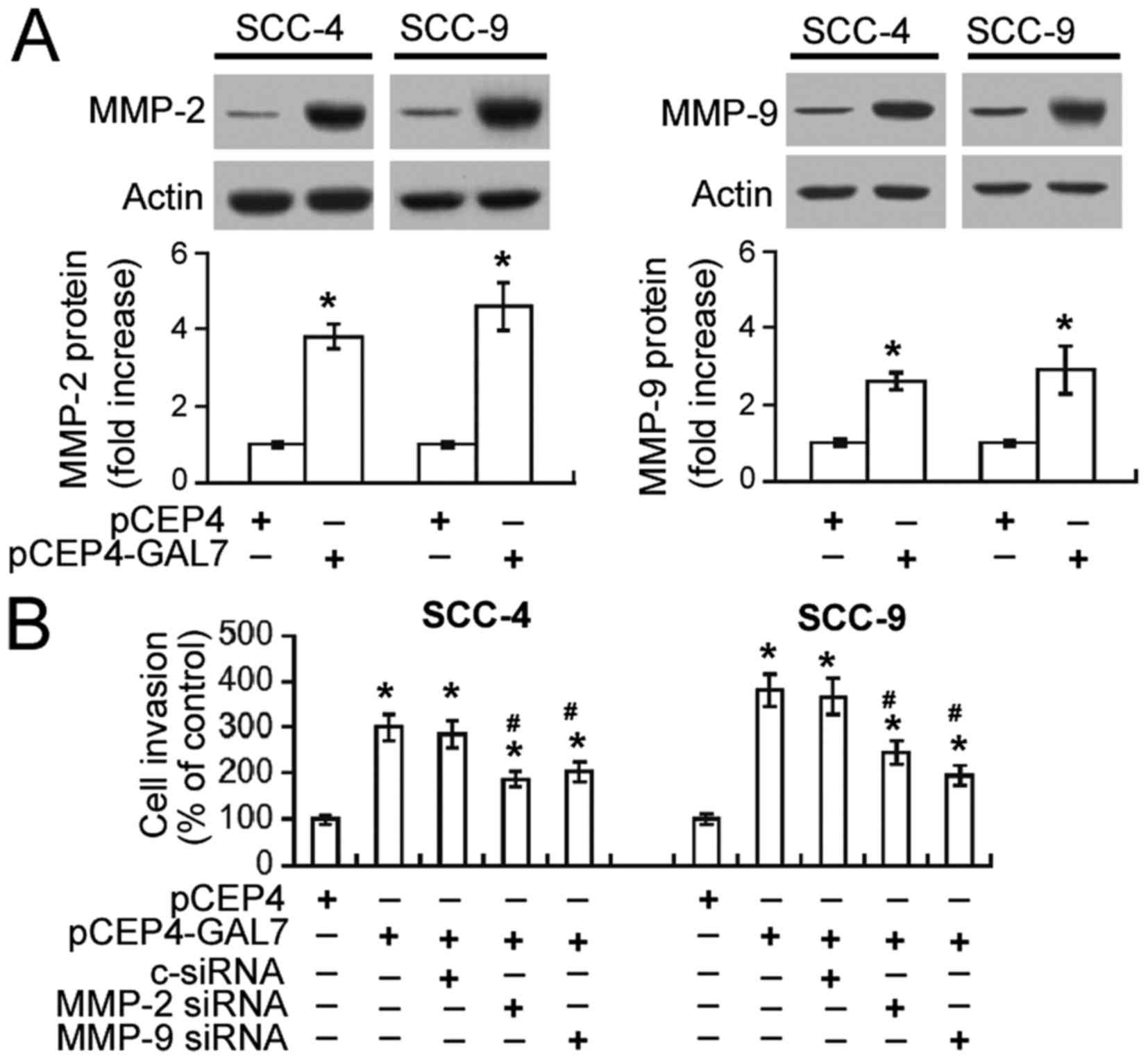
Upregulation of MMP-2 and MMP-9
mediates the pro-invasive activity of galectin-7
A possible association between galectin-7-mediated
invasiveness and MMP-2 and MMP-9 upregulation was investigated.
Western blot analysis demonstrated that galectin-7 overexpression
resulted in a 3–5-fold increase in MMP-2 protein and 2–3-fold
increase in MMP-9 protein expression in both SCC-4 and SCC-9 cells
(Fig. 4A). Transwell invasion assay
demonstrated that the invasiveness of SCC-4 and SCC-9 cells
overexpressing galectin-7 was significantly decreased by
co-transfection with MMP-2 or MMP-9-specific siRNA (P<0.05;
Fig. 4B).
Galectin-7 promotes OSCC cell invasion
via activation of ERK and JNK signaling
Finally, the signaling pathways involved in the
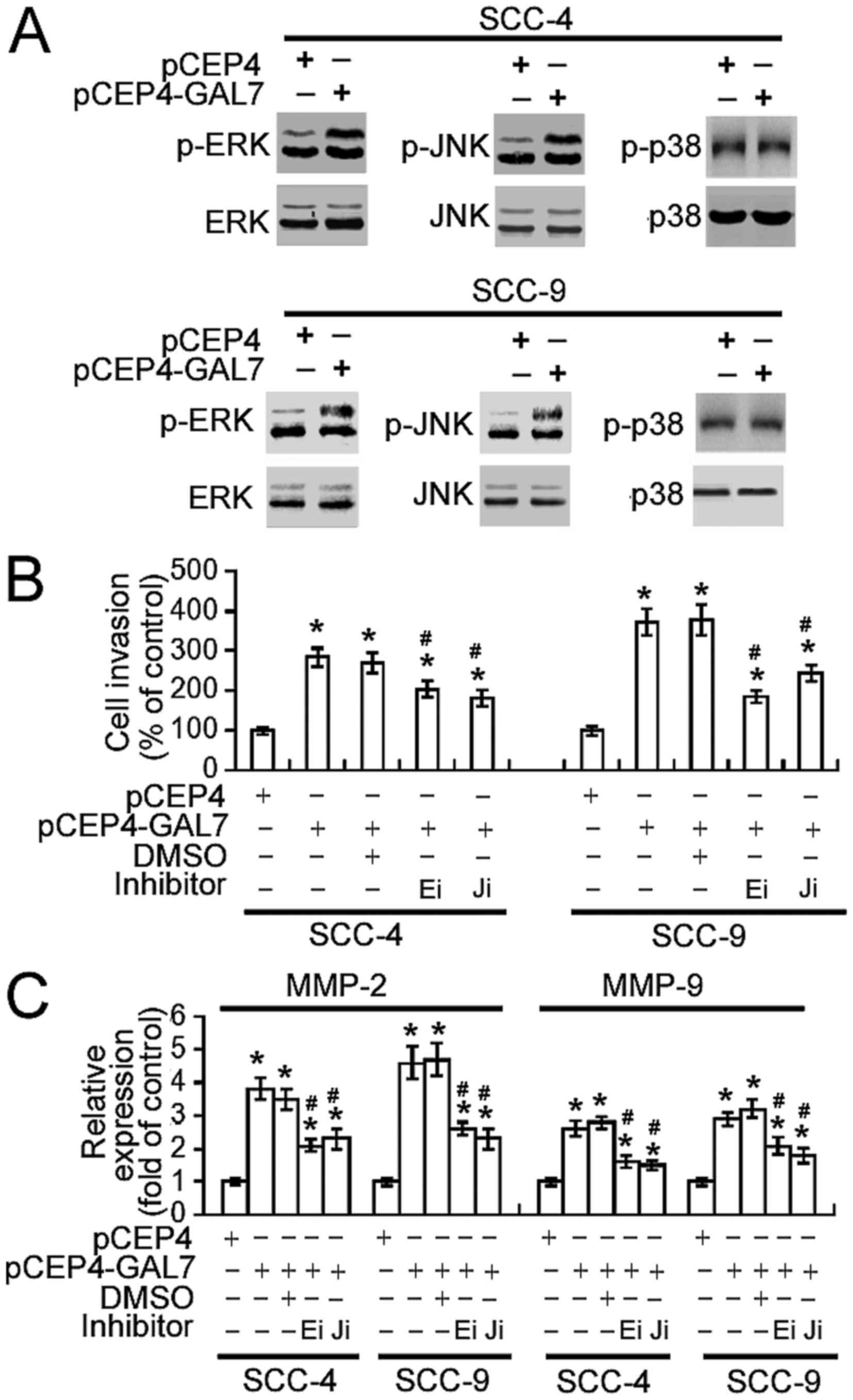
action of galectin-7 were investigated. As shown in Fig. 5A, increasing galectin-7 expression
markedly enhanced the phosphorylation of ERK1/2 and JNK1/2 in SCC-4
and SCC-9 cells, without altering total levels of ERK1/2 and
JNK1/2. No change in p38 phosphorylation levels was detected.
Notably, the pharmacological inhibition of ERK or JNK activity
significantly suppressed the invasiveness of
galectin-7-overexpressing SCC-4 and SCC-9 cells (P<0.05;
Fig. 5B) and abrogated the
upregulation of MMP-2 and MMP-9 (P<0.05; Fig. 5C).
Discussion
Matsukawa et al (18) reported previously that adenoviral
delivery of the galectin-7 gene may induce modest apoptosis and
reduce the viability of human OSCC HSC3 cells. However, knockdown
of galectin-7 using antisense galectin-7 oligonucleotides
demonstrated no significant effects on cell viability. In the
current study, the biological roles of galectin-7 in two other OSCC
cell lines were explored, and targeted reduction of galectin-7 via
siRNA technology did not alter viability and spontaneous apoptosis
in SCC-4 and SCC-9 cells. These results suggest that galectin-7 is
not required for the maintenance of OSCC cell viability. The
anti-viability effect elicited by overexpression of galectin-7 may
only reflect a non-specific cytotoxicity, as the potential
cytotoxic activity of galectin-7 overexpression on healthy human
cells was not tested in the current study.
The ability of galectin-7 to modulate cell behavior
seems to be cell-dependent. Previous studies have demonstrated that
overexpressing galectin-7 inhibits the proliferation of several
specific cancer cells such as gastric cancer cells (9) and colon carcinoma cells (19). However, in other cancer cells
including epithelial ovarian cancer (20), galectin-7 was involved in cell
proliferation, as its downregulation inhibited the proliferation of
A2780-PAR ovarian cancer cell.
Metastasis is the main cause of cancer-associated
mortality. Galectin-7 exhibits the ability to modulate the
metastatic phenotype of several types of cancer cells (10,12,21).
Demers et al (21)
demonstrated that ectopic expression of galectin-7 increases the
invasiveness of lymphoma, accelerates the development of thymic
lymphoma, and previously identified that overexpressing galectin-7
enhances the metastatic growth of breast cancer cells in the lungs
and bones in two different mouse models (10). Enforced expression of galectin-7 also
promotes the invasiveness of human HeLa cervical epithelial
adenocarcinoma cells (12). The
results of the present study are consistent with results from
previous studies, as they demonstrated that galectin-7 has the
ability to modulate the invasive properties of OSCC cells.
Knockdown of galectin-7 suppressed the migration and invasion of
SCC-4 and SCC-9 cells, whereas overexpressing galectin-7 increased
them. Taken together, these findings indicate that galectin-7 is a
potential target for the treatment of tumor dissemination in
OSCC.
Compelling evidence suggests that the induction of
MMPs plays a pivotal role in the OSCC invasiveness. For instance,
Bedal et al (22) have
previously reported that collagen XVI facilitates the invasion of
OSCC cells by inducing MMP-9 expression. It has previously been
suggested that the downregulation of MMP-2 and MMP-9 may account
for the decreased invasiveness of OSCC cells due to the knockdown
of BubR1, a critical component of spindle assembly checkpoint
(23). Inhibiting MMP-2 and MMP-9
expression has also been demonstrated to mediate the anti-invasive
effects of curcumin (a natural polyphenolic compound) in OSCC cells
(24). In line with its pro-invasive
activity, galectin-7 expression increases the expression of MMP-9
in several cancer cells (12,16,21). The
current study investigated the effects of MMP-2 and MMP-9 on
galectin-7 action in OSCC cells. Galectin-7 overexpression resulted
in the significant upregulation of MMP-2 and MMP-9. Most
importantly, silencing MMP-2 or MMP-9 significantly impaired the
invasiveness of OSCC cells that overexpressed galectin-7. Thus
MMP-2 and MMP-9 may be required for the galectin-7-mediated
invasiveness of OSCC cells.
To gain a better insight into the function of
galectin-7 in OSCC cell invasiveness, the signaling pathways
involved were analyzed. Since mitogen-activated protein kinase
(MAPK) pathways are implicated in the invasion of oral cancer cells
(25,26) and galectin-7 may activate p38 MAPK
signaling in cervical cancer cells (12), the current study investigated the
importance of MAPK signaling in mediating galectin-7 action. The
results of the present study demonstrated that galectin-7
overexpression leads to the phosphorylation and activation of ERKs
and JNKs, but not p38 MAPK, in SCC-4 and SCC-9 cells.
Interestingly, the pharmacological inhibition of ERK or JNK
activity significantly attenuated OSCC cell invasiveness induced by
galectin-7 overexpression. Moreover, galectin-7-mediated
upregulation of MMP-2 and MMP-9 was compromised by pretreatment
with the ERK or JNK inhibitors. Taken together, these results
suggest that galectin-7 promotes the invasiveness of OSCC cells
largely by inducing the expression of MMP-2 and MMP-9 via
activation of ERK and JNK signaling.
In conclusion, the current study provides novel
evidence demonstrating the pro-invasive activity of galectin-7,
which is associated with increased MMP-2 and MMP-9 expression, in
OSCC cells. Furthur studies are required to investigate the utility
of galectin-7 as a target for the treatment of metastatic OSCC.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziober AF, Falls EM and Ziober BL: The
extracellular matrix in oral squamous cell carcinoma: Friend or
foe? Head Neck. 28:740–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Künzel J, Mantsopoulos K, Psychogios G,
Grundtner P, Koch M and Iro H: Lymph node ratio as a valuable
additional predictor of outcome in selected patients with oral
cavity cancer. Oral Surg Oral Med Oral Pathol Oral Radiol.
117:677–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Nam SY, Choi SH, Cho KJ and Roh
JL: Prognostic value of lymph node density in node-positive
patients with oral squamous cell carcinoma. Ann Surg Oncol.
18:2310–2317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sasahira T, Kirita T and Kuniyasu H:
Update of molecular pathobiology in oral cancer: A review. Int J
Clin Oncol. 19:431–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magnaldo T, Fowlis D and Darmon M:
Galectin-7, a marker of all types of stratified epithelia.
Differentiation. 63:159–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rondanino C, Poland PA, Kinlough CL, Li H,
Rbaibi Y, Myerburg MM, Al-bataineh MM, Kashlan OB, Pastor-Soler NM,
Hallows KR, et al: Galectin-7 modulates the length of the primary
cilia and wound repair in polarized kidney epithelial cells. Am J
Physiol Renal Physiol. 301:F622–F633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
St-Pierre Y, Campion CG and Grosset AA: A
distinctive role for galectin-7 in cancer? Front Biosci (Landmark
Ed). 17:438–450. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Hwang JA, Ro JY, Lee YS and Chun
KH: Galectin-7 is epigenetically-regulated tumor suppressor in
gastric cancer. Oncotarget. 4:1461–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demers M, Rose AA, Grosset AA, Biron-Pain
K, Gaboury L, Siegel PM and St-Pierre Y: Overexpression of
galectin-7, a myoepithelial cell marker, enhances spontaneous
metastasis of breast cancer cells. Am J Pathol. 176:3023–3031.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Labrie M, Vladoiu MC, Grosset AA, Gaboury
L and St-Pierre Y: Expression and functions of galectin-7 in
ovarian cancer. Oncotarget. 5:7705–7721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JE, Chang WY and Cho M: Induction of
matrix metalloproteinase-9 by galectin-7 through p38 MAPK signaling
in HeLa human cervical epithelial adenocarcinoma cells. Oncol Rep.
22:1373–1379. 2009.PubMed/NCBI
|
|
13
|
Patel BP, Shah PM, Rawal UM, Desai AA,
Shah SV, Rawal RM and Patel PS: Activation of MMP-2 and MMP-9 in
patients with oral squamous cell carcinoma. J Surg Oncol. 90:81–88.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas GT, Lewis MP and Speight PM: Matrix
metalloproteinases and oral cancer. Oral Oncol. 35:227–233. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alves PM, Godoy GP, Gomes DQ, Medeiros AM,
de Souza LB, da Silveira EJ, Vasconcelos MG and Queiroz LM:
Significance of galectins-1, −3, −4 and −7 in the progression of
squamous cell carcinoma of the tongue. Pathol Res Pract.
207:236–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demers M, Biron-Pain K, Hébert J, Lamarre
A, Magnaldo T and St-Pierre Y: Galectin-7 in lymphoma: Elevated
expression in human lymphoid malignancies and decreased lymphoma
dissemination by antisense strategies in experimental model. Cancer
Res. 67:2824–2829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giulietti A, Overbergh L, Valckx D,
Decallonne B, Bouillon R and Mathieu C: An overview of real-time
quantitative PCR: Applications to quantify cytokine gene
expression. Methods. 25:386–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsukawa S, Morita K, Negishi A, Harada
H, Nakajima Y, Shimamoto H, Tomioka H, Tanaka K, Ono M, Yamada T
and Omura K: Galectin-7 as a potential predictive marker of chemo-
and/or radio-therapy resistance in oral squamous cell carcinoma.
Cancer Med. 3:349–361. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ueda S, Kuwabara I and Liu FT: Suppression
of tumor growth by galectin-7 gene transfer. Cancer Res.
64:5672–5676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ, Jeon HK, Lee JK, Sung CO, Do IG,
Choi CH, Kim TJ, Kim BG, Bae DS and Lee JW: Clinical significance
of galectin-7 in epithelial ovarian cancer. Anticancer Res.
33:1555–1561. 2013.PubMed/NCBI
|
|
21
|
Demers M, Magnaldo T and St-Pierre Y: A
novel function for galectin-7: Promoting tumorigenesis by
up-regulating MMP-9 gene expression. Cancer Res. 65:5205–5210.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bedal KB, Grässel S, Oefner PJ, Reinders
J, Reichert TE and Bauer R: Collagen XVI induces expression of MMP9
via modulation of AP-1 transcription factors and facilitates
invasion of oral squamous cell carcinoma. PLoS One. 9:e867772014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou CK, Wu CY, Chen JY, Ng MC, Wang HM,
Chen JH, Yuan SS, Tsai EM, Chang JG and Chiu CC: BubR1 acts as a
promoter in cellular motility of human oral squamous cancer cells
through regulating MMP-2 and MMP-9. Int J Mol Sci. 16:15104–15117.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee AY, Fan CC, Chen YA, Cheng CW, Sung
YJ, Hsu CP and Kao TY: Curcumin inhibits invasiveness and
epithelial-mesenchymal transition in oral squamous cell carcinoma
through reducing matrix metalloproteinase 2, 9 and modulating
p53-E-cadherin pathway. Integr Cancer Ther. 14:484–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu FY, Safdar J, Li ZN, Fang QG, Zhang X,
Xu ZF and Sun CF: CCR7 regulates cell migration and invasion
through MAPKs in metastatic squamous cell carcinoma of head and
neck. Int J Oncol. 45:2502–2510. 2014.PubMed/NCBI
|
|
26
|
Chen PN, Hsieh YS, Chiang CL, Chiou HL,
Yang SF and Chu SC: Silibinin inhibits invasion of oral cancer
cells by suppressing the MAPK pathway. J Dent Res. 85:220–225.
2006. View Article : Google Scholar : PubMed/NCBI
|