Introduction
Lung cancer is one of the most common types of
malignant tumor, with a rising global incidence. It is currently
the most lethal form of malignant tumor globally, with a low
five-year survival rate (1).
Furthermore, the number of lung cancer cases diagnosed annually is
increasing, of which non-small cell lung cancer (NSCLC) accounts
for ~80% (2). Adenocarcinoma is the
most common histological subtype of NSCLC (3). Although previous studies have
contributed considerably to the understanding of this type of
carcinoma, the development of effective targeted therapies is
required (3).
In patients who are diagnosed with NSCLC,
radiotherapy is one of the primary treatment options (4). However, the radioresistance of lung
cancer remains a significant therapeutic obstacle (5). Improving tumor radiosensitivity is an
effective way to increase the potency of radiotherapy (6). The combination of gene therapy and
conventional radiation is a promising approach in cancer treatment
(7).
The ubiquitously distributed protein cyclophilin A
(CyPA), also known as peptidylprolyl isomerase A, is a member of
the immunophilin family. CyPA possesses peptidyl prolyl cis-trans
isomerase (PPIase) activity, and is involved in numerous biological
processes including T-cell activation, protein folding, trafficking
and molecular chaperoning (8).
Previous studies have demonstrated that CyPA is overexpressed in
numerous types of cancer, and is an important factor in malignant
transformation and metastasis (9,10). The
role of CyPA in lung cancer has recently been the subject of
various studies. Campa et al (11) demonstrated that CyPA protein levels
were significantly raised in lung cancer tissue specimens, compared
with adjacent non-diseased lung tissues. Subsequent studies
revealed that the suppression of CyPA expression diminishes NSCLC
tumor growth through the regulation of matrix metallopeptidase 9
(12,13). However, the role of CyPA in the
radiosensitivity of lung adenocarcinoma remains to be
elucidated.
A previous study demonstrated that CyPA protein
expression in the tissue of lung adenocarcinoma tumors is
significantly upregulated following radiation therapy (14). Therefore, in order to further
investigate the underlying mechanisms of CyPA gene radiosensitivity
in lung adenocarcinoma cells, the current study utilized lentiviral
vectors packaged by virus particles to specifically silence the
CyPA gene.
Materials and methods
Materials and reagents
PAa lung adenocarcinoma cells were obtained from
Peking University Health Science Center (Beijing, China), and the
293FT human embryonic kidney cell line was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Lentiviral vectors [pLLU2G-green fluorescent protein (GFP)],
packaging systems (3rd generation lentivirus packing system) and
negative control virus particles (pLP1, pLP2, pLP/VSV-G and pLLU2G)
were obtained from Invitrogen (Thermo Fisher Scientific, Inc.)
Lipofectamine® 2000 transfection reagent and One
Shot® Stbl3™ chemically competent E. coli were
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The
QIAquick Gel Extraction Kit was purchased from Tiangen Biotech Co.,
Ltd. (Beijing, China). Dulbecco's modified Eagle's medium (DMEM),
fetal bovine serum (FBS) and diethylpyrocarbonate were all
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt,
Germany).
Construction of CyPA RNA interference
(RNAi) lentivirus vector
For the silencing of CyPA expression, DNA
oligonucleotides were designed based on the CyPA siRNA sequence
(5′-TCTCGAGTTTTTCTCGAGA-3′), and cloned into pLLU2G lentiviral
vectors to construct pLLU2G-CyPA small hairpin (sh)RNA plasmids,
according to the method reported previously (15). Briefly, DNA oligonucleotides were
ligated with plasmid pLLU2G and digested with HpaI and
XhoI to form double stranded DNA. The double stranded DNA
was then transformed into One Shot Stbl3™ Chemically Competent
E. coli (Thermo Fisher Scientific, Inc.). Negative control
virus particles (pLP1, pLP2, pLP/VSV-G and pLLU2G) from Invitrogen
(Thermo Fisher Scientific, Inc.) were used to monitor the
nonspecific reactions induced by the shRNA, and to optimize the
efficiency of virus transduction according to the manufacturer's
protocol.
Viral packaging
Lentiviral vectors were produced by the transient
transfection of 293T cells, as described previously (16). The 293FT cells (~5×106
cells) in logarithmic growth phase were inoculated into 10 cm
culture dishes and cultured for 24 h in a humidified 5%
CO2 atmosphere at 37°C. The vectors were subsequently
transfected into the 293FT cells using Lipofectamine®
2000 and incubated overnight under the same conditions. The
following day, DMEM containing 10% FBS was changed and the viral
supernatants were collected following 48 h under the same
conditions, filtered using 0.45 µm pore size filters and stored at
−80°C. For the determination of infectious titers, 293FT cells were
infected with lentivirus (CyPA shRNA and Control shRNA) (dilution,
1:10) and incubated overnight at 37°C with 5% CO2. The
cells were subsequently washed in PBS and cultured for an
additional 48 h under the same conditions. GFP-positive cells were
counted using a BD FACSVerse™ flow cytometer and BD FACSuite
software (version 1.0) (both BD Biosciences, Franklin Lakes, NJ,
USA).
Transduction of PAa lung
adenocarcinoma cells
PAa lung adenocarcinoma cells were inoculated into
6-well plates (1×105 cells/well) and divided into three
groups, including blank (no transfection), negative control
(transduction of the pLLU2G-eGFP plasmid) and CyPA-siRNA
(pLLU2G-CyPA-EGFP). Three replicates were performed for each group.
GFP expression was detected via fluorescence microscopy (Nikon
Corporation, Tokyo, Japan) to determine the infection efficiency.
The protein expression of CyPA was detected by western blot
analysis.
Western blot analysis of CyPA
Total cellular protein was extracted using an M-PER
Mammalian protein extraction kit (Thermo Fisher Scientific, Inc.).
Total protein (25 µg) was then separated by SDS-PAGE on a 15% gel
and transferred to a polyvinylidene difluoride membrane. The
membrane was blocked with 5% non-fat milk in Tris-buffered saline
with Tween 20 (TBST) for 1 h at 4°C, and incubated overnight at 4°C
with the primary antibody directed against CyPA (1:1,000 dilution;
cat. no. ab126738) or β-actin (1:2,000 dilution; cat. no. AM1021B)
(both Abgent Biotech Co., Ltd.). Following washing 3 times with
TBST, the membrane was incubated with a corresponding horseradish
peroxidase-conjugated goat anti-rabbit IgG (1: 7,500 dilution; cat.
no. 111-035-144; Jackson ImmunoResearch, Inc., Chicago, USA) for 1
h at 4°C. Protein bands were detected using the Pierce ECL Western
Blotting Substrate Kit (Thermo Fisher, Scientific, Inc.), followed
by exposing the membrane to Hyperfilm on the Kodak Digital Science™
Image Station 440CF (both Kodak, Rochester, NY, USA). This analysis
was repeated 3 times. Quantitative analysis of CyPA protein
expression was conducted using ImageJ software (version 1.38e;
National Institutes of Health, Bethesda, MD, USA) (n=3; mean ±
standard deviation).
Clonogenic survival assay
Lung adenocarcinoma cell suspensions in the
logarithmic growth phase were inoculated into 25 mm2
culture bottles at various densities (600, 1,000, 4,000, 8,000 and
10,000 cells). The blank group, negative control group and
CyPA-siRNA group were each irradiated with doses of 0, 2, 4, 6 and
8 Gy (dose rate, 1 Gy/min) at the 60Co Radiation Center
of Beijing Normal University (Beijing, China). The experiments were
repeated three times and duplicates were set up for each group.
Following irradiation, the cells were placed in a
37°C incubator and cultured for 10 days to form clones. The
colonies were fixed by treatment with 100% methanol for 5 min and
subsequently stained with 0.5% crystal violet for 5 min at room
temperature. Colonies of PAa cells exposed to various radiation
doses in each group were counted using Gel-Pro Analyzer software
(version 4.0; Media Cybernetics, Inc., Rockville, MD, USA).
Colonies with >50 cells were counted as colony-forming units.
The clone formation rate (%) was calculated according to the
following formula: (Number of colonies formed/number of cells
seeded) ×100. The cell survival fraction (SF) was calculated as
follows: (Number of colonies formed following treatment/number of
cells seeded) × inoculation efficiency. The inoculation (plate)
efficiency was calculated as follows: Number of colonies
counted/number of cells inoculated. The multi-target single-hit
model was used to evaluate the radiation sensitivity of CyPA-siRNA
cells. D0 is the mean lethal dose resulting from the
multi-target model. Dq is defined as the quasithreshold
dose.
Cell cycle and apoptosis analysis by
flow cytometry
Lung adenocarcinoma cells (1.5×106/ml) in
the logarithmic growth phase were suspended in RPMI-1640 medium
supplemented with 10% fetal calf serum (Sigma-Aldrich; Merck
Millipore) and inoculated into 25 mm2 culture bottles. A
total of three groups were set up as aforementioned. Following
adherent growth for 24 h, the cells were exposed to 60Co
radiation of 0 or 2 Gy. Following radiation, the cells were
continuously cultured for 24 h at 37°C. For each radiation dose, 3
parallel experimental bottles were established and the experiment
was repeated 3 times.
The cells were harvested 24 h following radiation
and centrifuged at 200 × g for 5 min at room temperature.
The supernatant was discarded, the cells were washed with PBS twice
and then fixed with cold anhydrous ethanol at 4°C for 12 h. Cells
were then filtered with 400 mesh nylon filters and washed with PBS
twice. Finally, the cells were incubated with 10 ng/ml RNase A and
500 µg/ml propidium iodide on ice for 30 min, and analyzed with the
FACScan™ System (BD Biosciences). Cell cycle phases were analyzed
using CellQuest™ software (version 5.7; BD Biosciences).
Statistical analysis
Statistically significant differences between the
groups were determined using the Student's t-test (two groups) or
one-way analysis of variance, followed by a post hoc Dunnett's T3
test (multiple groups). The data were analyzed using SPSS version
20.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression analysis of CyPA in stably
transfected cell lines
To silence the CyPA gene, CyPA-specific shRNA
plasmids were constructed and transfected into PAa cells. A total
of four plasmids, termed shRNA-1, shRNA-2, shRNA-3 and shRNA-4,
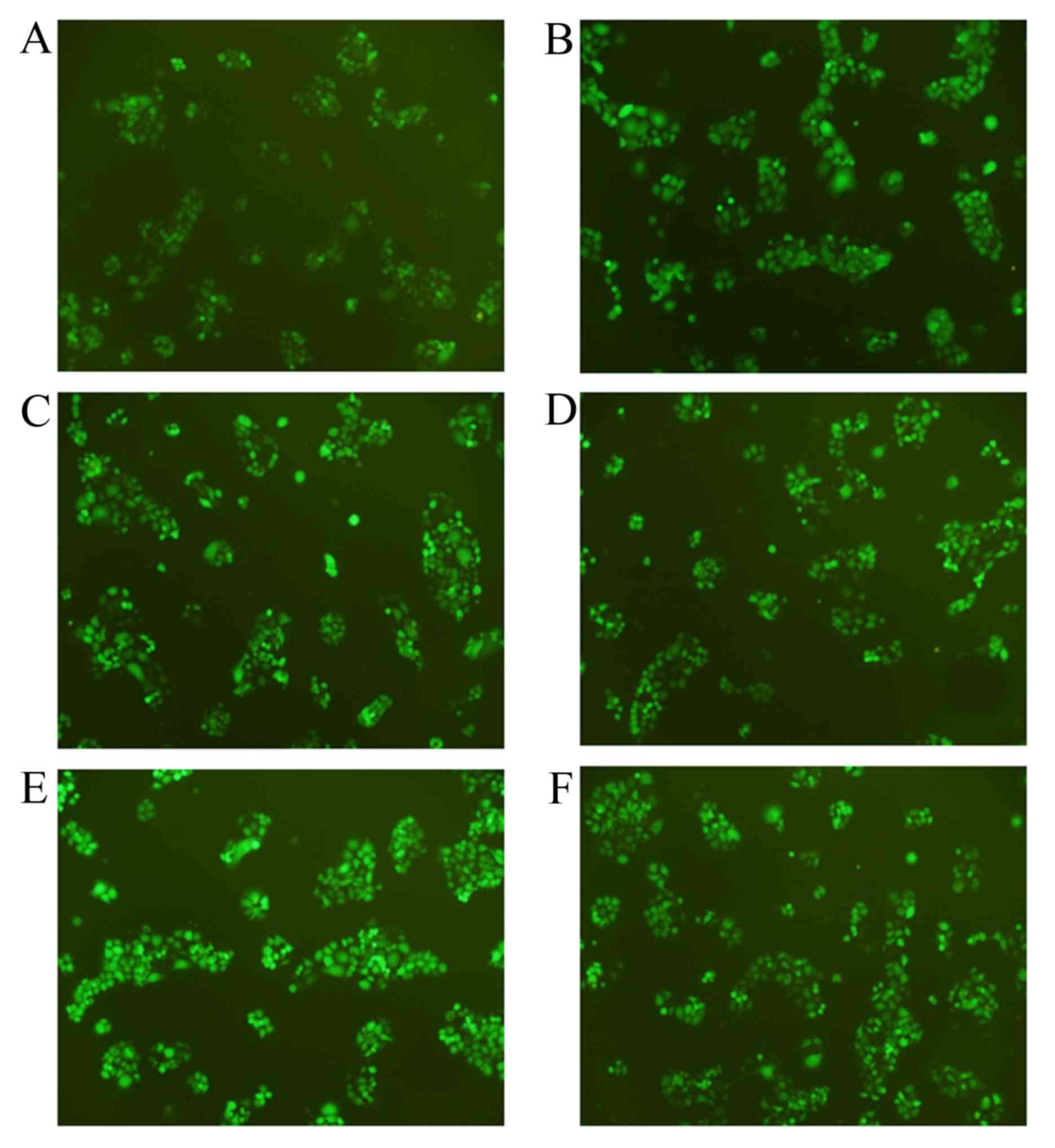
were used. Fig. 1 presents the stable
transfected cell lines under fluorescence microscopy, with a
relatively high transfection efficiency being achieved.
To further determine the silencing of the CyPA gene,
western blot analysis of protein expression in the stable
transfected PAa cell lines was performed. β-actin was used as the
internal control. As presented in Fig.
2A, the expression of CyPA was low or undetectable in the cell
lines transfected with CyPA-shRNA-1, −2 and −3 compared with the
blank group, indicating an effective knockdown of the CyPA gene.
CyPA expression was then normalized to the internal control and the
relative expression levels were quantitatively analyzed (Fig. 2B). The protein expression of CyPA in
the cell line transfected with CyPA-shRNA-1, −2 and −3 was
significantly lower, compared with the blank group without
transfection (CyPA-shRNA-1, P=0.0085; CyPA-shRNA-2, P=0.035;
CyPA-shRNA-3, P=0.024). There was no significant difference between
the CyPA-shRNA-4 group and the blank group (P=0.257). Among the
three effective shRNA vectors (shRNA-1, −2 and −3), shRNA-3
resulted in relatively higher transfection efficiency and lower
CyPA expression levels (Fig. 2B). In
the shRNA-3 group, CyPA protein expression was knocked down by 85%
compared with the blank group. Therefore, the cell line transfected
with the CyPA shRNA-3 vector was utilized for the subsequent
experiments.
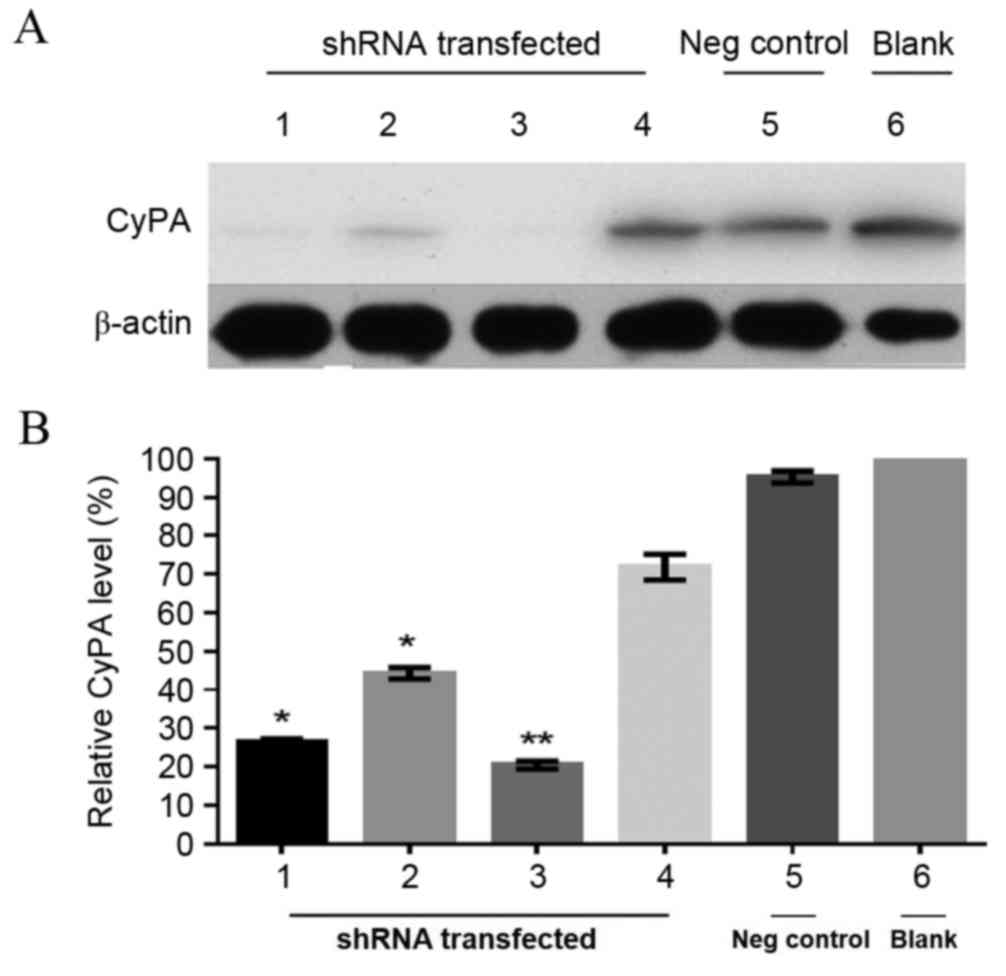 | Figure 2.Knockdown of CyPA expression in the
transfected PAa cell line. (A) Representative western blot of CyPA
expression levels in PAa cell lines with and without CyPA-shRNA
transfection. β-actin was used as an internal loading control. (B)
Relative expression of CyPA. Protein levels of CyPA were normalized
to β-actin and the data were expressed as the percentage of the
blank control. N=3 (mean ± standard deviation). *P<0.05 and
**P<0.01, compared with the blank group. Lane 1, CyPA-shRNA-1;
lane 2, CyPA-shRNA-2; lane 3, CyPA-shRNA-3; lane 4, CyPA-shRNA-4;
lane 5, Negative control (Transfection with the vector without CyPA
shRNA); lane 6, Blank (PAa cell line without transfection). PAa,
cyclophilin A-silencing lung adenocarcinoma; CyPA, cyclophilin A;
shRNA, small hairpin RNA; Neg, negative. |
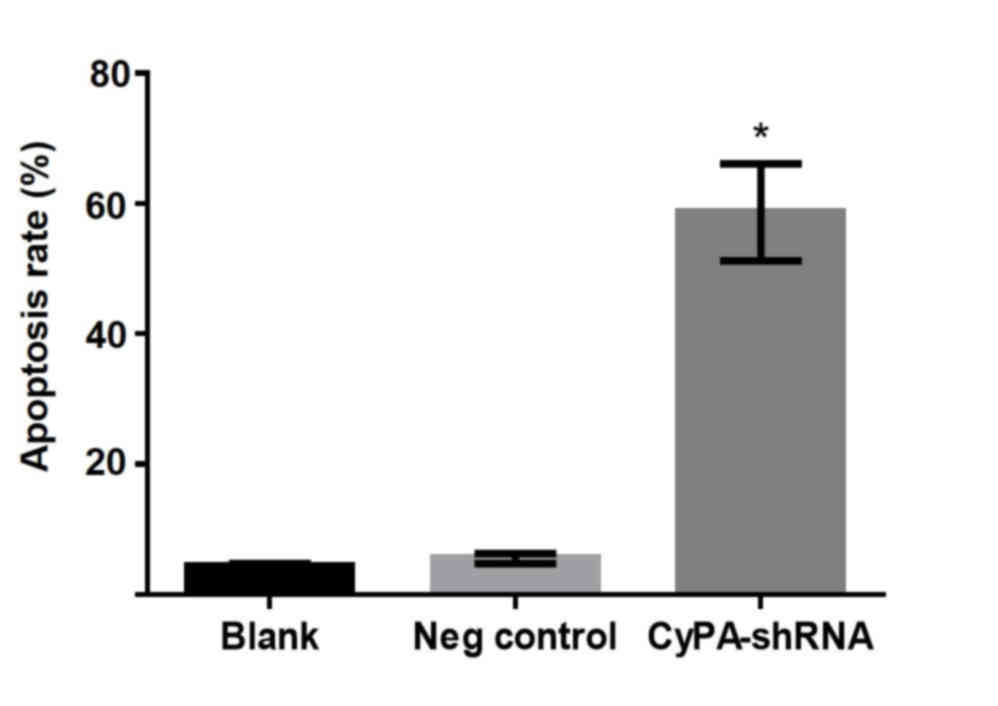
Silencing of CyPA gene significantly
enhances the apoptosis of PAa cells
To investigate the effect of CyPA silencing on lung
cell growth without radiation, the in vitro apoptosis of PAa
cells treated with CyPA shRNA-3 was analyzed using flow cytometry.
During the culture period, few apoptotic cells (<10%) were
present in the blank and negative control groups (Fig. 3). By contrast, a significant increase
in apoptotic damage was observed in CyPA-silenced cells, with an
average cell apoptosis rate of 55.5% (P=0.0145).
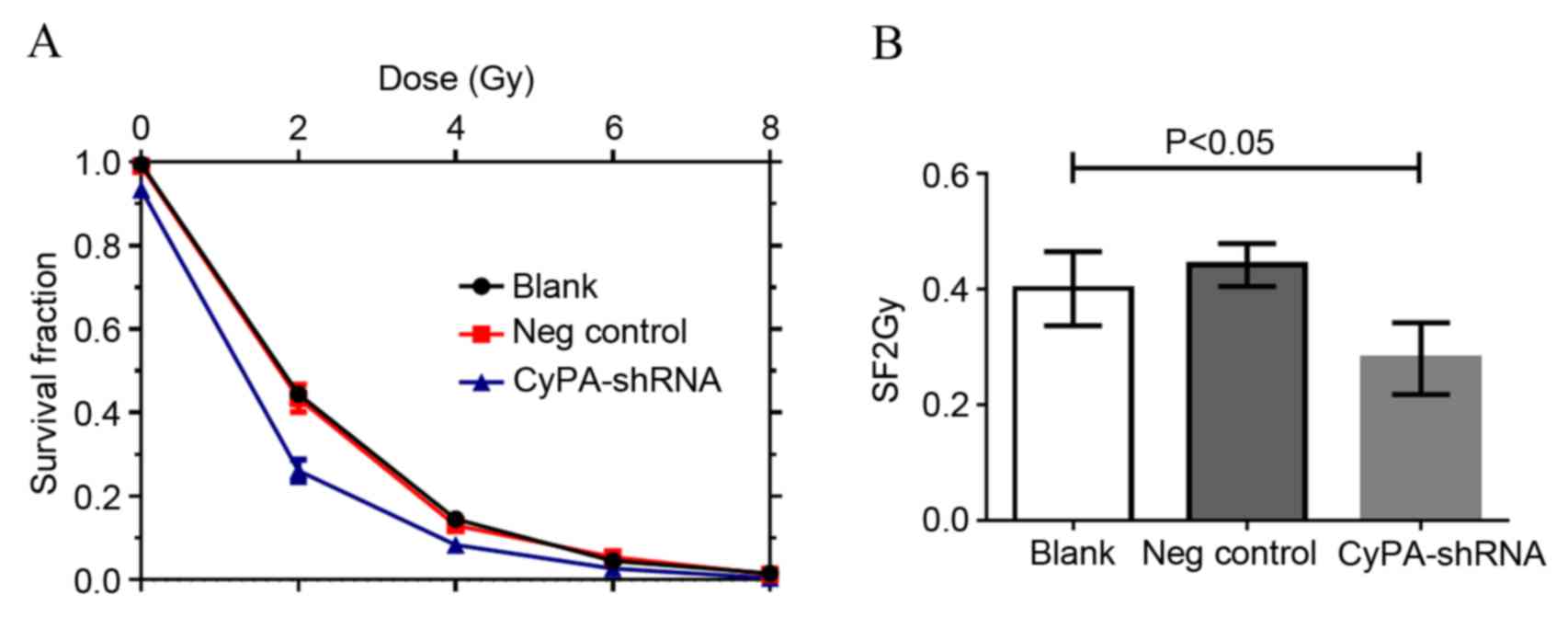
CyPA gene silencing enhances the
radiosensitivity of PAa cells
The effect of CyPA silencing on the sensitivity of
PAa cells to radiation was subsequently assessed using a clonogenic
cell survival assay. Irradiation resulted in a marked
dose-dependent apoptotic effect in PAa cells (Fig. 4). The number of colonies decreased
with the increase in radiation dose for all three groups. The
CyPA-silenced cell line exhibited a significantly decreased
survival fraction compared with the blank group at 2 Gy, indicating
greater radiosensitivity (P=0.0308; Fig.
4B). Multi-target single-hit model fitting survival curves
provided a sensitization enhancement ratio of 1.157±0.005.
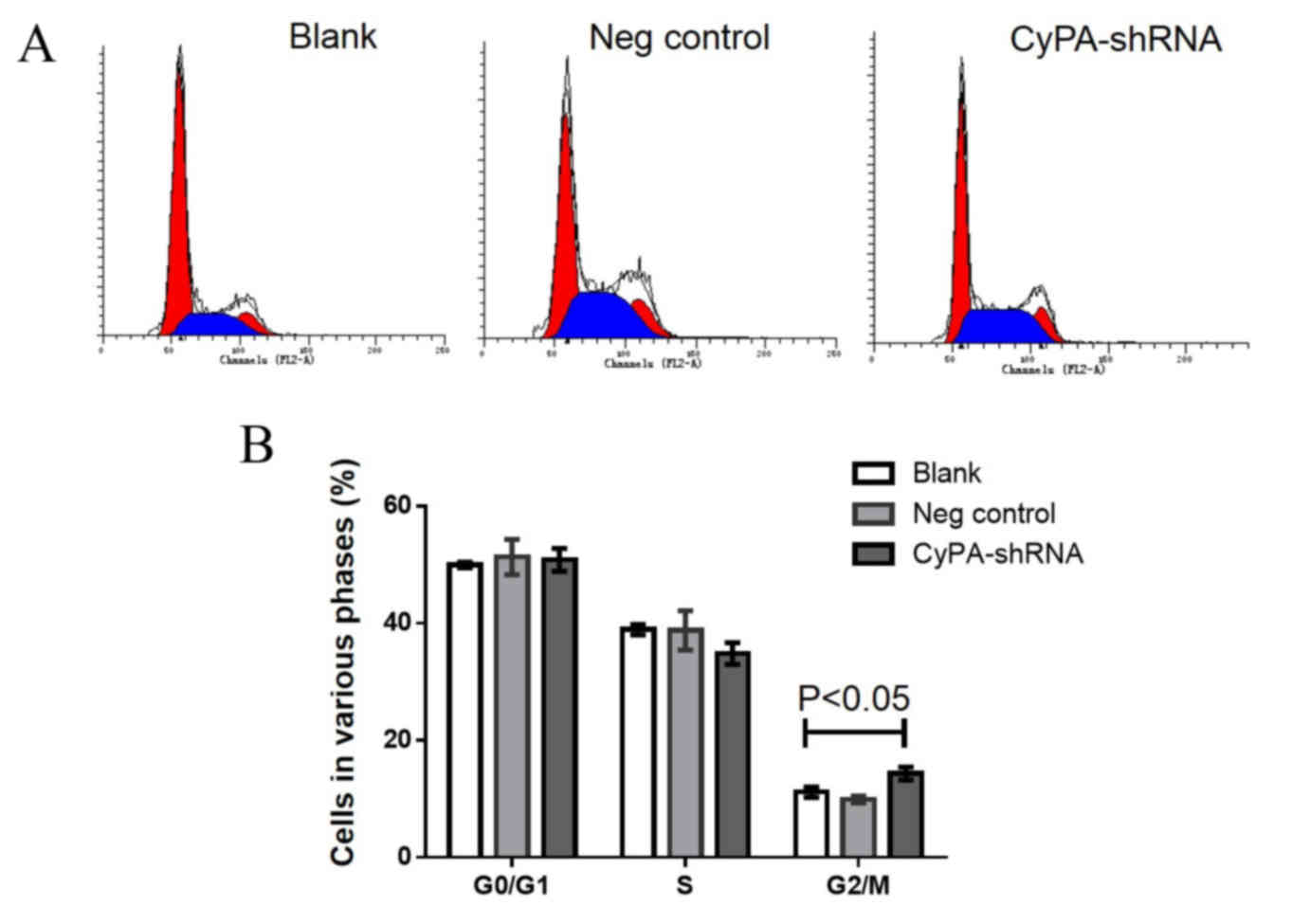
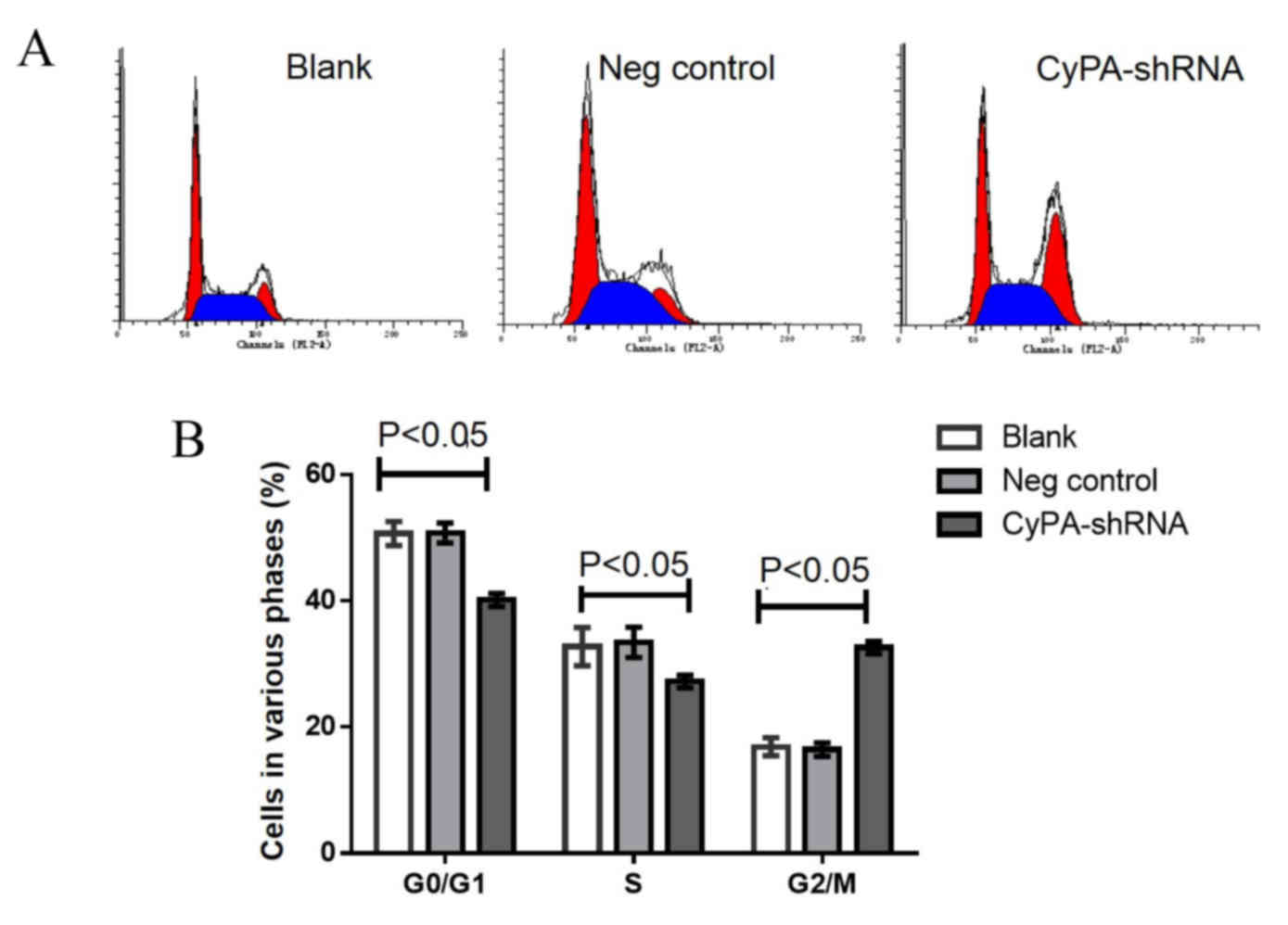
Increased cell cycle arrest at the
G2/M phase occurs in CyPA-silenced PAa cells prior to
and following radiation
Cell cycle analysis using flow cytometry was
performed to determine whether CyPA gene silencing affects the cell
cycle distribution of PAa cells prior to radiation. The
representative cell cycle distribution is presented in Fig. 5A. No significant difference was
observed in G0/G1 and S phase cells among the
three groups (Fig. 5B). However, the
number of cells at the G2/M phase was significantly
increased in the CyPA-silenced group, compared with the control
group (P=0.035).
To investigate whether CyPA silencing has a greater
effect on the cell cycle distribution of PAa cells post-radiation,
the cell cycle distribution was analyzed following irradiation with
60Co at 2 Gy. Significantly fewer cells were at the
G0/G1 and S phase in the CyPA-silenced group,
compared with the blank and control groups (all P<0.05; Fig. 6). Following radiation at 2 Gy, the
percentage of G2/M phase cells in the CyPA-silenced
group was 32.15±0.86, whereas only 18.37±0.15% of blank group cells
were in the G2/M phase.
Discussion
Since the development of RNAi in animals,
personalized gene therapy utilizing this technique has been
evaluated in various human diseases, with >2,000 clinical trials
implementing RNAi-mediated gene therapy completed or ongoing
worldwide by 2013 (17). During these
trials, engineered viruses were often exploited to efficiently
deliver therapeutic genes to the target cells (17). In the present study, the selection of
shRNA sequences was evaluated based on the effective silencing of
the CyPA gene and transduction efficiency in PAa cells, and CyPA
shRNA-3 was selected.
CyPA is important during the pathogenesis of cancer,
functioning as a ‘molecular switch’ due to its PPIase activity
(18). Previous studies have
demonstrated aberrant CyPA overexpression in numerous types of
cancer cell (19–21). During the present study, a stable
CyPA-shRNA knockdown PAa cell line was initially developed to
investigate the role of CyPA gene in lung adenocarcinoma. It was
observed that CyPA knockdown significantly enhanced the apoptosis
of PAa cells. This is concordant with the results of a previous
report, demonstrating that CyPA is an essential promoter for tumor
growth (13). These findings suggest
that CyPA may be a potential novel target for the treatment of lung
adenocarcinoma.
Radiotherapy is a common therapeutic choice for lung
adenocarcinoma (4).
Gene-radiotherapy, the combination of traditional radiation and
targeted gene therapy is a recent breakthrough in cancer treatment
(22). In a previous study, proteomic
analysis of pulmonary adenocarcinoma demonstrated an upregulation
of CyPA following radiotherapy (14).
In the present study, the effect of CyPA knockdown on the
radiosensitivity of PAa cells was investigated using
fluorescence-activated cell sorting analysis and the multi-target
single-hit mathematical model. It was observed that the survival
fraction at 2 Gy (SF2), D0 and Dq decreased following
CyPA-silencing. Furthermore, the Dq sensitization enhancement ratio
was >1. It is known that a decrease of D0 is
associated with an enhanced radiosensitizing effect, whereas a
decrease in Dq is associated with weaker cellular repair following
sublethal injury (23), and the SF2
value is negatively correlated with radiosensitivity (24). The aforementioned results, therefore,
demonstrate that RNAi-mediated gene silencing of CyPA reduced
sublethal repair injury and improved the radiosensitivity of human
PAa lung adenocarcinoma cells. To the best of our knowledge, the
present study is the first to demonstrate that silencing of CyPA
leads to a significant increase in radiosensitivity in lung
adenocarcinoma cells.
The radiosensitivity of tumor cells is closely
associated with the distribution of cells within the proliferation
cycle. Cells in the G2/M phase exhibit the highest
radiosensitivity, followed by the G0/G1
phase, with the S phase being the least radiosensitive stage
(25). In the current study, an
increase in the number of cells in the G2/M phase in the
CyPA-silenced groups, compared with the blank and negative control
groups, was observed. Furthermore, CyPA silencing was demonstrated
to synergize with radiation, leading to marked G2/M
phase arrest in PAa cells and a significant reduction in the
proportion of cells in the G0/G1 and S phase.
Taken together, these results indicate that CyPA silencing is able
to elevate the radiosensitivity of PAa cells via G2/M
phase arrest and the promotion of cell apoptosis.
A previous report demonstrated that the
overexpression of CyPA in cancer cells may prevent hypoxia and
anticancer drug-induced cell apoptosis through the upregulation of
the extracellular signal-regulated kinase (ERK)1/2 signaling
pathway (18). In addition,
disruption of the ERK1/2 signaling pathway sensitizes prostate
cancer cells to radiation (26).
Therefore, in the present study, it is likely that he silencing of
CyPA disturbed the ERK1/2 signaling cascade, thus, resulting in an
increase in PAa cell radiosensitivity.
In conclusion, the current study demonstrated that
lentiviral vectors are able to specifically silence the CyPA gene
and increase cell apoptosis. In addition, RNAi-mediated
downregulation of CyPA enhanced the radiosensitivity of lung
adenocarcinoma cells, and CyPA silencing also significantly induced
G2/M phase arrest. These data provide compelling
evidence that the combination of CyPA gene silencing and
irradiation may represent a novel potential strategy for the
treatment of lung adenocarcinoma.
Acknowledgements
The present study was supported by grants from The
National Key Research and Development Program of China (grant no.
2016YFC0100105), the National Science Foundation of China (grant
nos. 81628008, 81571641 and 81072925) and an internal grant from
the China-Japan Friendship Hospital (grant no. 2014-3-MS-18).
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Houston KA, Henley SJ, Li J, White MC and
Richards TB: Patterns in lung cancer incidence rates and trends by
histologic type in the United States, 2004–2009. Lung Cancer.
86:22–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baker S, Dahele M, Lagerwaard FJ and Senan
S: A critical review of recent developments in radiotherapy for
non-small cell lung cancer. Radiat Oncol. 11:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomez-Casal R, Bhattacharya C, Ganesh N,
Bailey L, Basse P, Gibson M, Epperly M and Levina V: Non-small cell
lung cancer cells survived ionizing radiation treatment display
cancer stem cell and epithelial-mesenchymal transition phenotypes.
Mol Cancer. 12:942013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma W, Ma CN, Li XD and Zhang YJ: Examining
the effect of gene reduction in miR-95 and enhanced
radiosensitivity in non-small cell lung cancer. Cancer Gene Ther.
23:66–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torres-Roca JF and Stevens CW: Predicting
response to clinical radiotherapy: Past, present, and future
directions. Cancer Control. 15:151–156. 2008.PubMed/NCBI
|
|
8
|
Thapar R: Roles of Prolyl Isomerases in
RNA-mediated gene expression. Biomolecules. 5:974–999. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nigro P, Pompilio G and Capogrossi MC:
Cyclophilin A: A key player for human disease. Cell Death Dis.
4:e8882013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng W, Xin Y, Xiao Y, Li W and Sun D:
Cyclophilin A enhances cell proliferation and xenografted tumor
growth of early gastric cancer. Dig Dis Sci. 60:2700–2711. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campa MJ, Wang MZ, Howard B, Fitzgerald MC
and Patz EJ Jr: Protein expression profiling identifies macrophage
migration inhibitory factor and cyclophilin a as potential
molecular targets in non-small cell lung cancer. Cancer Res.
63:1652–1656. 2003.PubMed/NCBI
|
|
12
|
Qian Z, Zhao X, Jiang M, Jia W, Zhang C,
Wang Y, Li B and Yue W: Downregulation of cyclophilin A by siRNA
diminishes non-small cell lung cancer cell growth and metastasis
via the regulation of matrix metallopeptidase 9. BMC Cancer.
12:4422012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Howard BA, Furumai R, Campa MJ, Rabbani
ZN, Vujaskovic Z, Wang XF and Patz EF Jr: Stable RNA
interference-mediated suppression of cyclophilin A diminishes
non-small-cell lung tumor growth in vivo. Cancer Res. 65:8853–8860.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang JC, Zhao PC, Zhang HZ and Wang H: A
proteomical study on the radiosensitized target molecules of
fuzheng zengxiao formula in pulmonary adenocarcinoma nude mice
model. J Tradit Chin Med. 31:3–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Joseph G, Pollok K, Berthoux L,
Sastry L, Luban J and Cornetta K: G2 cell cycle arrest and
cyclophilin A in lentiviral gene transfer. Mol Ther. 14:546–554.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dull T, Zufferey R, Kelly M, Mandel RJ,
Nguyen M, Trono D and Naldini L: A third-generation lentivirus
vector with a conditional packaging system. J Virol. 72:8463–8471.
1998.PubMed/NCBI
|
|
17
|
Sampsonas F, Ryan D, McPhillips D and
Breen DP: Molecular testing and personalized treatment of lung
cancer. Curr Mol Pharmacol. 7:22–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brazin KN, Mallis RJ, Fulton DB and
Andreotti AH: Regulation of the tyrosine kinase Itk by the
peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci USA.
99:1899–1904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi KJ, Piao YJ, Lim MJ, Kim JH, Ha J,
Choe W and Kim SS: Overexpressed cyclophilin A in cancer cells
renders resistance to hypoxia-and cisplatin-induced cell death.
Cancer Res. 67:3654–3662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Guo H, Dong D, Wu H and Li E:
Expression and prognostic relevance of cyclophilin A and matrix
metalloproteinase 9 in esophageal squamous cell carcinoma. Diagn
Pathol. 8:2072013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obchoei S, Weakley SM, Wongkham S,
Wongkham C, Sawanyawisuth K, Yao Q and Chen C: Cyclophilin A
enhances cell proliferation and tumor growth of liver
fluke-associated cholangiocarcinoma. Mol Cancer. 10:1022011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaliberov SA, Kaliberova LN, Yan H, Kapoor
V and Hallahan DE: Retargeted adenoviruses for radiation-guided
gene delivery. Cancer Gene Ther. 23:303–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhuang HQ, Sun J, Yuan ZY, Wang J, Zhao
LJ, Wang P, Ren XB and Wang CL: Radiosensitizing effects of
gefitinib at different administration times in vitro. Cancer Sci.
100:1520–1525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Björk-Eriksson T, West C, Karlsson E and
Mercke C: Tumor radiosensitivity (SF2) is a prognostic factor for
local control in head and neck cancers. Int J Radiat Oncol Biol
Phys. 46:13–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang T, Carraway RE, LaRoche D and
FitzGerald TJ: Disruption of ERK1/2 Sensitizes radiation resistance
prostate cancer cells to paclitaxel and ionizing radiation. Int J
Radiat Oncol Biol Phys. 90:(Suppl). S8062014. View Article : Google Scholar
|