Introduction
Breast carcinoma refers to the malignant tumor of
breast epithelial cells and accounts for over 20% of all malignant
tumors diagnosed in women. Thus, it is a major cause of mortality
that threatens the health of women (1). To improve the quality of life of breast
carcinoma patients, various studies have been carried out combining
surgical operation and post-operation adjuvant therapy (2). The etiological agents that lead to
breast carcinoma and the mechanisms behind these causative agents
are relatively complicated. Factors that increase the likelihood of
breast cancer include family history, contact with radioactive
rays, certain chemical substances, proto-oncogene activation and
stimulation of biotic factors, which can all lead to the malignant
proliferation or oncogenic mutation of breast epithelial tissue
(3).
The formation of an adjuvant therapeutic schedule
for breast carcinoma patients requires judgment which takes into
account the combination of clinical classification and stage of the
patients. Currently, we administer post-operation chemotherapy to
treat breast carcinoma patients, the purpose of which is to reduce
the postoperative recurrence as much as possible and prolong the
disease-free survival and five-year survival rate. In terms of
chemotherapy cocktails, we primarily administer taxotere +
adriamycin + cyclophosphamide (TAC) and the taxotere + epirubicin +
cyclophosphamide (TEC) plans (4–8),
adriamycin amycin + cyclophosphamide + paclitaxel (AC-P) plan,
adriamycin amycin + cyclophosphamide + taxol (AC-T) plan and
adriamycin amycin + cyclophosphamide (AC) plan. The mechanism of
action of chemotherapy drugs is to curb DNA proliferation, mitosis
or other biological processes through interference into the
different phases of the cell cycle. Therefore, in most cases, these
drugs lack specificity and damage normal tissues and cells of the
human body (3,4). Previous findings showed that the
combination of chemotherapy drugs and targeted therapeutic drugs
can lead to good outcome for the treatment of breast carcinoma
(4–6).
Estrogen receptor β (ERβ) gene encodes
estrogen receptor β. Estrogen is of great significance in normal
physiology, aging and the treatment of many diseases (9). The main function of the estrogen nuclear
receptor is the transcription factor for the activation of ligand,
which can mediate the genetic transcription of tissues and organs
regulated and controlled by this hormone (10).
Since Mufudza et al (11) cloned the gene of estrogen receptor α
(ERα), there have been numerous studies conducted on this estrogen
receptor showing that, in normal breast tissue, there is only 7–10%
of ERα expression and it is present mostly in the breast cells that
are in their non-proliferation phase (11). However, the expression levels of ERα
vary based on the menstrual cycle. Clinical studies have shown that
the expression levels of ERα are closely associated with the degree
of differentiation and grade of malignancy and location of the
transfer of breast cancer cells (11–14).
Therefore, some scholars consider that ERα can be treated as one of
the factors in the evaluation of prognosis of breast carcinoma.
However, due to the fact that ERα is only expressed in a small
quantity of breast epithelial cells, the sensitivity and
specificity of determining the prognosis of breast carcinoma by ERα
is poor. ERβ is the new member of the super family of steroid
hormone receptors. It has six functional zones: A-F (5,15). Leygue
et al found ERβ mRNA expression in breast (16). Additionally, the ERβ mRNA was detected
in breast carcinoma samples and the mammary epithelial cells of
several types of individuals. The study by Lanfranchi and Brind
showed that the ratio of ERα and ERβ in breast carcinoma patients
varies as breast carcinoma develops (17). However, whether ERβ can be treated as
the evaluation index for breast carcinoma treatment and prognosis
remains to be determined.
Clinical data and pathological samples of 120 breast
carcinoma patients were collected to study the expression levels of
the ERβ gene in breast carcinoma and to examine its clinical
relevance in neoadjuvant therapy.
Materials and methods
Sample selection
In the present study, we retrospectively analyzed
the clinical data of 120 female patients with breast carcinoma who
were hospitalized at the Department of Breast Surgery of the
Affiliated Hospital of Shandong Academy of Medical Sciences. We
collected and categorized clinical data including factors such as
age, disease time, childbearing history, and menstrual history.
According to the clinical performance, imagological examination,
and pathological examination of patients, we classified and
identified the stages of the breast carcinoma in patients and
performed corresponding neoadjuvant chemotherapy TEC plans as well
as an operation plan. Inclusion criteria were: Patients that were
diagnosed with breast carcinoma pathologically. Exclusion criteria
were: i) Presence of malignant tumors in other systems, excluding
those with the breast carcinoma transferred to niduses; ii)
patients without definite diagnosis; iii) patients with cognitive
disorder or mental problems; iv) failure in getting the samples of
the patients due to certain reasons; v) uncooperative patients or
family members; vi) patients that quit from this study; and vii)
patients with poor general condition, and thus unsuitable for the
examination and treatment.
Neoadjuvant therapeutic schedule
The neoadjuvant therapeutic schedule, i.e., TEC
plan, refers to the chemotherapeutic schedule of taxotere +
epirubicin + cyclophosphamide cocktail. We carried out the TEC plan
for 3–4 cycles before the operation with 3 weeks for each cycle.
After chemotherapy, we performed a modified radical operation for
breast carcinoma. Taxotere (Rhône-Poulenc Rorer, Paris, France) 75
mg/m2 d1, (Famaxin; Pfizer, Inc., New York, NY, USA):
100 mg/m2 d1, and cyclophosphamide (Baxter, Deerfield,
IL, USA) 500 mg/m2 d1 were administered.
Immunohistochemical methods
Breast carcinoma paraffin sections were dewaxed in
water and 3% H2O2 then incubated for 10 min
at 20°C. The section was washed with distilled water and soaked in
PBS for 5 min twice. The tissue was incubated with 5% PBS diluted
goat serum (Life Technologies, Carlsbad, CA, USA) and sealed, and
then incubated for 10 min at 20°C. The goat serum was discarded and
the primary rabbit polyclonal ERβ antibody (dilution, 1:100; cat.
no. ab60709; Abcam, Cambridge, MA, USA) was added. The tissue was
incubated for 1–2 h at 37°C or stored at 4°C overnight. We added
the proper amount of the working goat monoclonal biotin-labeled
anti-human IgG secondary antibody (dilution, 1:1,000; cat. no.
NEF803001EA; PerkinElmer, Inc., Waltham, MA, USA) to the sample and
incubated it for 10 min at 37°C. HRP was added (S7571;
Sigma-Aldrich, St. Louis, MO, USA), incubated for 10 min at 37°C
and 1–2 drops of DAB Plus Chromogen (TA-125-HD) was added to 1 ml
DAB Plus Substrate (TL-125-HDX) (both from Thermo Fisher
Scientific, Waltham, MA, USA). We blended and added drops to the
section, incubated the section for 10 min at 37°C, and washed and
sealed the section.
Statistical analysis
SPSS 20.0 software (Chicago, IL, USA) was used to
conduct statistical analysis. We described the continuous variable
of the normal distribution using mean and standard deviation. When
the continuous variables show skewed distribution, we described it
using the median and quartile range. The absolute percentage was
used for the classified variables. The Student's t-test was applied
for the continuous variables. For a comparison of classified
variables, the Pearson's χ2 test was applied. The
univariate analysis was applied to analyze the correlation between
ERβ and the other factors.
Results
Clinical description and general
conditions
We collected the data including age, weight,
menstrual period, disease course and other information regarding
general clinical conditions for analysis. Most of the patients were
aged 50–60 years. The age, weight, menstrual period and disease
course of patients with breast carcinoma showed no significant
statistical differences (P>0.05) (Table I). We also statistically analyzed the
childbirth history, contact with radioactive rays and family
history (Table I). Results of
statistical analysis of the pathological classification of breast
carcinoma are shown in Table I.
 | Table I.Collection and analysis of the general
clinical data of patients in the control and disease group (mean ±
SD). |
Table I.
Collection and analysis of the general
clinical data of patients in the control and disease group (mean ±
SD).
| Groups | Cases (n) | Age (years) | Weight (kg) | Menstrual period
(day) | Disease course
(months) |
|---|
| Control | 60 | 53.8±8.4 | 69.7±12.4 | 28.4±2.5 | 5.2±1.1 |
| Disease | 60 | 55.2±9.2 | 68.2±3.8 | 31.2±1.1 | 6.8±2.3 |
| T-value | – | 0.78 | 0.32 | 0.85 | 0.44 |
| P-value | – | 0.25 | 0.75 | 0.45 | 0.58 |
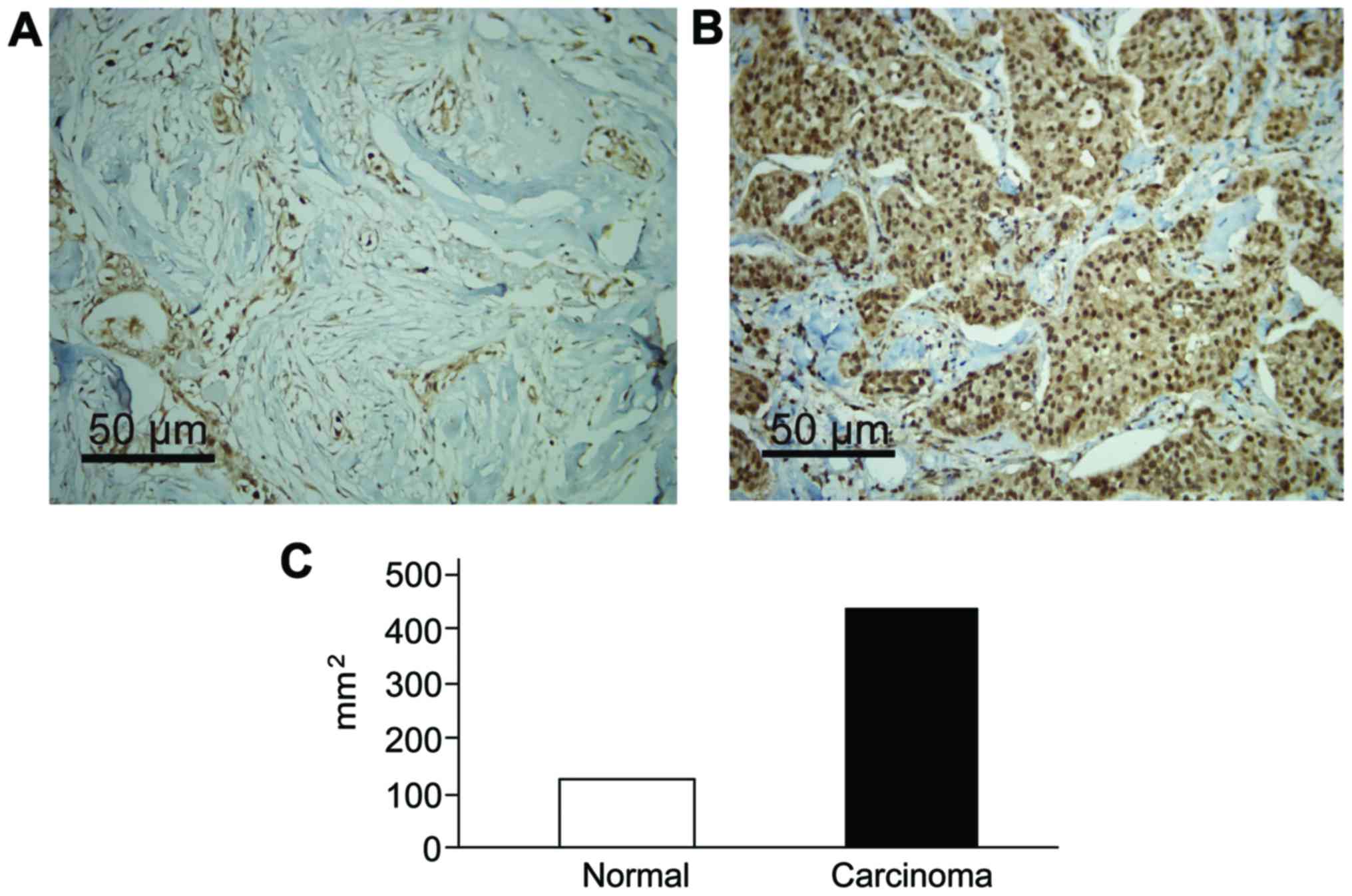
Expression of ERβ in normal and breast
carcinoma tissues
There were different degrees of ERβ expression in
normal and breast carcinoma tissues. Immunohistochemical results
revealed that ERβ gene was significantly increased in the
lesion tissues, and that the results were statistically significant
(P<0.05) (Fig. 1).
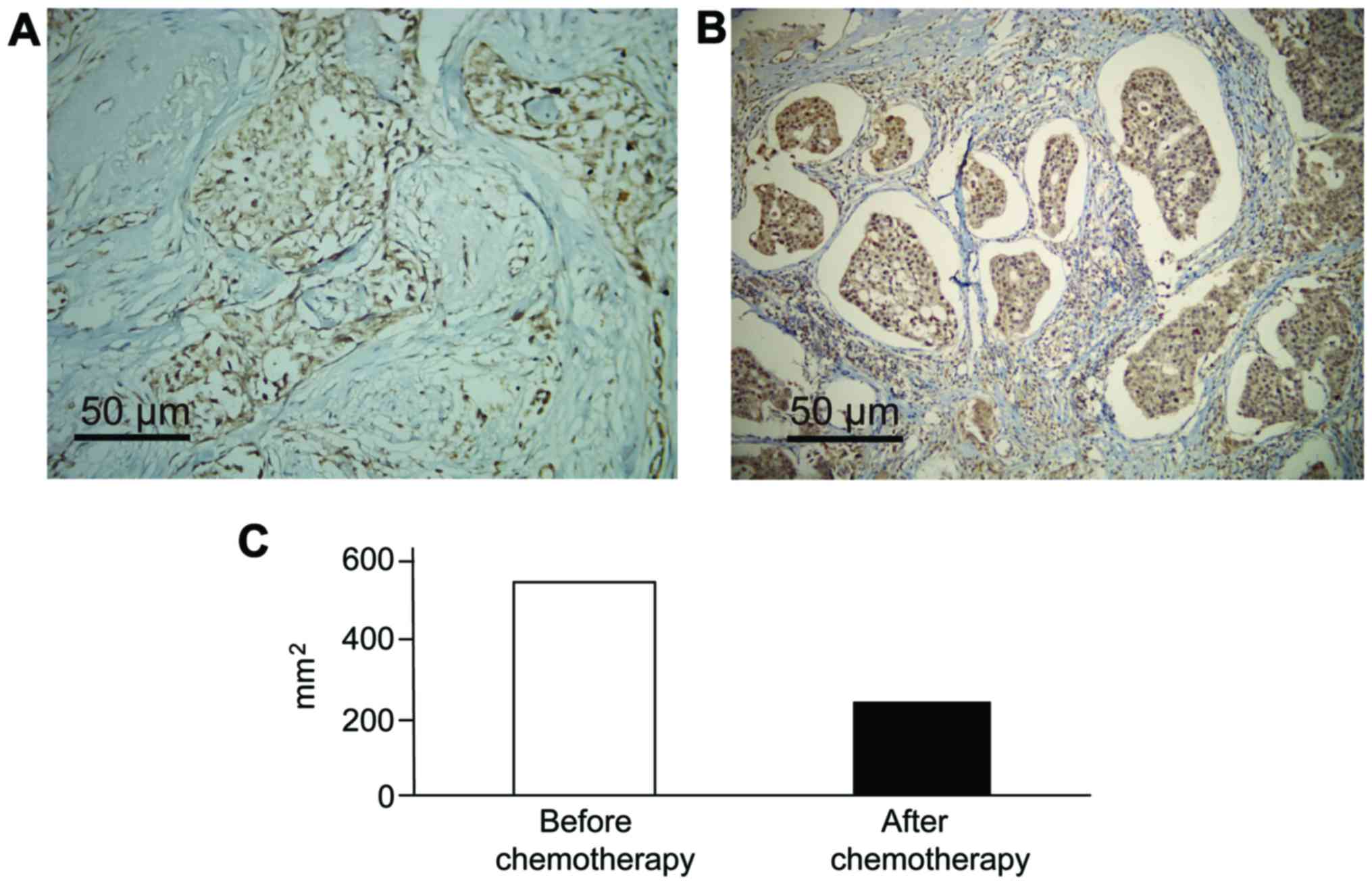
ERβ level before and after neoadjuvant
chemotherapy
Prior to the operation, we carried out neoadjuvant
chemotherapy for 3–4 cycles for patients in the experimental group.
After the operation, we stained the breast carcinoma tissue
immunohistochemically. We found that, after the administration of
neoadjuvant chemotherapy, the ERβ expression level in the breast
carcinoma tissues of the patients was greatly decreased, which
showed statistical significance (Fig.
2) (P<0.05).
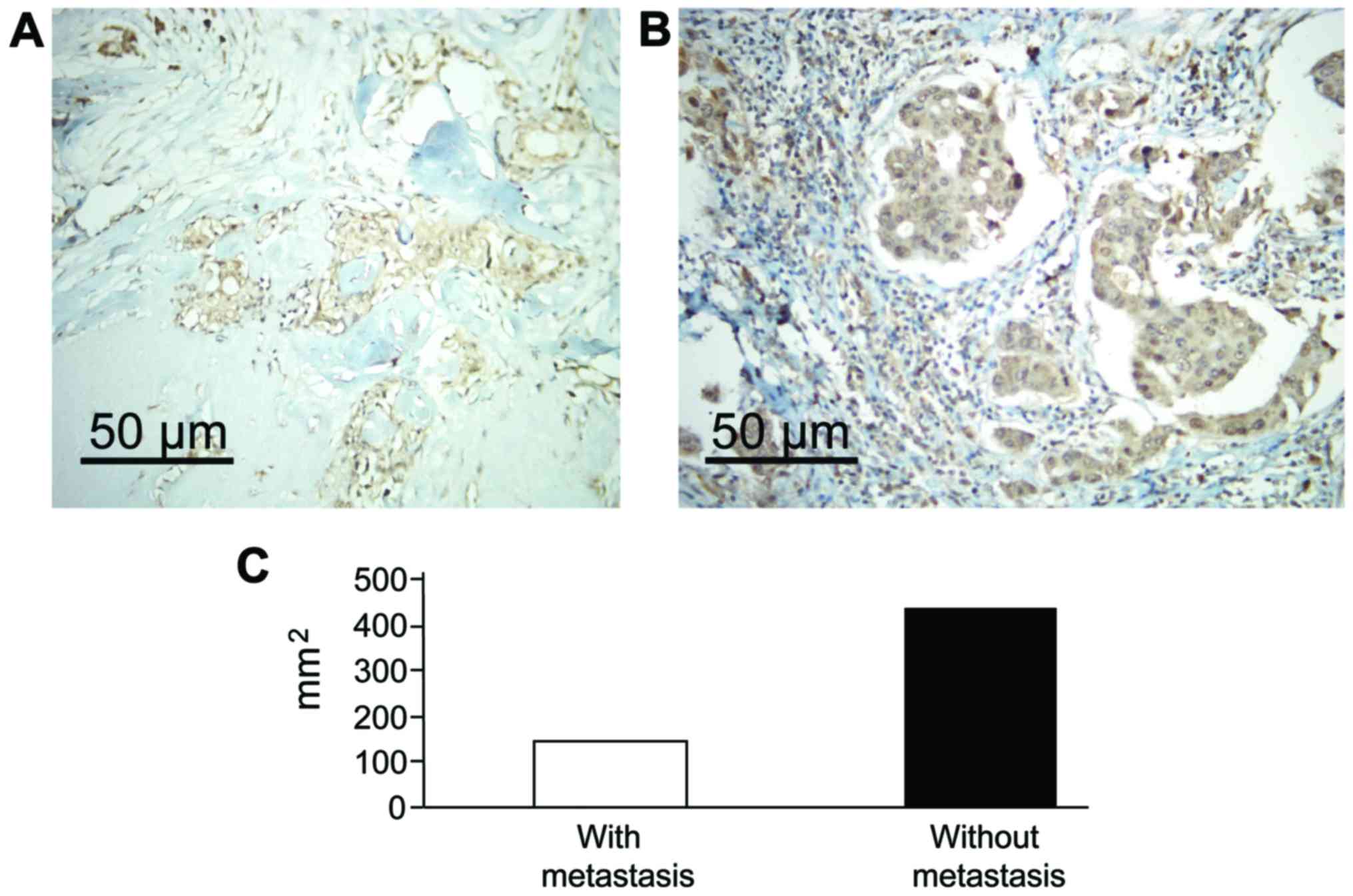
ERβ level of the patients after the
recurrence of breast carcinoma or before and after metastasis
We collected tissue samples of patients that had
recurrence of, or had metastasis after the operation, and stained
for ERβ immunohistochemically. The results revealed that the ERβ
levels for patients who have recurrence after the operation were
significantly higher than that after the operation (P<0.05). The
ERβ expression level was significantly increased in lymphatic
metastasis, and the results were statistically significant
(Fig. 3) (P<0.05).
Discussion
The ever increasing detection rate of patients with
breast carcinoma is mainly due to the upgrade of the testing
apparatus and awareness of breast cancer in women. In terms of the
treatment of breast carcinoma, in the past, surgical operation
combined with post-operation chemotherapy were often applied to
curb the growth and proliferation of cancer cells to the most
optimal degree. However, the medical field is constantly evolving
and has new opinions regarding current treatment methods about
breast carcinoma. Investigators are examining whether it is
possible to apply targeted chemotherapy prior to surgery to
markedly decrease the size of the tumor to avoid physical and
mental damage to the women. In addition, targeted treatment based
on targeted gene specificity can significantly improve prognosis
and survival of the patients. Targeted drugs that were used in the
past are mainly from the HERB (12)
receptor family at the surface of cancer cells. However, as the
disease develops, the drug resistance of the targeted therapy drugs
increase, which is one of the major challenges in the clinical
treatment of breast carcinoma. Based on this, we designed and
carried out the present study. For breast carcinoma patients who
have a definite diagnosis, we arranged for three to four cycles of
pre-operation ‘adjuvant chemotherapy’ (TEC plan) (13) before the surgery. Additionally, we
measured the expression levels of ERβ gene before and after
the operation to provide a theoretical basis for the development of
new types of targeted drugs for treatment.
In the present study, we found that, among the 120
breast carcinoma patients, there were different degrees of
expression of the ERβ gene when looking at the healthy and
breast carcinoma tissues of the patients. However, the expression
in the breast carcinoma tissues significantly increases, showing
significant statistical difference (P<0.05). The high expression
of ERβ also indicates that the cell proliferation conditions are
exuberant in the body of breast carcinoma patients (15). Past studies, such as that conducted by
Leygue et al (16), found the
expression of ERβ mRNA in the breast carcinoma tumor samples and
the mammary epithelial cells of several types of people. In
addition, Leygue et al (16)
showed that the ratio of ERα and ERβ in the breast carcinoma
patients varies as breast cancer develops. Compared to surrounding
normal breast tissues, the ERα mRNA/ERβ mRNA ratio in ERα-positive
breast carcinoma tissues significantly increases. This discovery
indicates that ERβ may play a part in the occurrence and
development of the breast carcinoma (17–19).
However, the ability to discern the occurrence and development of
breast carcinoma and prognosis by analyzing the expression of ERβ
is still not thoroughly researched and lacks the absolute
specificity of the cells in the tissue (20). Whether the expression levels of ERβ
are related to the differentiation level of cells in tissue
requires further research and verification (21). Even so, this study has provided
reasonable theoretical support for the diagnosis, treatment,
prognosis evaluation, recurrence, transfer and other adverse events
of breast carcinoma. It has also suggested a reasonable research
direction for the development of targeted treatment drugs. To
establish ERβ as a biomarker, the ability to check ERβ expression
level by serology can significantly increase the detection rate of
ERβ. This allows physicians to reasonably evaluate the regression
and prognosis of tumors in clinical application according to the
specific expression levels.
In the present study, we designed the pre-operation
neoadjuvant chemotherapy (TEC plan) for 3–4 cycles to test the
expression levels of ERβ gene in cancer tissues of patients
and identified a correlation between the ERβ level and the clinical
stages of breast carcinoma and lymphatic metastasis, and the
results were statistically significant (P<0.05). We hypothesized
that among patients who have lymphatic metastasis and poor
pathological analysis, the number of cancer cells of executant
proliferation greatly increase. If we reverse this process, we can
come to the conclusion that the higher the ERβ level is, the higher
the stage of cancer is; however, we lack experimental proof. Our
results indicate that the expression levels of ERβ are not related
to the pathological classification of breast carcinoma. After the
patients received neoadjuvant chemotherapy, the expression levels
of the ERβ gene significantly decrease compared to levels before
the neoadjuvant chemotherapy and the results were statistically
significant (P<0.05). This indicates that there may be some
value of using ERβ levels for clinical reference in evaluating the
effect of chemotherapeutic drugs. However, we only focused on the
in vitro study of ERβ in clinical breast carcinoma patients.
There is no support of our findings in animal experiments. The
related targeted therapeutic drugs remain to be developed.
Theoretical support is necessary for issues concerning whether the
targeted therapeutic drugs can improve the survival rate and
disease-free survival period in animal models of breast
carcinoma.
Additionally, our results did not show any
correlation between the size of cancer and ERβ expression levels.
This is different from the results of past studies. Zu et al
(19) studied this issue and
suggested that in gastric adenocarcinoma, there is a correlation
between the size and prognosis of the cancer and the expression of
tumor markers. However, Yang et al (20) indicated that there was no significant
correlation between the size of the tumor and the proliferation and
metabolism degree of the cancer cells. Combined with our study
results, we think that, in breast carcinoma, the size of tumor in
the breasts do not have significant correlation with the metabolism
of the tumor. In malignant breast tumors, there are differences in
differentiation degree of cancer cells. We found that, in the
undifferentiated or low-differentiated carcinoma patients, often,
the size of the tumor is not large and in most cases, there have
already had distant metastasis or lymphatic metastasis as
previously reported (11).
Through the collection and study of the samples
before and after the operation, we recognize the limitations of our
study. For example, in our early studies, we found that the
expression of ERβ gene varies according to the dynamic change and
the meaning of such changes during the treatment process of
patients. However, further in vitro and in vivo
experiments are needed for verification. Furthermore, during the
occurrence and development of breast carcinoma, the function of the
ERβ gene, i.e., the working mechanism, still remains to be
explored. Even though there has been no theoretical support for
these study results in the study of breast carcinoma, it has
provided good insights for our future study.
In conclusion, the ERβ gene is of important
clinical value for the evaluation of recurrence, transfer and death
of breast carcinoma patients. We can treat ERβ as the evaluation
index for the neoadjuvant chemotherapy when observing its clinical
effects. In the targeted therapy and corresponding drug development
for breast carcinoma, ERβ can act as one of the specific drug
targets.
References
|
1
|
Howell A, Anderson AS, Clarke RB, Duffy
SW, Evans DG, Garcia-Closas M, Gescher AJ, Key TJ, Saxton JM and
Harvie MN: Risk determination and prevention of breast cancer.
Breast Cancer Res. 16:4462014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bodai BI and Tuso P: Breast cancer
survivorship: a comprehensive review of long-term medical issues
and lifestyle recommendations. Perm J. 19:48–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyd NF, Martin LJ, Bronskill M, Yaffe MJ,
Duric N and Minkin S: Breast tissue composition and susceptibility
to breast cancer. J Natl Cancer Inst. 102:1224–1237. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Z, Qiao JX, Shetty A, Wu G, Huang Y,
Davidson NE and Wan Y: Regulation of estrogen receptor signaling in
breast carcinogenesis and breast cancer therapy. Cell Mol Life Sci.
71:15492014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Lindström LS, Foo JN, Rafiq S,
Schmidt MK, Pharoah PD, Michailidou K, Dennis J, Bolla MK, Wang Q,
et al: kConFab Investigators: 2q36.3 is associated with prognosis
for oestrogen receptor-negative breast cancer patients treated with
chemotherapy. Nat Commun. 5:40512014.PubMed/NCBI
|
|
6
|
Seneviratne S, Campbell I, Scott N,
Kuper-Hommel M, Round G and Lawrenson R: Ethnic differences in
timely adjuvant chemotherapy and radiation therapy for breast
cancer in New Zealand: a cohort study. BMC Cancer. 14:8392014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, Andre F,
Bergh J, et al: Panel members: Personalizing the treatment of women
with early breast cancer: highlights of the St Gallen International
Expert Consensus on the Primary Therapy of Early Breast Cancer
2013. Ann Oncol. 24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei J, Rudolph A, Moysich KB, Rafiq S,
Behrens S, Goode EL, Pharoah PP, Seibold P, Fasching PA, Andrulis
IL, et al: kConFab Investigators: Assessment of variation in
immunosuppressive pathway genes reveals TGFBR2 to be associated
with prognosis of estrogen receptor-negative breast cancer after
chemotherapy. Breast Cancer Res. 17:182015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omoto Y and Iwase H: Clinical significance
of estrogen receptor β in breast and prostate cancer from
biological aspects. Cancer Sci. 106:337–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastore MB, Jobe SO, Ramadoss J and
Magness RR: Estrogen receptor-α and estrogen receptor-β in the
uterine vascular endothelium during pregnancy: functional
implications for regulating uterine blood flow. Semin Reprod Med.
30:46–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mufudza C, Sorofa W and Chiyaka ET:
Assessing the effects of estrogen on the dynamics of breast cancer.
Comput Math Methods Med. 2012:4735722012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crooke PS, Justenhoven C, Brauch H,
Dawling S, Roodi N, Higginbotham KS, Plummer WD, Schuyler PA,
Sanders ME, Page DL, et al: GENICA Consortium: Estrogen metabolism
and exposure in a genotypic-phenotypic model for breast cancer risk
prediction. Cancer Epidemiol Biomarkers Prev. 20:1502–1515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cardaci S and Ciriolo MR: TCA cycle
defects and cancer: when metabolism tunes redox state. Int J Cell
Biol. 2012:1618372012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colditz GA: Relationship between estrogen
levels, use of hormone replacement therapy, and breast cancer. J
Natl Cancer Inst. 90:814–823. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leygue E, Dotzlaw H, Watson PH and Murphy
LC: Altered expression of estrogen receptor-α variant messenger
RNAs between adjacent normal breast and breast tumor tissues.
Breast Cancer Res. 2:64–72. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanfranchi A and Brind J: Breast Cancer:
Risks and Prevention (4th). Breast Cancer Prevention Institute. New
York: 2007.
|
|
18
|
Zhou B, Moodie A, Blanchard AA, Leygue E
and Myal Y: Claudin 1 in breast cancer: new insights. J Clin Med.
4:1960–1976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zu H, Wang F, Ma Y and Xue Y:
Stage-stratified analysis of prognostic significance of tumor size
in patients with gastric cancer. PLoS One. 8:e545022013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XR, Chang-Claude J, Goode EL, Couch
FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A,
et al: Associations of breast cancer risk factors with tumor
subtypes: a pooled analysis from the Breast Cancer Association
Consortium studies. J Natl Cancer Inst. 103:250–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Green LE, Dinh TA and Smith RA: An
estrogen model: the relationship between body mass index,
menopausal status, estrogen replacement therapy, and breast cancer
risk. Comput Math Methods Med. 2012:7923752012. View Article : Google Scholar : PubMed/NCBI
|