Introduction
Conventional treatments for the clinical management
of cancer include surgery, chemotherapy and radiotherapy. The
majority of patients with high-stage cancer or who have undergone
tumor surgery require chemotherapy. However, some patients cannot
tolerate the treatment or exhibit drug resistance, and the efficacy
of systemic chemotherapy remains unsatisfactory (1). Only 60–70% of malignant tumors can be
treated with radiotherapy, and certain patients with recurrent or
metastatic disease only qualify for palliative treatment due to
tolerance to other treatments, complications and/or malignant tumor
metastasis (2,3). Other treatment options include
immunotherapy and traditional Chinese medicine, including Ginkgol
C17:1.
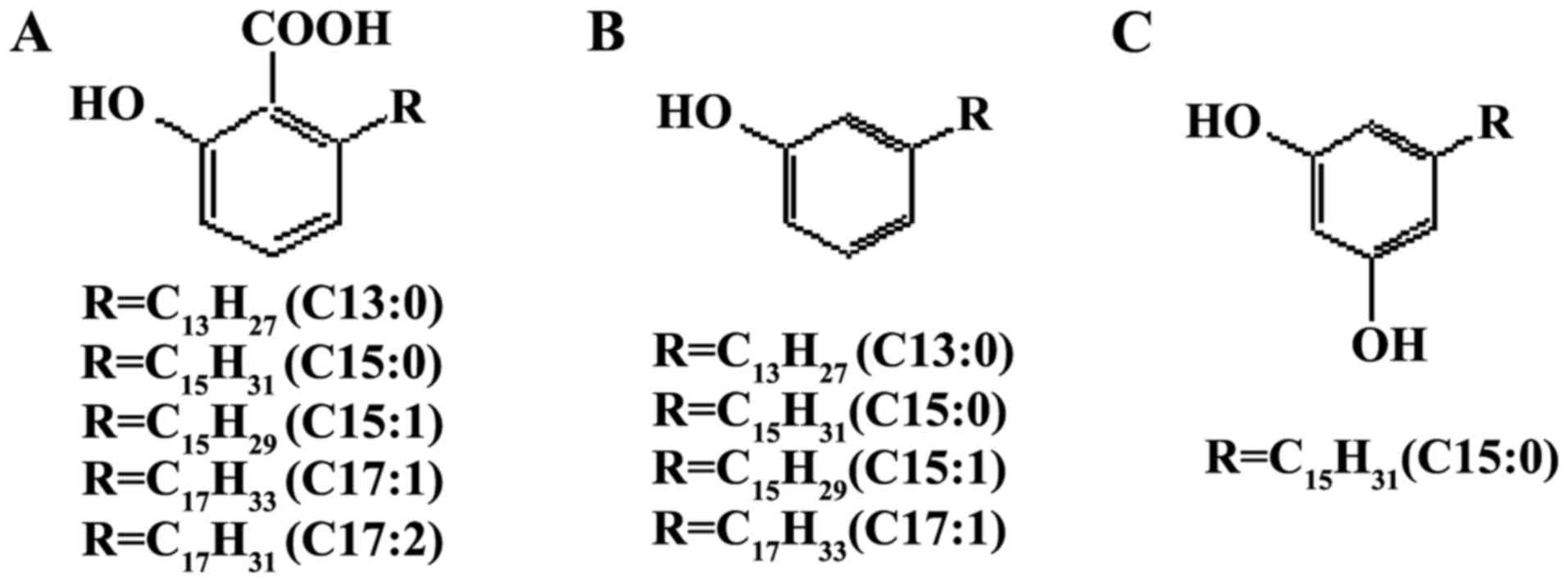
Ginkgols are the active compounds with an anticancer
effect from Ginkgo biloba. Three different classes of
alkylphenols, including Ginkgolic acids, ginkgols and bilobols
(Fig. 1), occur in various parts of
Ginkgo biloba. Ginkgols are also termed 3-alkylphenols or
cardanols, and their alkyl side chains vary between 13 and 17
carbons in length with 0–2 double bonds (4). Ginkgols have been reported to exhibit a
wide range of pharmacological effects, including antibacterial
(5,6),
antifeeding (7), antioxidant
(8) and anti-apoptotic activities, on
tumor cells in vitro (9).
Ginkgols are composed of four homolog monomers with
different alkyl side chains: C13:0, C15:0, C15:1 and C17:1
(10). Among the ginkgol monomers,
Ginkgol C17:1 has been shown to exert the strongest inhibitory
effect on cell viability in a number of human cancer cells
(9,11).
Ginkgol C17:1 was isolated, purified and identified
by the authors of the present study. Considering the results from
our previous studies (9,12), the present study aimed to
systematically investigate the proliferation, migration and
invasion of tumor cells following treatment with various
concentrations of Ginkgol C17:1. Furthermore, the present study
explored the possible mechanisms of the signaling pathways
underlying the effects of Ginkgol C17:1.
Materials and methods
Cancer cell lines and culture
Human hepatoma carcinoma HepG2 and human colon
epithelial carcinoma SW480 cells were obtained from the Institution
of Cell Biology of the Chinese Academy of Sciences (Shanghai,
China) and cryopreserved at the School of Medicine, Jiangsu
University (Zhenjiang, China). The HepG2 and SW480 cells were
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere containing 5% CO2. The medium was
changed every 2 days and the cells were maintained at
subconfluence.
Reagents
Trypsin-EDTA solution was purchased from Gibco;
Thermo Fisher Scientific, Inc. The horseradish peroxidase
(HP)-conjugated secondary antibodies, goat anti-mouse polyclonal
IgG (cat. no. A0216; dilution, 1:10,000) and goat anti-rabbit
polyclonal IgG (cat. no. A0208; dilution, 1:10,000) were purchased
from Beyotime Institute of Biotechnology (Haimen, China). The
enhanced chemiluminescence (ECL) reagents were bought from GE
Healthcare Life Sciences (Chalfont, UK). MTT was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Mouse
monoclonal immunoglobulin G (IgG) anti-as homolog gene family,
member A (RhoA; cat. no. sc-418; dilution, 1:1,000) and β-actin
antibody (cat. no. sc-47778; dilution, 1:1,000) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Polyclonal rat
anti-rabbit matrix metalloproteinase (MMP)-7 antibody (cat. no.
S247; dilution, 1:1,000) was purchased from Bioworld Technology,
Inc. (St. Louis Park, MN, USA). Rabbit polyclonal antibodies
against protein kinase B (Akt; cat. no. IM001-0359; dilution,
1:1,000) and phosphorylated (p)-Akt (cat. no. IM001-0270; dilution,
1:1,000) were purchased from ExCel Biology Co., Ltd. (Shanghai,
China). Ginkgol C17:1 (high performance liquid chromatography
(HPLC) purity of >96.5%) was donated by the School of Food and
Biological Engineering, Jiangsu University (7).
MTT assay
The cells were pooled and diluted to a cell density
of 105 cells/ml, then dispensed into a 96-well plate to
a final volume of 100 µl per well. Following incubation for 12 h at
37°C in 5% CO2, the cells were treated with various
concentrations (5, 10, 20, 40 or 80 µg/ml) of Ginkgol C17:1in the
same final volume of 100 µl per well for an extended incubation of
24 h at 37°C. A total of 10 µl MTT solution (5 mg/ml) was then
added to each well and incubated for another 4 h at 37°C.
Subsequent to the removal of the growth medium, 100 µl dimethyl
sulfoxide (DMSO) was added and mixed thoroughly. The absorbance was
measured at an optical density of 490 nm using a microplate reader
(Bio-ad Laboratories, Inc., Hercules, CA, USA). Positive (DMEM and
1% DMSO) and negative controls (DMEM alone) were included in all
assays.
Migration assay
A cell migration assay was performed using a
Transwell Boyden chamber, with 8.0-µm polyethylene terephthalate
(PET) membrane 24-well cell culture inserts (Corning Incorporated,
Corning, NY, USA). In total, 500 µl DMEM containing 10% FBS was
added to the bottom chamber. Exponential phase HepG2 or SW480 cells
were collected, and 5×104 cells were seeded into the
upper chamber in 300 µl serum-free medium containing various
concentrations (20, 40 or 80 µg/ml) of Ginkgol C17:1. The cells
were allowed to migrate through the PET membrane to the bottom
chamber. Following a 24-h incubation at 37°C in 5% CO2,
the cells that had not migrated were removed from the upper chamber
using cotton swabs, and the cells that had migrated to the lower
side of the membrane were fixed in 4% paraformaldehyde and stained
with Giemsa solution for 10 min. The level of migration was
determined by counting the cell numbers under a light microscope at
a magnification of ×200. The migrated cells were counted in 5
randomly selected fields per insert, and the mean values were
calculated. All experiments were performed in triplicate for each
migration condition.
In vitro invasion assay
For the peridium basement membrane preparation, 5
mg/ml Matrigel (Corning Matrigel basement membrane matrix; cat. no.
356234; Corning Incorporated) was thawed at 4°C, kept on ice and
diluted in cold serum-free DMEM to a final concentration of 1 mg/ml
using pre-cooled pipette tips. A total of 50 µl diluted Matrigel
was then added to the upper chamber of each pre-cooled Transwell
insert. The wells without lids were maintained at 37°C for ~2 h
until the Matrigel dried completely. Subsequently, for the
hydration basement membrane preparation, gelled Matrigel was gently
washed with warm serum-free culture medium, after which 50 µl
serum-free DMEM medium was added to the Transwell insert. The
hydration basement membrane was left at 37°C for 30 min. Finally,
the assay protocol was the same as described for the cell migration
assay.
Western blotting
HepG2 and SW480 cells were cultured with 20, 40 or
80 µg/ml Ginkgol C17:1 for 24 h, reached a confluency of ~80% in
the 6-well plates, they were lysed in lysis buffer (50 mM Tris, 150
mM NaCl, 1 mM EDTA and 1% Triton X-100; pH 7.4), washed three times
with cold PBS and treated with 1 mM phenylmethylsulfonyl fluoride
(Shanghai Bogoo Biotechnology Co., Ltd., Shanghai, China) for 30
min on the ice. Following transfer into an Eppendorf tube (Corning
Incorporated), centrifugation at 12,000 × g was performed
for 5 min at 4°C. Finally, the supernatant was taken as the whole
cell protein extract.
The sample proteins were run on 10.0 or 12.5% SDS
polyacrylamide gels, and were transferred onto polyvinylidene
difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., USA). The
PVDF membranes were initially blocked with 5% milk in TBS-T (80 g/l
NaCl, 2 g/l KCl, 30 g/l Tris and 0.1% Tween-20; pH 7.4) for 1 h at
room temperature. The membranes were then incubated with the
primary antibodies at 4°C overnight. Following subsequent
incubation of the membranes with HP-conjugated secondary antibodies
for 1 h at room temperature, the ECL reagents were applied to
reveal the positive bands on the membrane, according to the
protocol of the manufacturer. The bands were detected using the
Typhoon 9400 imager (GE Healthcare Life Sciences, Piscataway, NJ,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed with the Student's t-test in
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). An independent
samples t-test was used to compare the relative cell numbers in the
viability, migration and invasion assays. P<0.05 was considered
to indicate a statistically significant difference.
Results
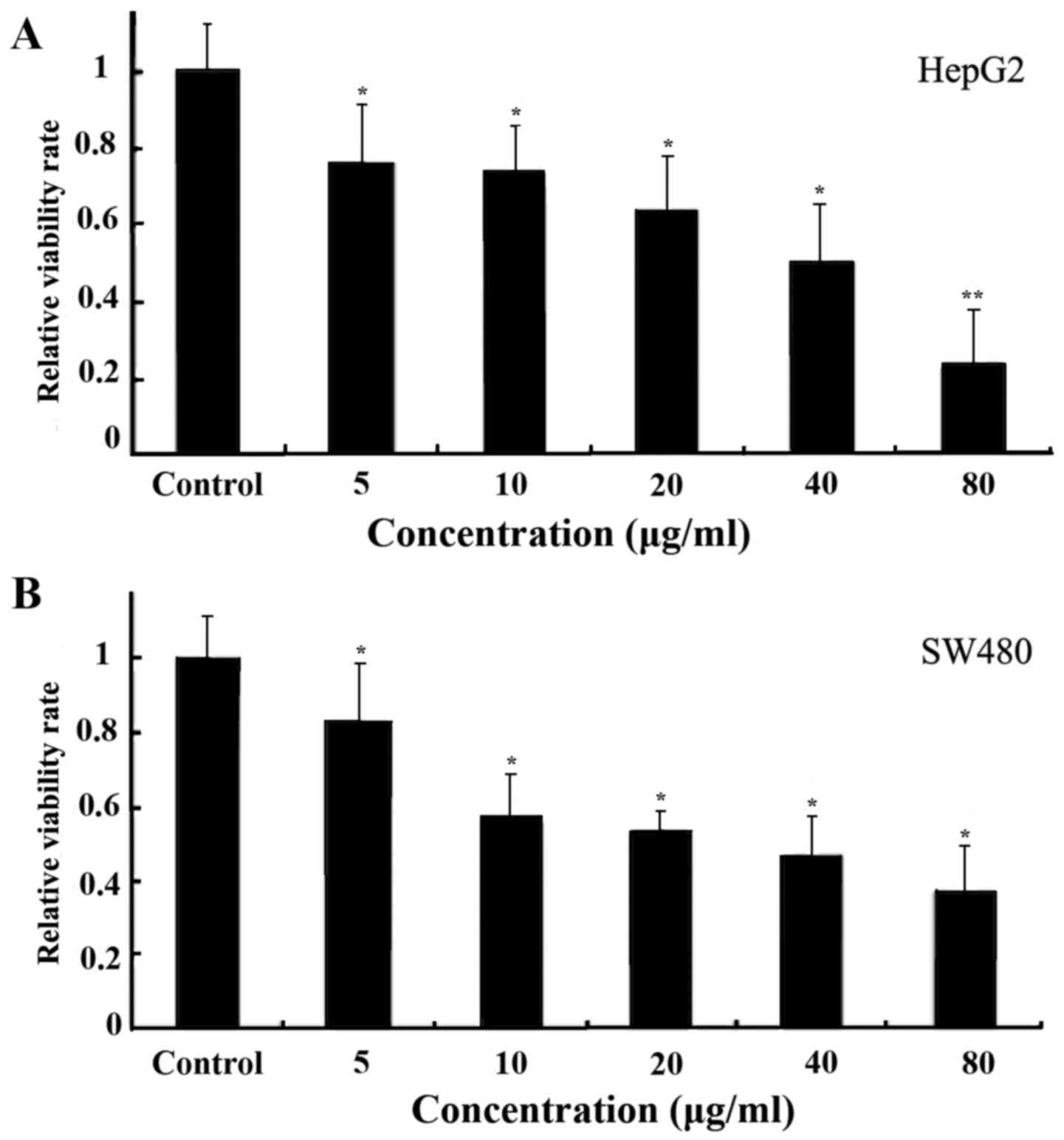
Ginkgol C17:1 reduces the viability of
tumor cells
The HepG2 (Fig. 2A)
and SW480 (Fig. 2B) cells were
treated with Ginkgol C17:1 for 24 h at concentrations of 5, 10, 20,
40 or 80 µg/ml. Cell viability was detected using the MTT assay.
The results revealed that the concentrations of Ginkgol C17:1
exhibited a dose-dependent inhibitory effect on the viability of
the cancerous cell lines.
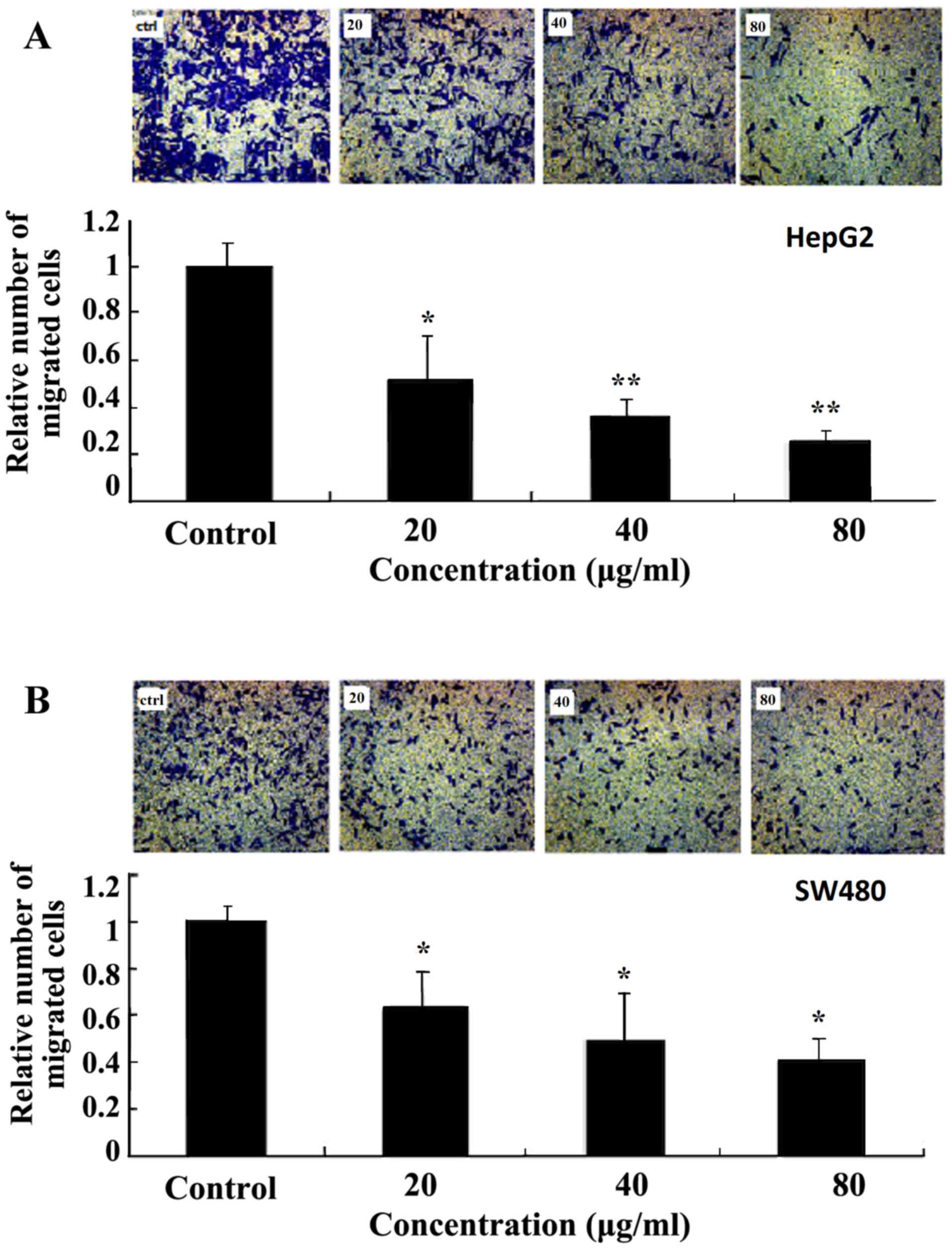
Ginkgol C17:1 suppresses the migration
of tumor cells
The effect of Ginkgol C17:1 on the migration of
HepG2 (Fig. 3A) and SW480 (Fig. 3B) cells was evaluated using a
Transwell Boyden chamber. The cellular migration was induced using
10% FBS as a chemotactic factor to increase the basal migration of
tumor cells in the control group. Treatment of the cells with 20,
40 or 80 µg/ml Ginkgol C17:1 for 24 h exhibited a dose-dependent
inhibitory effect on cell migration.
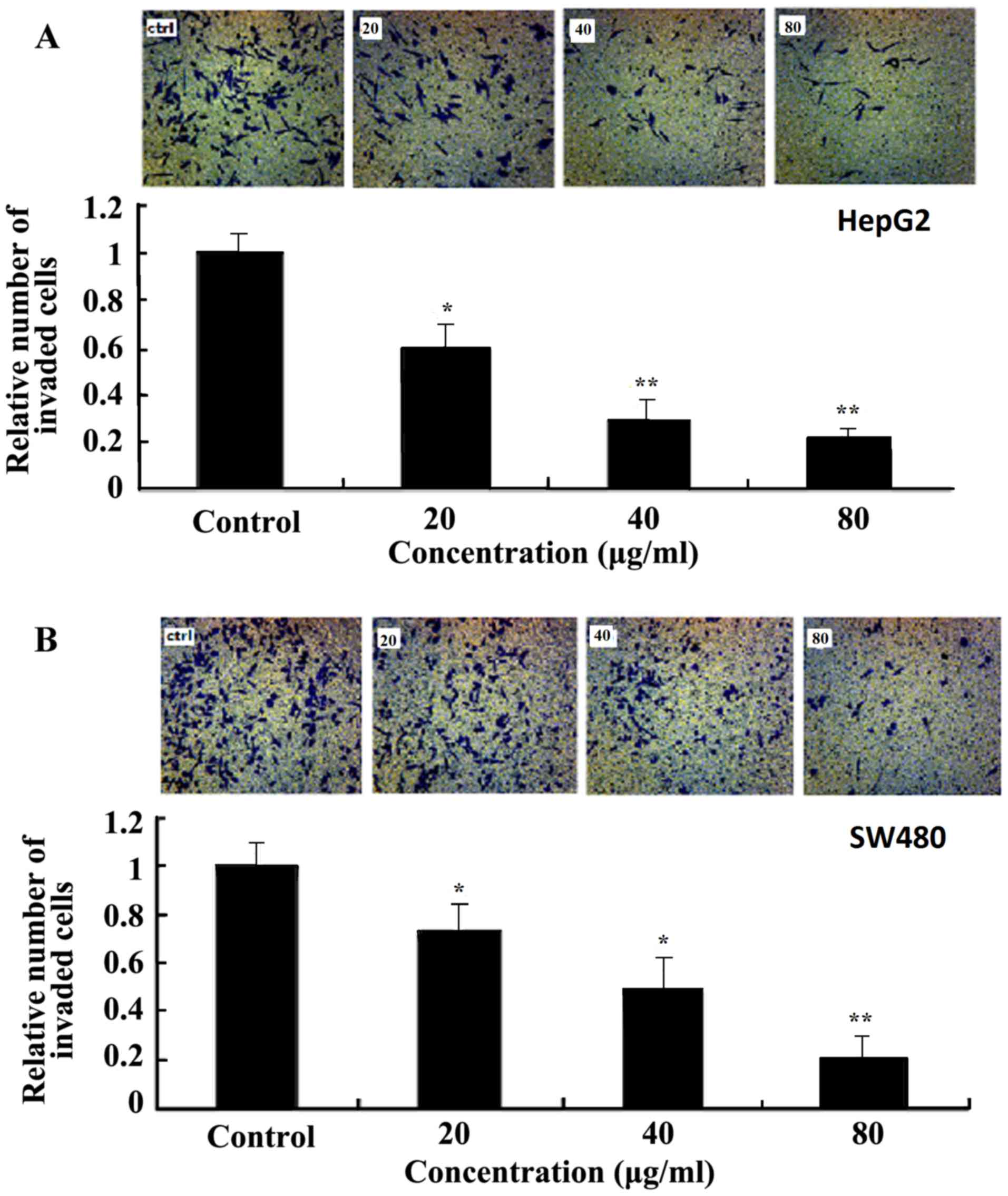
Ginkgol C17:1 suppresses the invasion
of tumor cells
The effect of Ginkgol C17:1 on tumor cell invasion
was examined using a Matrigel invasion assay with a modified
Transwell Boyden chamber. Subsequent to treatment with various
concentration of Ginkgol C17:1 for 24 h, the number of HepG2
(Fig. 4A) and SW480 (Fig. 4B) cells that passed through the
Matrigel basement membrane matrix was markedly lower compared with
the control group, and Ginkgol C17:1 exhibited a dose-dependent
inhibitory effect on cancer cell invasion.
Ginkgol C17:1 effects the protein
expression of several signaling pathway components
Several signaling pathways, including the
mitogen-activated protein kinase/extra cellular signal-regulated
kinase (EK), reactive oxygen species/EK and phosphatidylinositol
3-kinase (PI3K)/Akt pathways have been reported to be involved in
the invasion, metastasis and prognosis of cancer (13,14).
However, it remains unknown whether these pathways contribute to
the anticancer effects of Ginkgol C17:1. In the present study, the
expressions of MMP-7, RhoA, Akt and p-Akt were analyzed by western
blotting. Following treatment of HepG2 (Fig. 5A) and SW480 cells (Fig. 5B) with various concentration of
Ginkgol C17:1 for 24 h, the total amount of Akt remained the same,
while the protein expressions of MMP-7, RhoA and p-Akt were
markedly decreased.
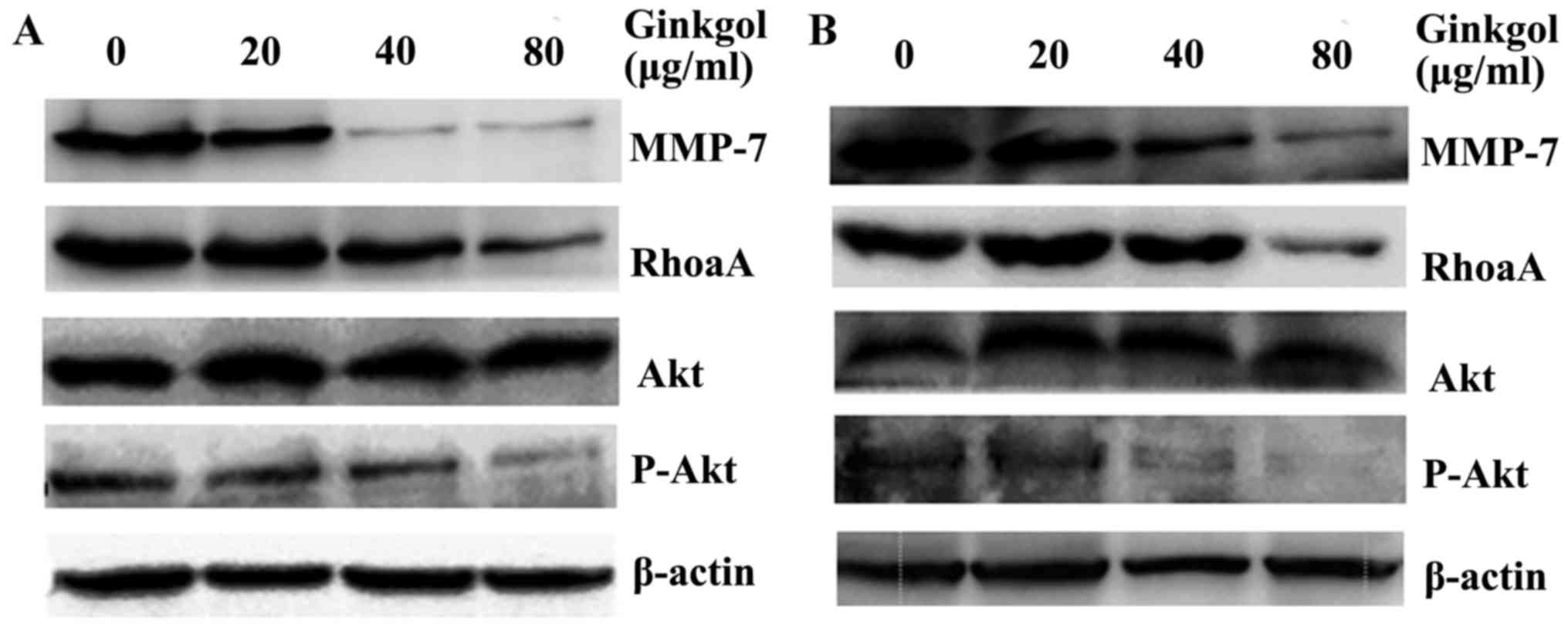 | Figure 5.Ginkgol C17:1 affects the protein
levels of MMP-7, RhoA and p-Akt in cancer cells. Subsequent to the
treatment of (A) HepG2 and (B) SW480 cells with 20, 40 or 80 µg/ml
Ginkgol C17:1 for 24 h, whole cell extracts were prepared for the
detection of the protein levels of MMP-7, RhoA, Akt and p-Akt by
western blotting. β-actin expression served as a loading control.
MMP, matrix metalloproteinase; RhoA, ras homolog gene family,
member A; p, phosphorylated; Akt, protein kinase B. |
Discussion
Ginkgo biloba L. is the only surviving member
of the plant order Ginkgoales and serves as one of the oldest
living seed plant groups of medicinal, spiritual and horticultural
importance worldwide (15). Studies
investigating the antitumor characteristics of ginkgo alkylphenols
have focused on ginkgo acids (GAs). Liu and Zeng (16) found that HepG2 cells were more
sensitive to the cytotoxicity of GAs than primary rat hepatocytes.
GAs significantly inhibited the growth of Hep-2 cells, arrested
cells at the G0/G1 phase, decreased the level of B-cell lymphoma 2
(Bcl-2)/Bcl-2-like protein 4 (Bax) (17) and induced the apoptosis of pituitary
gland tumor cells by increasing the radiosensitivity of the cells
(18).
However, GAs are not stable, but are easily
decarboxylated to form ginkgols subsequent to exposure to heat and
acid or base catalysis. The Ginkgol C17:1 monomer can be isolated
and the structure of the monomer has been confirmed by HPLC-diode
array detection, gas chromatography-mass spectrometry, proton
nuclear magnetic resonance (NM) and carbon-13-NM analysis (9). Our previous study revealed that Ginkgol
C17:1 exhibited stronger thermal stability compared with GAs and
exerted stronger anticancer effects compared with the monomers of
Ginkgol and GAs, with respect to the apoptosis of tumor cells
(9).
In the present study, the effects of Ginkgol C17:1
on the biological behaviors of human tumor cells were
systematically investigated, in terms of cell proliferation,
migration and invasion. Consistent with a previous study using
other cancer cells (9), the present
study found a dose-dependent inhibition of cell proliferation in
human hepatoma carcinoma HepG2 cells and colon cancer SW480 cells.
In addition, the results of the present study revealed that Ginkgol
C17:1 inhibited the cell migration and invasion capabilities of
HepG2 and SW480 cells in a dose-dependent manner.
The protein expressions of RhoA, MMP-7, Akt, and
p-Akt were analyzed by western blotting to investigate the
underlying mechanisms of the inhibitory effects of Ginkgol C17.1 on
cancer cells. The results revealed that, subsequent to a 24 h
treatment with Ginkgol C17:1, the expressions of RhoA and MMP-7
proteins were markedly reduced. Although the total amount of Akt
protein remained unchanged, the expression of p-Akt was markedly
decreased.
RhoA activation is associated with invasive growth
and metastasis. The best known effector of RhoA is Rho kinase
(ROCK), which appears to serve a key role in regulating the force
and velocity of actomyosin crossbridging in smooth muscle and
non-muscle cells by inhibiting the myosin phosphatase-mediated
dephosphorylation of the regulatory chain of myosin II (19). The inhibition of RhoA reduces the
level of tumor cell invasion and metastasis, and these effects may
be mediated by the RhoA target protein ROCK (20). Since RhoA protein expression decreased
subsequent to Ginkgol C17:1 treatment, the present study
hypothesized that Ginkgol C17:1 suppresses the development and
growth of tumors by inhibiting the RhoA/ROCK pathway.
MMP-7 is associated with invasive tumor growth and
distant metastasis in several types of cancer (21). Although MMP-7 is rarely expressed in
normal tissues, the level of expression rises during the malignant
transformation of tumors, inflammatory diseases (22) and epithelial-mesenchymal transition
(23); thus serving an important role
in the process of malignancy (24).
MMP-7 markedly enhances chondrosarcoma cell motility and invasion
due to its upregulation in response to stress exposure, which may
promote lung colonization of chondrosarcomas in vivo
(25). MMP-7 was also found to be an
indicator of poor survival and a high recurrence rate in patients
with colorectal cancer (26). In the
present study, the marked decrease in the level of MMP-7 following
Ginkgol C17:1 treatment confirmed that Ginkgol C17:1 inhibits the
migration and invasion of tumor cells by inhibiting MMP-7.
The PI3K/Akt signaling pathway has been found to
serve an important role in the survival, proliferation and
apoptosis of tumor cells (27,28).
p-Akt-positive expression is significantly associated with distant
metastasis and unfavorable prognosis (29,30). While
the protein expression of total Akt in cancer cells did not change
following treatment with Ginkgol C17:1 in the present study, the
level of p-Akt was markedly decreased, confirming the inhibitory
effect of Ginkgol C17:1 on the activation of the PI3K/Akt signaling
pathway.
In conclusion, the present study investigated the
inhibitory effect of Ginkgol C17:1 on cancer cell proliferation and
migration and revealed that Ginkgol C17:1 inhibited the invasion
capacity of hepatocellular and colon cancer cells. The underlying
mechanisms for the suppression of tumor biological behaviors may be
associated with the reduced activities of the RhoA/ROCK, MMP-7 and
PI3K/Akt pathways. These results indicate that Ginkgol C17:1 maybe
a novel candidate for cancer therapy. Additional investigation is
required, for example, to understand the interaction of Ginkgol
C17:1 with the cancer cells and the subsequent cascade with respect
to altered cancer biology.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81372404),
the Specialized Research Fund for Senior Personnel Program of
Jiangsu University (grant no. 11JDG129), the Postdoctoral
Foundation of China (grant no. 2012M521018) and the Postdoctoral
Foundation of Jiangsu province (grant no. 1201025B).
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermorken JB and Specenier P: Optimal
treatment for recurrent/metastatic head and neck cancer. Ann Oncol.
21:(Suppl 7). vii252–vii261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sawicka E, Mirończuk A, Wojtukiewicz MZ
and Sierko E: Chemoradiotherapy for locally advanced pancreatic
patients: is it still an open question? Contemp Oncol (Pozn).
20:102–108. 2016.PubMed/NCBI
|
|
4
|
van Beek TA and Montoro P: Chemical
analysis of Ginkgo biloba leaves and extracts, and
phytopharmaceuticals. J Chromatogr A. 1216:2002–2032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boonsai P, Phuwapraisirisan P and Chanchao
C: Antibacterial activity of a cardanol from Thai Apis mellifera
Propolis. Int J Med Sci. 11:327–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng ZL, Wei Y, Xu DM, Hao SH and Hu JY:
Effect of 2-allylphenol against Botrytis cinerea Pers., and its
residue in tomato fruit. Crop Protection. 26:1711–1715. 2007.
View Article : Google Scholar
|
|
7
|
Shi QT, Liu H, Zhang YZ, Gu S and Liu LY:
Study on antifeeding activity of ginkgo phenol to cannage
caterpillar. Biomass Chem Engineer. 43:13–16. 2009.
|
|
8
|
Trevisan MT, Pfundstein B, Haubner R,
Würtele G, Spiegelhalder B, Bartsch H and Owen RW: Characterization
of alkyl phenols in cashew (Anacardium occidentale) products and
assay of their antioxidant capacity. Food Chem Toxicol. 44:188–197.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang XM, Wang YF, Li YY and Ma HL: Thermal
stability of ginkgolic acids from Ginkgo biloba and the effects of
ginkgol C17:1 on the apoptosis and migration of SMMC7721 cells.
Fitoterapia. 98:66–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan WH, Shen ZB, Wang CZ and Yu Q:
Isolation and identification of alkylphenols from Ginkgo biloba
leaves. Chem Ind Forest Prod. 21:1–6. 2001.
|
|
11
|
Lee JS, Cho YS, Park EJ, Kim JW, Oh WK,
Lee HS and Ahn JS: Phospholipase Cgamma1 inhibitory principles from
the sarcotestas of Ginkgo biloba. J Nat Prod. 61:867–871. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YF, Yang XM, Li YY, Huang BZ, Guo CY
and Xing CH: Inhibitory effect of ginkgols on SMMC-7721 liver
cancer cells in vitro and liver cancer H22-braring mice in vivo. J
Jiangsu Univ (Med Edit). 23:233–237. 2013.
|
|
13
|
Law AY and Wong CK: Stanniocalcin-2
promotes epithelial-mesenchymal transition and invasiveness in
hypoxic human ovarian cancer cells. Exp Cell Res. 316:3425–3434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KH, Cho YS, Park JM, Yoon SO, Kim KW
and Chung AS: Pro-MMP-2 activation by the PPARgamma agonist,
ciglitazone, induces cell invasion through the generation of ROS
and the activation of ERK. FEBS Lett. 581:3303–3310. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh B, Kaur P, Gopichand Singh RD and
Ahuja PS: Biology and chemistry of Ginkgo biloba. Fitoterapia.
79:401–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZH and Zeng S: Cyctotoxicity of
ginkgolic acids in HepG2 cells and primary rat hepatocytes. Toxicol
Lett. 187:131–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou C, Li X, Du W, Feng Y, Kong X, Li Y,
Xiao L and Zhang P: Antitumor effects of ginkgolic acids in human
cancer cell occur via cell cycle arrest and decrease the Bcl-2/Bax
ratio to induce apoptosis. Chemotherapy. 56:393–402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sukumari-Ramesh S, Singh N, Jensen MA,
Dhandapani KM and Vender JR: Anacardic acid induces
caspase-independent apoptosis and radiosensitizes pituitary adenoma
cells. J Neurosurg. 114:1681–1690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wettschureck N and Offermanns S:
Rho/Rho-kinase mediated signaling in physiology and
pathophysiology. J Mol Med (Berl). 80:629–638. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han JC, Li XD, Du J, Xu F, Wei YJ, Li HB
and Zhang YJ: Elevated matrix metalloproteinase-7 expression
promotes metastasis in human lung carcinoma. World J Surg Oncol.
13:52015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng F, Yang H, Jack C, Zhang H, Moller A,
Spivey D, Page RC, Tierney DL and Crowder MW: Biochemical
characterization and zinc binding group (ZBGs) inhibition studies
on the catalytic domain of MMP7 (cdMMP7). J Inorg Biochem.
165:7–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simic P, Williams EO, Bell EL, Gong JJ,
Bonkowski M and Guarente L: SIRT1 suppresses the
epithelial-to-messenchymal transition in cancer and organ fibrosis.
Cell Rep. 3:1175–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li KJ, Hua G and Li H: Mechanism research
progress and protection of light skin damage. J Dermatol Venereol.
32:24–27. 2010.
|
|
25
|
Guan PP, Yu X, Guo JJ, Wang Y, Wang T, Li
JY, Konstantopoulos K, Wang ZY and Wang P: By activating matrix
metalloproteinase-7, shear stress promotes chondrosarcoma cell
motility, invasion and lung colonization. Oncotarget. 6:9140–9159.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Omar A Ahmed Haji, Haglund C, Virolainen
S, Häyry V, Atula T, Kontio R, Salo T, Sorsa T and Hagström J:
MMP-7, MMP-8, and MMP-9 in oral and cutaneous squamous cell
carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol.
119:459–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Zhang Y, Jia K, Dong Y and Ma W:
Metformin inhibits the proliferation of A431 cells by modulating
the PI3K/Akt signaling pathway. Exp Ther Med. 9:1401–1406.
2015.PubMed/NCBI
|
|
28
|
Itoh N, Semba S, Ito M, Takeda H, Kawata S
and Yamakawa M: Phosphorylation of Akt/PKB is required for
suppression of cancer cell apoptosis and tumor progression in human
colorectal carcinoma. Cancer. 94:3127–3134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meuillet EJ: Novel Inhibitors of AKT:
Assessment of a different approach targeting the pleckstrin
homology domain. Curr Med Chem. 18:2727–2742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia S, Feng Z, Qi X, Yin Y, Jin J, Wu Y,
Wu H, Feng Y and Tao M: Clinical implication of Sox9 and activated
Akt expression in pancreatic ductal adenocarcinoma. Med Oncol.
32:3582015. View Article : Google Scholar : PubMed/NCBI
|