Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequent causes of cancer-associated mortality globally due to a
high incidence and poor prognosis (1). This is particularly high in China due to
the 10% hepatitis B virus infection rate in the general population
(2). The majority of patients with
HCC are diagnosed at intermediate or advanced stages of disease,
excluding them from potentially curative treatment options,
including resection, local ablation or liver transplantation
(3). For intermediate or advanced
HCC, chemotherapy is not routinely used as, in addition to severe
adverse reactions, including myelosuppression, nausea and vomiting,
the response rate is ~10–20% (4).
Therefore, there is an urgent requirement to improve the efficacy
of chemotherapy and simultaneously decrease the associated adverse
reactions.
Traditional Chinese medicine (TCM) emphasizes the
importance of inhibiting tumor growth and alleviating the adverse
reactions of chemotherapy and/or radiotherapy in order to improve
patient quality of life (5). TCM
physicians often use Fuzheng Guben herbs and Qingre Jiedu herbs
during cancer treatment (6). Fuzheng
Guben herbs, including Ganodorma lucidum, Astragalus
membranaceus, Ginseng species and Fructus lycii,
have been observed to strengthen the immune response through the
activation of T and B lymphocytes, macrophages, natural killer (NK)
cells and dendritic cells, and promoting the production of
cytokines, including interleukins (IL), tumor necrosis factors
(TNF) and interferon (7).
Additionally, these herbs have been demonstrated to protect bone
marrow from cyclophosphamide (CTX) and cytosine arabinoside, and
prevent a chemotherapy-induced decrease in white blood cells (WBC),
red blood cells (RBC) and platelets (PLT) in the peripheral blood
(8). According to TCM, heat is
considered an important causative factor in HCC, and Qingre Jiedu
has been administered to clear heat and detoxify the body (6). Previous pharmacological studies have
demonstrated that Qingre Jiedu herbs (including Hedyotis
diffusa Willd, Prunella vulgaris, Lobelia chinensis
Lour and Sophora flavescens) contain anthraquinones,
polysaccharides, flavonoids, alkaloids and triterpenoids that are
able to inhibit tumor cell proliferation, induce cell apoptosis and
suppress angiogenesis (9–11). Matrine from Sophora flavescens
is able to induce the apoptosis of HepG2 cells via the upregulation
of tumor protein 53, B cell lymphoma-2 (Bcl-2)-associated X protein
(Bax) and Fas, and the downregulation of Bcl-2 and c-Myc (12).
Fuzheng Qingjie granules (FZQJ) are composed of
Fuzheng Guben and Qingre Jiedu herbs (13). The four Fuzheng Guben herbs are
Astragalus membranaceus, Ligustrum lucidum,
Ganoderma lucidum and Rhizoma dioscoreae, and the two
Qingre Jiedu herbs are Hedyotis diffusa Willd and
Prunella vulgaris (13). FZQJ
granules are used to treat the symptoms that are commonly observed
post-chemotherapy or radiotherapy, including thirst, night sweats,
constipation, insomnia, loss of appetite and weakness. A previous
study demonstrated that FZQJ may induce apoptosis of HepG2 cells
via activating p38 mitogen-activated protein kinases (MAPKs) and
inducing mitochondria-dependent apoptosis (13).
CTX is biotransformed principally in the liver to
active alkylating metabolites, which cross-link tumor cell DNA
(14) in order to interfere with the
growth of susceptible rapidly proliferating malignant cells. CTX is
used in combination with other antineoplastic drugs to treat a
variety of susceptible malignancies, including lymphoma (15) and myeloma (16), ovarian (17), nasopharyngeal (18) and liver cancer (19). However, CTX is also associated with
severe toxicities, including diarrhea, nausea, vomiting, bone
marrow suppression, haemorrhagic cystitis, fatigue, night sweat,
hair loss, immunosuppression and impaired hepatic and renal
function (20).
The present study investigated whether FZQJ is able
to potentiate the anticancer effects of CTX in hepatoma 22 (H22)
tumor-bearing mice, and potentially alleviate CTX-associated
toxicities.
Materials and methods
Preparation of FZQJ decoction
FZQJ was manufactured and provided by the Department
of Pharmacy, The Second Affiliated Hospital, Fujian University of
Traditional Chinese Medicine (Fuzhou, China). FZQJ granules were
dissolved in distilled water to produce a solution with a final
concentration of 0.3 g/ml, which was stored at 4°C until use.
Mouse xenograft experiments
A total of 50 male specific pathogen free Institute
of Cancer Research mice (6-weeks-old, 22–25 g) were purchased from
Guangdong Animal Center (Guangzhou, China). All animals lived in a
light/dark cycle. Food and tap water were available ad libitum. The
room temperature (RT) was maintained at 23±2°C and humidity was
approximately 60%. The mice were inoculated subcutaneously on the
right side of theirs back with 5×106 H22 cells in
Matrigel/Dulbecco's Modified Eagle's medium with gentamycin (BD
Biosciences, Franklin Lakes, NJ, USA). H22 cells were purchased
from the Shanghai Institute of Life Science (Chinese Academy of
Sciences, Shanghai, China) and cultured in RPMI-1640 culture medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C
with 5% CO2. Mice were randomly divided into 4 groups
(n=10 per group) as follows: Vehicle group (oral distilled water),
CTX group (Baxter Oncology GmbH, Halle, Saxony, Germany,
intraperitoneally; 40 mg/kg/day), FZQJ group (oral; 6 g/kg/day) and
combination group (CTX 40 mg/kg/day intraperitoneally plus FZQJ 6
g/kg/day orally). Mice in the vehicle control group were inoculated
with H22 cells and administered distilled water orally. In
addition, another ten mice without H22 cells served as the blank
group. At day seven, all the mice were sacrificed by cervical
dislocation and peripheral blood was collected by ocular
enucleation. The tumors were dissected by a surgical operation and
weighed.
Thymus index
The thymus was collected from the mice by a surgical
operation and weighed. The thymus index was calculated according to
the following formula: Thymus index (mg/10 g) = thymus weight
(mg)/body weight (g) × 10.
Routine blood analysis
WBC, lymphocyte (LY) and RBC cell numbers as well as
PLT and hemoglobin (Hb) concentrations, were examined with EDTA-K2
anticoagulated whole blood using an automatic hemocytometer (Sysmex
Corporation, Kobe, Japan). This process was performed ≥3 times for
each blood sample.
T and NK immune cells
Anticoagulated whole blood was stained with a
fluorescein isothiocyanate-conjugated cluster of differentiation
(CD) 3 monoclonal antibody (mAb; cat. no. 100203; dilution, 1:200,
BioLegend, Inc., San Diego, CA, USA), in combination with a
phycoerythrin (PE)-conjugated CD8a mAb (cat. no. 100707; dilution,
1:80; BioLegend, Inc.), or PE-conjugated CD4 mAb (cat. no. 100509;
dilution, 1:200; BioLegend, Inc.) or PE-conjugated CD49b mAb (cat.
no., 108907; dilution, 1:80; BioLegend, Inc.), incubated at RT for
15 min in the dark and subsequently lysed using BD FACS™ lysing
solution (BD Biosciences). A FACSCalibur™ flow cytometer with
CellQuest software (version 5.1; BD Biosciences) was utilized to
analyze the percentage of CD3+ T cells, CD4+
T cells, CD8+ T cells and NK cells present. Absolute
counts of CD3+ T cells, CD4+ T cells,
CD8+ T cells and NK cells were calculated according to
the following formula: Absolute cell number = the percentage of
cells × the number of LYs. All experiments were performed ≥3
times.
Cytokine assays
The serum levels of IL-2 and TNF-α in H22
tumor-bearing mice were determined using a γ radioimmunoassay
counter (Dongya Immunological Technique Institute, Beijing, China),
with 125I-IL-2 and 125I-TNF-α
radioimmunoassay kits according to the protocol of the manufacturer
(Dongya Immunological Technique Institute).
Hepatic and renal functions of H22
tumor-bearing mice
To determine the safety of FZQJ, the serum levels of
alanine transaminase (ALT), aspartate aminotransferase (AST), blood
urea nitrogen (BUN) and creatinine (CRE) were assessed according to
the manufacturer's protocol with a FLEX mode automatic biochemical
analyzer (TBA-120FR, Toshiba, Kawasaki, Japan).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
The tumor tissues were fixed in 4% paraformaldehyde
at RT for 36 h. Following dehydration, the fixed tissues were
embedded in paraffin. The samples were sectioned at thickness of 5
µm. Subsequently, the sections were deparaffinized with ≥99% xylene
baths at RT, 10 min each, and then rehydrated in graded ethanol
solutions of 100, 95, 70, and 50% (v/v). The apoptotic cells of the
tumors were detected using a TUNEL assay, according to the
manufacturer's protocol (Fuhzou Maixin Biotech Co., Ltd., Fuzhou,
China). TUNEL-positivity indicates that the cells exhibit the DNA
damage that results from apoptotic cascades (21). These cells possessed a pyknotic
nucleus with dark brown staining, and were counted in 10 random
fields at ×200 magnification. The apoptotic index was reported as
the number of TUNEL-positive cells/total number of cells
scored.
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA). Data were presented as
the mean ± standard deviation. For multiple comparisons, the data
were analyzed using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
FZQJ potentiates the anticancer effect
of CTX
To evaluate whether FZQJ is able to potentiate the
anticancer effect of CTX, the tumor xenograft weight of each mouse
was examined at seven days post-treatment. As presented in Fig. 1A, compared with the vehicle group,
tumor weight significantly decreased in all three groups
(P<0.01). No significant difference was observed between the CTX
group and FZQJ group (P=0.156). Notably, when these drugs were
administered simultaneously, the inhibitory rate was higher,
compared with CTX alone (P=0.027). Therefore, FZQJ may potentiate
the anticancer effect of CTX.
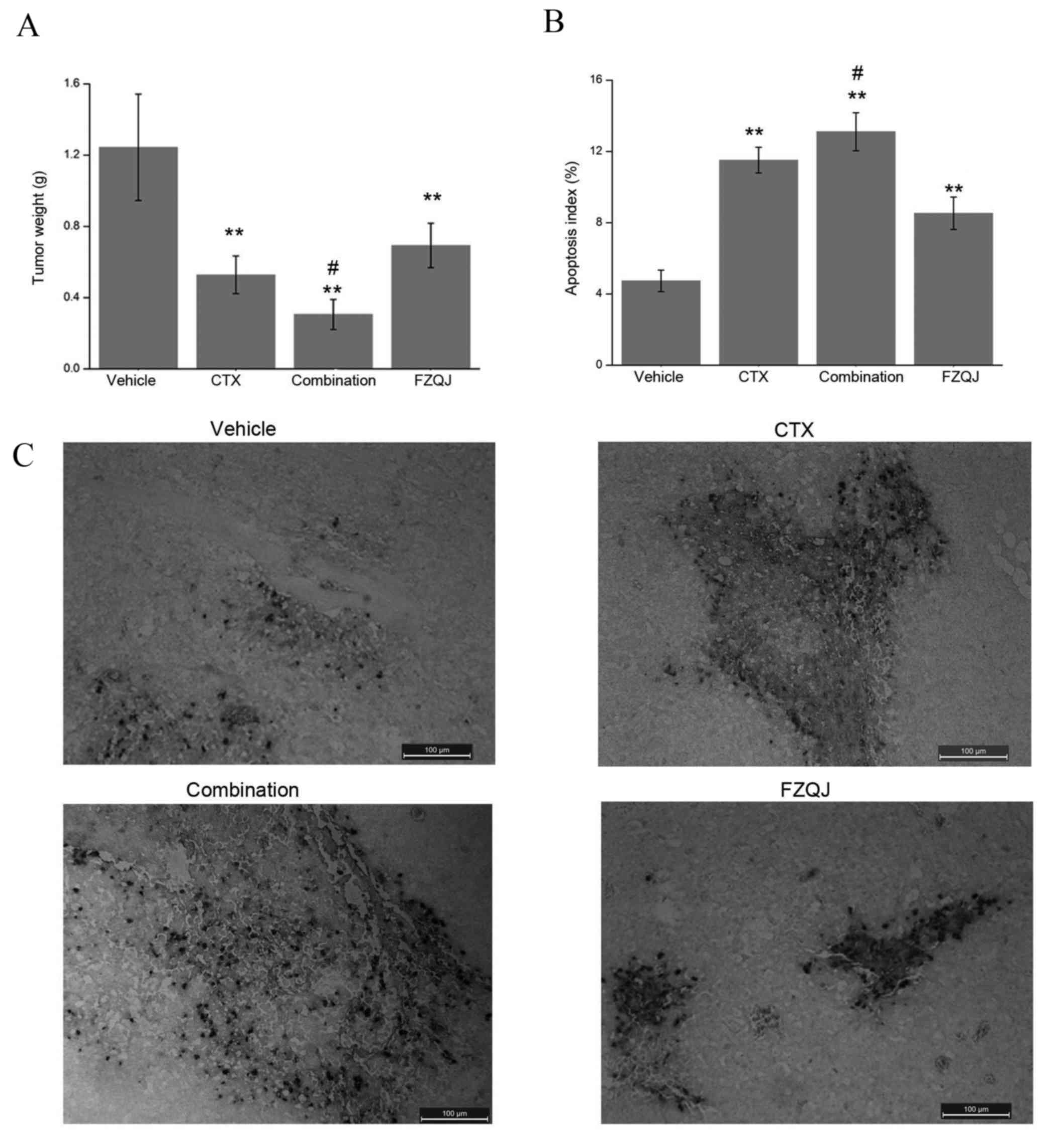 | Figure 1.Comparisons of tumor weight and
apoptosis index. (A) Comparison of tumor weight between the
vehicle, CTX, combination and FZQJ groups. **P<0.01, compared
with the vehicle group; #P<0.05, compared with the
CTX group. (B) Apoptosis index was calculated by dividing the
number of TUNEL-positive cells by the total number of cells in the
field. **P<0.01, compared with the vehicle group;
#P<0.05, compared with the CTX group. (C)
Representative images of TUNEL assays (magnification, × 200; scale
bar, 100 µm). TUNEL-positive cells are stained dark brown. CTX,
cyclophosphamide; FZQJ, Fuzheng Qingjie; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick-end labeling. |
In addition, TUNEL assays were performed in order to
assess the apoptotic cells in H22 neoplastic tissue (Fig. 1B), in which the TUNEL-positive cells
were stained dark brown. The apoptosis index in all three treated
groups was significantly higher, compared with the vehicle group
(P<0.01; Fig. 1C). Compared with
the CTX group, the apoptosis percentage was higher in the
combination group (P=0.049). These data demonstrated that FZQJ may
be able to potentiate the anticancer effect of CTX through the
induction of apoptosis.
FZQJ alleviates CTX-induced peripheral
blood cell and body weight decreases
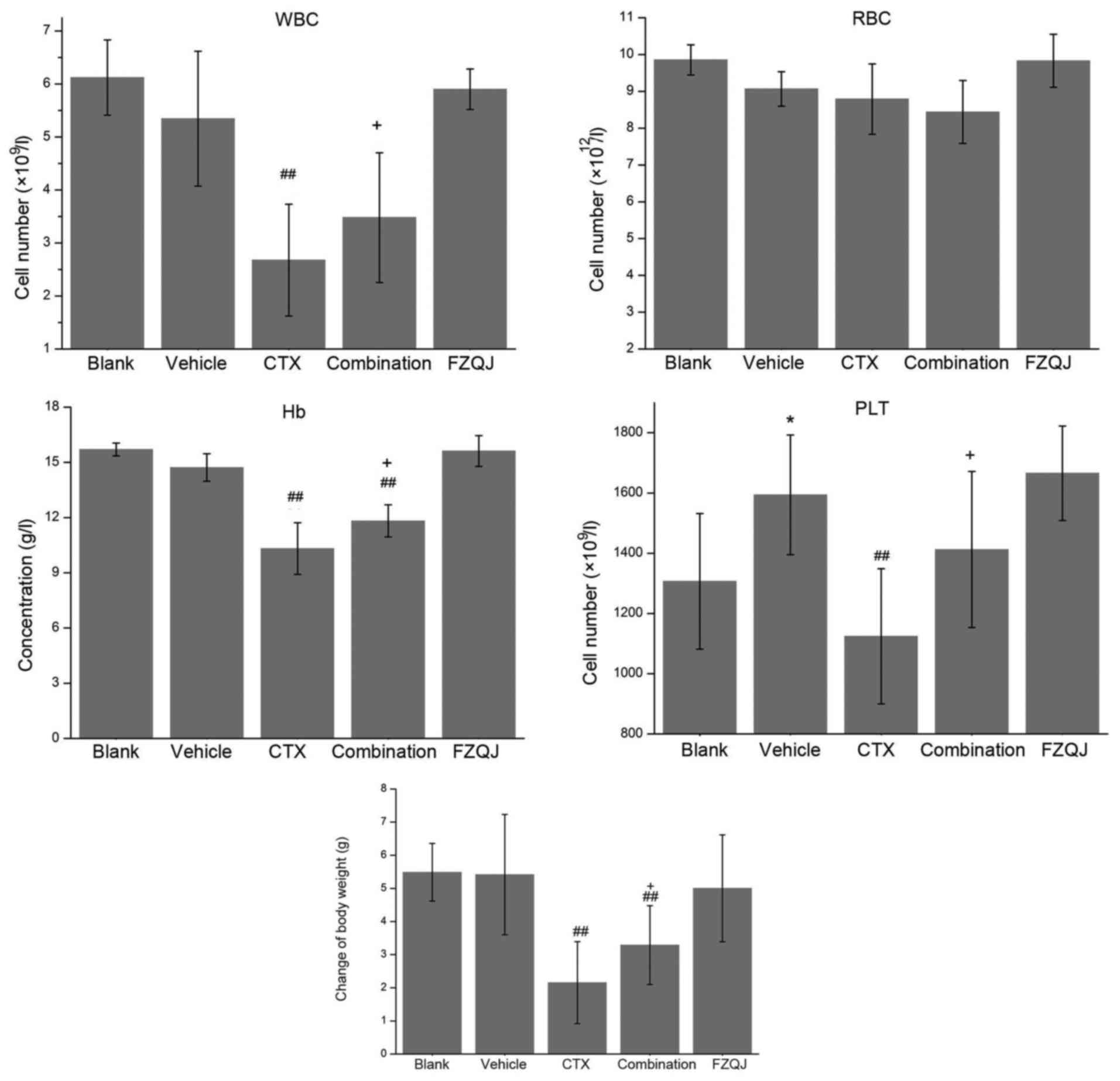
To evaluate whether FZQJ is able to alleviate the
adverse effects of CTX, the numbers of WBCs, RBCs, PLTs, the
concentration of Hb in the peripheral blood and the body weight of
the mice over the course of treatment were examined. As presented
in Fig. 2, PLT counts were higher in
the vehicle group, compared with the blank group (P=0.048). The
levels of WBCs, RBCs, PLTs and Hb were similar between the vehicle
group and the FZQJ group (5.34±1.27) × 109/l vs.
(5.90±0.38) × 109/l, (9.07±0.47) × 1012/l vs.
(9.83±0.72) × 1012/l, (1594.00±198.66) ×
109/l vs. (1665.60±156.87) × 109/l,
14.72±0.74 vs. 15.62±0.83 g/l, respectively, indicating that FZQJ
granules are non-toxic to bone marrow. As hypothesized, CTX
markedly decreased the WBC and PLT counts, as well as the
concentration of Hb, due to its induction of bone marrow
suppression. By contrast, when CTX and FZQJ were simultaneously
administered, these parameters were all significantly improved
(WBCs, P=0.048; PLTs, P=0.047; Hb, P=0.016). Similarly, a
significant difference was not observed with respect to the body
weight of the mice between the vehicle group and the FZQJ group.
And CTX notably induced body weight loss (P<0.01). The
CTX-induced body weight decrease was prevented by FZQJ
(P=0.050).
FZQJ improves immune function in H22
tumor-bearing mice
To evaluate the effect of FZQJ on the immune
function of H22 tumor-bearing mice, the subpopulations of
lymphocyte cells, thymus index, serum IL-2 and TNF-α levels were
examined. As presented in Fig. 3A,
the thymus index in the H22 tumor-bearing mice was significantly
decreased, compared with the blank group (P=0.021). The thymus
index was further decreased when CTX was administered (P=0.000),
whilst FZQJ was able to increase the thymus index of non-treated
(P=0.030) and CTX-treated H22 tumor-bearing mice (P=0.049).
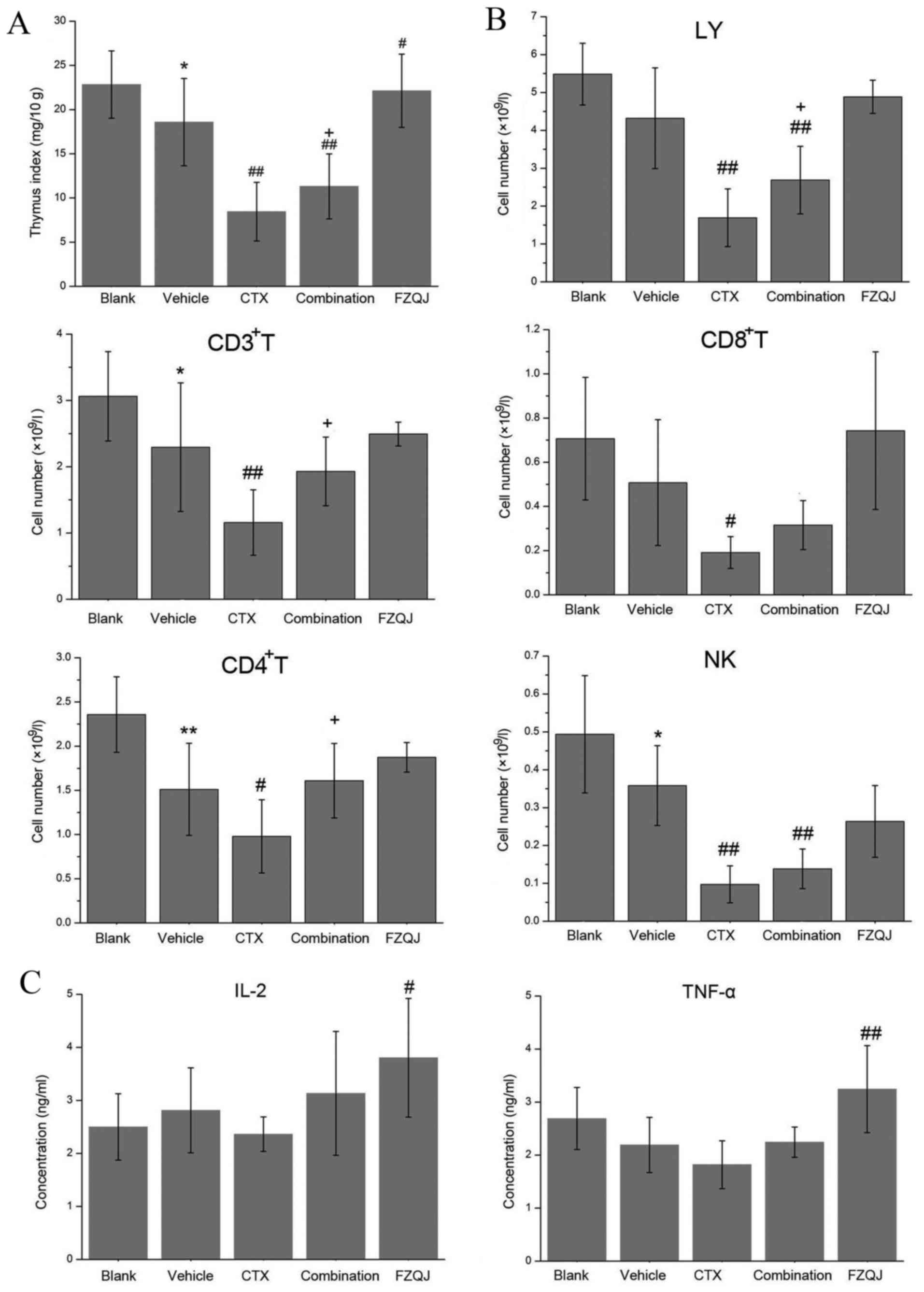 | Figure 3.Comparison of immune function in H22
tumor-bearing mice. (A) Comparison of thymus index. (B) Comparison
of LY, CD3+ T, CD4+ T, CD8+ T and NK cell counts. (C) Comparison of
expression levels of IL-2 and TNF-α in sera as evaluated by a
radioimmunoassay. *P<0.05 and **P<0.01, compared with the
blank group; #P<0.05 and ##P<0.01,
compared with the vehicle group; +P<0.05, compared
with the CTX group. CTX, cyclophosphamide; FZQJ, Fuzheng Qingjie;
LY, lymphocytes; CD, cluster of differentiation; IL, interleukin;
TNF, tumor necrosis factor; NK, natural killer. |
In addition, the absolute counts of CD3+
T, CD4+ T and NK cells in the vehicle group were notably
lower, compared with those in the blank group (P=0.039, 0.004,
0.045 respectively; Fig. 3B), an
effect that tended to be reversed by the administration of FZQJ. As
hypothesized, CTX markedly decreased the numbers of LYs,
CD3+ T, CD4+ T, CD8+ T and NK
cells (Fig. 3B). The addition of FZQJ
to CTX increased the numbers of LYs, CD3+ T and
CD4+ T cells. As presented in Fig. 3C, it was observed that the levels of
IL-2 and TNF-α were highest in the FZQJ groups (P=0.049 and 0.006
respectively, compared with the vehicle group), and IL-2 and TNF-α
serve a key role in cellular immunity (22–23).
Taken together, these data demonstrate that CTX is
able to impede cellular immune function, and that FZQJ granules are
able to prevent CTX-induced immune suppression in H22 tumor-bearing
mice.
FZQJ exhibits no hepatic and renal
toxicity
As presented in Table
I, similar levels of ALT, BUN and CRE were observed between the
vehicle group and the FZQJ group. CTX induced a marked increase of
AST as predicted, and FZQJ was not able to prevent the CTX-induced
deterioration of hepatic function. The results demonstrate that
FZQJ was unable to alleviate CTX-induced hepatic injury.
 | Table I.Hepatic and renal functions of
H22-tumor bearing mice. |
Table I.
Hepatic and renal functions of
H22-tumor bearing mice.
| Group | ALT, IU/l | AST, IU/l | BUN, mmol/l | CRE, µmol/l |
|---|
| Normal | 45.25±9.36 | 223.75±23.14 | 7.73±1.52 | 18.53±2.10 |
| Vehicle | 50.20±7.53 | 220.80±30.36 | 7.70±1.23 | 19.38±3.08 |
| CTX | 49.20±9.93 |
429.00±55.84a | 7.48±1.41 | 16.68±2.91 |
| Combination
(CTX+FZQJ) | 53.00±9.56 |
433.75±54.99a | 7.02±0.99 | 18.30±2.79 |
| FZQJ | 52.00±8.37 | 225.00±34.73 | 6.94±1.34 | 17.28±2.05 |
Discussion
FZQJ has previously been used as adjuvant treatment
during chemotherapy for gastrointestinal malignancies (24). The present study demonstrated that
FZQJ is able to potentiate the anticancer efficacy of CTX, and
prevent CTX-induced immune suppression and body weight loss without
overt hepatorenal toxicity in H22 tumor-bearing mice. The
underlying mechanisms for these processes may include FZQJ-induced
tumor cell apoptosis and stimulated IL-2 and TNF-α production to
enhance cellular immune function.
Numerous studies have demonstrated that Fuzhegn
Guben herbs and their ingredients are able to enhance the
anticancer effects of chemotherapy and/or radiotherapy whilst
reducing certain side effects (5,25). For
example, ginsenoside Rg3 combined with CTX decreased cell
susceptibility to drug resistance and improved survival time in
C57/BL6 mice with Lewis lung carcinoma (26). Shenqi Fuzheng injection has also been
demonstrated to improve the immune function of patients with breast
cancer receiving neoadjuvant chemotherapy (27). The present study observed that FZQJ
produced an antineoplastic effect; however, this was less
pronounced, compared with CTX. Notably, FZQJ was able to
significantly potentiate the antineoplastic effect of CTX, with the
absence of associated and overt side effects. The antineoplastic
effect of FZQJ may be associated with the induction of H22 cell
apoptosis. However the mechanism underlying the apoptosis-inducing
action of FZQJ remains to be elucidated. Previous studies have
demonstrated that FZQJ-induced hepatoma cell apoptosis occurs via
the regulation of Bcl-2 and Bax expression in vitro
(13). In the present study, FZQJ was
observed to stimulate IL-2 and TNF-α production. TNF-α is able to
induce mitochondrial-mediated apoptosis via the activation of Bcl-2
family proteins, reactive oxygen species, C-Jun, C-Jun N terminal
kinases and cathepsin B (28–31). IL-2 is able to enhance the antitumor
efficacy of TNF-α, despite being less cytotoxic itself (32). In addition, Hedyotis diffusa
Willd and Prunella vulgaris in FZQJ granules were reported
to induce cell apoptosis via modulation of the IL-6/signal
transducer and activator of transcription 3, MAPK and
mitochondria-dependent signaling pathways (33,34). The
present and previous studies indicated that FZQJ granules were able
to induce H22 cell apoptosis via IL-6/stat 3, MAPK and
mitochondria-dependent pathway as well as stimulating IL-2 and
TNF-α production.
The current study also observed fewer
CD3+ T, CD4+ T and NK cells in the peripheral
blood of the vehicle group, compared with that of the blank group.
The results indicated that cellular immune function was impaired
once the blank mice were inoculated subcutaneously with H22 cells.
Conversely, mice in the vehicle group exhibited a higher PLT count.
This was in accordance with the thrombocytosis observed in patients
with hepatic tumors (35). A possible
underlying mechanism may be associated with tumor cell-stimulated
production of thrombopoietin and IL-6, which promote PLT
proliferation and activation (35,36). When
CTX alone was administrated for seven days continuously, the WBC,
PLT, LY, CD4+ T helper, CD8+ T
cytotoxic/suppressor, CD3+ T and NK cells, concentration
of Hb, thymus index and mouse body weight all decreased, indicating
that CTX induced bone marrow suppression and a gastrointestinal
reaction, which are common symptoms in patients receiving this
treatment. The present study also demonstrated that FZQJ not only
exhibited no toxicity, but that it also significantly increased the
numbers of blood cells, the thymus index and body weight.
Therefore, FZQJ may combat CTX-induced anemia and the decreased
cellular immune function, as well as alleviate certain side effects
of this treatment in the gastrointestinal tract.
Pharmacological studies have demonstrated that
Astragalus membranaceus, Ligustrum lucidum,
Ganoderma lucidum and Rhizoma dioscorea contain
potent immune stimulants, for example polysaccharides, which
trigger the production of numerous cytokines in vivo,
including IL-2, IL-12 and TNF-α, which may activate T and B cells
(37–41) and modulate the balanced association
between Th1 and Th2 cytokines (42,43).
Concordantly, the present study demonstrated that FZQJ was able to
upregulate the expression of IL-2 and TNF-α, which may be
responsible for the observed increase in the T and NK cell counts.
In a previous study, Astragalus membranaceus was able to
increase serum megakaryocyte colony-stimulating activity and
accelerate the recovery of hematopoiesis following bone marrow
suppression in anemic mice, which may provide an explanation for
the improvement of bone marrow suppression observed following FZQJ
administration in CTX-treated mice (44). Finally, Hedyotis diffusa Willd,
a component of FZQJ, was also reported to be capable of protecting
the gastrointestinal mucosa (45),
which may explain the observed improvement in CTX-induced body
weight loss.
In conclusion, FZQJ not only improves the anticancer
efficacy of CTX, but may also alleviate its adverse effects.
Therefore, FZQJ may provide a promising adjuvant treatment during
chemotherapy and/or radiotherapy for HCC.
Acknowledgements
This study was supported by the Natural Science
Foundation of Fujian Province (grant nos. 2015J01689 and
2014J01421).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
Hepatocellular carcinoma
|
|
TCM
|
Traditional Chinese medicine
|
|
FZQJ
|
Fuzheng Qingjie
|
|
CTX
|
cyclophosphamide
|
|
NK cell
|
natural killer cell
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
HRP
|
horseradish peroxidase
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
|
H&E
|
hematoxylin and eosin
|
|
WBC
|
white blood cells
|
|
RBC
|
red blood cells
|
|
PLT
|
platelet
|
|
Hb
|
hemoglobin
|
References
|
1
|
Bosch FX, Ribes J, Cléries R and Díaz M:
Epidemiology of hepatocellular carcinoma. Clin Liver Dis.
9:191–211, v. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka M, Katayama F, Kato H, Tanaka H,
Wang J, Qiao YL and Inoue M: Hepatitis B and C virus infection and
hepatocellular carcinoma in China: A review of epidemiology and
control measures. J Epidemiol. 21:401–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
European Association for the Study of the
Liver; European Organisation for Research and Treatment of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wörns MA, Weinmann A, Schuchmann M and
Galle PR: Systemic therapies in hepatocellular carcinoma. Dig Dis.
27:175–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Li J, Ji Y, An P, Zhang S and Li
Z: Traditional herbal medicine: A review of potential of inhibitory
hepatocellular carcinoma in basic research and clinical trial. Evid
Based Complement Alternat Med. 2013:2689632013.PubMed/NCBI
|
|
7
|
Zhang J and Shan BE: Recent advances on
traditional Chinese medicine in immunoregulation and anticancer
mechanism. Chin J Immunol. 22:385–388. 2006.(In Chinese).
|
|
8
|
Wu QX and Xu ZY: Advances on traditional
Chinese medicine in myelosuppression. Zhejiang J Tradit Chin Med.
45:618–621. 2010.(In Chinese).
|
|
9
|
Shi Y, Wang CH and Gong XG:
Apoptosis-inducing effects of two anthraquinones from Hedyotis
diffusa WILLD. Biol Pharm Bull. 31:1075–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin SY, Shen CY, Jiang JP, Wu LQ, Dai TY,
Qian WB and Meng HT: Apoptosis of multiple myeloid cells induced by
polysaccharides extracts from Hedyotis diffusa and its mechanism.
Zhonghua Xue Ye Xue Za Zhi. 34:337–340. 2013.(In Chinese).
PubMed/NCBI
|
|
11
|
Zhou QX, Liu F, Zhang JS, Lu JG, Gu ZL and
Gu GX: Effects of triterpenic acid from Prunella vulgaris L. on
glycemia and pancreas in rat model of streptozotozin diabetes. Chin
Med J (Engl). 126:1647–1653. 2013.PubMed/NCBI
|
|
12
|
Si WK, Cheng A, Li P, Liu B, Gao LH and
Yao J: Study on apoptosis of human hepatoma cell line HepG2 induced
by matrine. Acta Academiae Medicinae Militaris Tertiae. 23:816–820.
2001.(In Chinese).
|
|
13
|
Chen XZ, Li JN, Zhang YQ, Cao ZY, Liu ZZ,
Wang SQ, Liao LM and Du J: Fuzheng Qingjie recipe induces apoptosis
in HepG2 cells via P38 MAPK activation and the
mitochondria-dependent apoptotic pathway. Mol Med Rep. 9:2381–2387.
2014.PubMed/NCBI
|
|
14
|
Lee CS and Gibson NW: DNA interstrand
cross-links induced by the cyclopropylpyrroloindole antitumor agent
bizelesin are reversible upon exposure to alkali. Biochemistry.
32:9108–9114. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pisani F, Sciuto R, Dessanti ML,
Giannarelli D, Kayal R, Rea S, Marchesi F and Marino M: Long term
efficacy and safety of Fludarabine, Cyclophosphamide and Rituximab
regimen followed by (90)Y-ibritumomab tiuxetan consolidation for
the treatment of relapsed grades 1 and 2 follicular lymphoma. Exp
Hematol Oncol. 4:172015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fosså A, Muer M, Kasper C, Welt A, Seeber
S and Nowrousian MR: Bolus vincristine and epirubicin with
cyclophosphamide and dexamethasone (VECD) as induction and salvage
treatment in multiple myeloma. Leukemia. 12:422–426. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cain JM, Collins C, Petersdorf S, Figge
DC, Tamimi HK, Greer BE and Livingston RB: Phase II study of
high-dose cisplatin, etoposide, and cyclophosphamide for refractory
ovarian cancer. Am J Obstet Gynecol. 174:1688–1694. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsujii H, Kamada T, Tsuji H, Takamura A,
Matsuoka Y, Usubuchi H and Irie G: Improved results in the
treatment of nasopharyngeal carcinoma using combined radiotherapy
and chemotherapy. Cancer. 63:1668–1672. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang TC, Man S, Lee CR, Xu P and Kerbel
RS: Impact of metronomic UFT/cyclophosphamide chemotherapy and
antiangiogenic drug assessed in a new preclinical model of locally
advanced orthotopic hepatocellular carcinoma. Neoplasia.
12:264–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fraiser LH, Kanekal S and Kehrer JP:
Cyclophosphamide toxicity. Characterising and avoiding the problem.
Drugs. 42:781–795. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lozano GM, Bejarano I, Espino J, González
D, Ortiz A, García JF, Rodríguez AB and Pariente JA: Relationship
between caspase activity and apoptotic markers in human sperm in
response to hydrogen peroxide and progesterone. J Reprod Dev.
55:615–621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taniguchi T and Minami Y: The IL-2/IL-2
receptor system: A current overview. Cell. 73:5–8. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao ZY and Lan L: Experience of Professor
Du Jian in fighting against gastrointestinal cancer by integrative
therapy. J Fujian Univ Tradit Chin Med. 21:51–53. 2011.(In
Chinese).
|
|
25
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo- or radio-therapy for cancer. Biosci Trends.
4:297–307. 2010.PubMed/NCBI
|
|
26
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai Z, Wan X, Kang H, Ji Z, Liu L, Liu X,
Song L, Min W and Ma X: Clinical effects of shenqi fuzheng
injection in the neoadjuvant chemotherapy for local advanced breast
cancer and the effects on T-lymphocyte subsets. J Tradit Chin Med.
28:34–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin XM and Ding WX: Death receptor
activation-induced hepatocyte apoptosis and liver injury. Curr Mol
Med. 3:491–508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagai H, Matsumaru K, Feng G and Kaplowitz
N: Reduced glutathione depletion causes necrosis and sensitization
to tumor necrosis factor-alpha induced apoptosis in cultured mouse
hepatocytes. Hepatology. 36:55–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Lo CR and Czaja MJ: NF-kappaB
inhibition sensitizes hepatocytes to TNF-induced apoptosis through
a sustained activation of JNK and c-Jun. Hepatology. 35:772–778.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guicciardi ME, Deussing J, Miyoshi H,
Bronk SF, Svingen PA, Peters C, Kaufmann SH and Gores GJ: Cathepsin
B contributes to TNF-alpha-mediated hepatocyte apoptosis by
promoting mitochondrial release of cytochrome c. J Clin Invest.
106:1127–1137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Owen-Schaub LB, Crump WL III, Morin GI and
Grimm EA: Regulation of lymphocyte tumor necrosis factor receptors
by IL-2. J Immunol. 143:2236–2241. 1989.PubMed/NCBI
|
|
33
|
Lin J, Li Q, Chen H, Lin H, Lai Z and Peng
J: Hedyotis diffusa Willd. extract suppresses proliferation and
induces apoptosis via IL-6-inducible STAT3 pathway inactivation in
human colorectal cancer cells. Oncol Lett. 9:1962–1970.
2015.PubMed/NCBI
|
|
34
|
Woo HJ, do Jun Y, Lee JY, Woo MH, Yang CH
and Kim YH: Apoptogenic activity of
2α,3α-dihydroxyurs-12-ene-28-oic acid from Prunella vulgaris var.
lilacina is mediated via mitochondria-dependent activation of
caspase cascade regulated by Bcl-2 in human acute leukemia Jurkat T
cells. J Ethnopharmacol. 135:626–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carrington PA, Carr TF, Stevens RF and
Evans DI: Thrombocytosis associated with solid tumors in children.
Pediatr Hematol Oncol. 9:289–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mantadakis E, Tsalkidis A and
Chatzimichael A: Thrombocytosis in childhood. Indian Pediatr.
45:669–677. 2008.PubMed/NCBI
|
|
37
|
Yang B, Xiao B and Sun T: Antitumor and
immunomodulatory activity of Astragalus membranaceus
polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol.
62:287–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun Y, Hersh EM, Talpaz M, Lee SL, Wong W,
Loo TL and Mavligit GM: Immune restoration and/or augmentation of
local graft versus host reaction by traditional Chinese medicinal
herbs. Cancer. 52:70–73. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun Y, Hersh EM, Lee SL, McLaughlin M, Loo
TL and Mavligit GM: Preliminary observations on the effects of the
Chinese medicinal herbs Astragalus membranaceus and Ligustrum
lucidum on lymphocyte blastogenic responses. J Biol Response Mod.
2:227–237. 1983.PubMed/NCBI
|
|
40
|
Yue GG, Chan BC, Han XQ, Cheng L, Wong EC,
Leung PC, Fung KP, Ng MC, Fan K, Sze DM and Lau CB:
Immunomodulatory activities of Ganoderma sinense polysaccharides in
human immune cells. Nutr Cancer. 65:765–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kohguchi M, Kunikata T, Watanabe H, Kudo
N, Shibuya T, Ishihara T, Iwaki K, Ikeda M, Fukuda S and Kurimoto
M: Immuno-potentiating effects of the antler-shaped fruiting body
of Ganoderma lucidum (Rokkaku-Reishi). Biosci Biotechnol Biochem.
68:881–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen SM, Tsai YS, Lee SW, Liu YH, Liao SK,
Chang WW and Tsai PJ: Astragalus membranaceus modulates Th1/2
immune balance and activates PPARγ in a murine asthma model.
Biochem Cell Biol. 92:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Shan A, Liu T, Zhang C and Zhang
Z: In vitro immunomodulatory effects of an oleanolic acid-enriched
extract of Ligustrum lucidum fruit (Ligustrum lucidum supercritical
CO2 extract) on piglet immunocytes. Int Immunopharmacol.
14:758–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X and Zhu B: Effect of Astragalus
membranaceus injection on megakaryocyte hematopoiesis in anemic
mice. Hua Xi Yi Ke Da Xue Xue Bao. 32:590–592. 2001.(In Chinese).
PubMed/NCBI
|
|
45
|
Wang GY, Li ZB, Shi JX, Wang H, Gao QF,
Gen LF, Zhu JJ and Zhao QS: Oldenlandia diffusa protects against
indomethacin-induced injury of the gastrointestinal mucosa in rats.
Hebei J Tradit Chin Med. 23:70–71. 2001.(In Chinese).
|