Introduction
Gliomas are the most common type of primary brain
tumor (1,2). The World Health Organization
classification system groups gliomas into four histological grades
as follows: Astrocytomas, oligodendrogliomas, ependymomas and
oligo-astrocytomas (mixed gliomas) (3). Astrocytomas are subdivided as follows:
Pilocytic, grade I; diffuse, grade II; anaplastic, grade III; and
glioblastoma multiforme, grade IV (3). Malignant gliomas account for 80% of all
gliomas and are subcategorized into grade III/IV tumors (4). The incidence of gliomas has increased
from 5.9/100,000 people in 1973 to 6.61/100,000 people in 2016,
primarily due to improved radiological diagnosis (5,6). Despite
combined treatment regimens, however, it remains an incurable
disease and the prognosis is poor (7). Thorough investigation is required to
improve our understanding of its biological characteristics and
identify a novel molecular target for clinical therapy.
In 1993, the identification of a small endogenous
regulatory RNA molecule in Caenorhabditis elegans led to the
description of a family of numerous short single-stranded
ribonucleic acids (between 20 and 22 nucleotides) termed microRNAs
(miRNAs/miRs) (8). These molecules
are critical post-transcriptional regulators of gene expression in
complex organisms. It is not unexpected, therefore, that miRNAs are
themselves tightly regulated to allow for gene expression to be
shaped in a temporally restrained and tissue-specific manner, which
is required for properly structured organismal development and
growth (9–11). miR-548 is a poorly-conserved
primate-specific miRNA gene family, which is involved in the
regulation of the actin cytoskeleton, the mitogen-activated protein
kinase signaling pathway, ubiquitin-mediated proteolysis, glioma,
colorectal cancer and non-small cell lung cancer (12).
Upregulation of miR-548c-3p was also identified in
human embryonic stem cells and in unfractionated
castration-resistant prostate cancer (13). Overexpression of miR-548c-3p was also
reported in the blood of patients with gastric cancer (14). The higher expression of miR-548c-3p
may be more definite in the case of Helicobacter
pylori-negative gastric cancer (15). These results suggested that
miR-548c-3p is involved in tumor progression. In the present study,
miR-548c-3p was identified to be downregulated in glioma tissues
and cell lines; however, the underlying molecular mechanism remains
unclear.
The proto-oncogene c-Myb (c-Myb) codes for a
transcription factor that is a member of the Myb family (16,17). The
transcription factor Myb has a key role in stem and progenitor cell
regulation within the colonic crypts, bone marrow and a neurogenic
region of the adult brain (16). In
various types of human cancer, mutations have been identified in
the Myb gene: Overexpression of c-Myb contributes to transformation
in pediatric T-cell acute lymphocyte leukemia, pancreatic tumors
and colon tumors (18,19). Rearrangements and amplifications of
Myb have been identified in ~25% of diffuse cerebral gliomas
(20). These results suggest that
c-Myb is involved in glioma tumorigenesis.
In the present study, the potential effect of
miR-548c-3p on glioma tumorigenesis was investigated. miR-548c-3p
was identified to be downregulated in human malignant glioma
tissues. Furthermore, c-Myb was identified to be the direct target
of miR-548c-3p and mediated the biological effect of miR-548c-3p.
These results suggest that miR-548c-3p may be important in the
regulation of glioma development and may lead to clinical
applications in the treatment of glioma.
Materials and methods
Cell culture
The human glioma T98G, U87 and U251 cell lines, and
the human embryonic kidney (HEK)-293 cells (CRL-1573), were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and grown in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare,
Life Sciences Logan, UT, USA) at 37°C in a humidified atmosphere
with 5% CO2. Human brain tissues and glioma specimens
were obtained from 11 patients (5 males and 6 females; age, 35–55
years) treated at the Eye Hospital, Wenzhou Medical University
(Wenzhou, China) between September 2012 and July 2013. All patients
provided written informed consent. All studies and procedures
involving human tissue were approved by the Wenzhou Medical
University Institutional Review Board. Patient samples were used in
accordance with The Declaration of Helsinki.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The T98G cells (1×105) were seeded in
6-well plates and total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A 10-ng
sample of total RNA was transcribed into cDNA using a
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), and the miR-548c-3p
expression level was quantified using a TaqMan® MicroRNA
Assay kit (cat. no. 479537_MIR; Ambion; Thermo Fisher Scientific,
Inc.), which included primers for has-miR-548c-3p. All procedures
were performed according to the manufacturer's protocols. The
thermocycling conditions were as follows: 95°C for 5 min, followed
by 40 cycles of 95°C for 5 sec, 58°C for 10 sec and 72°C for 5 sec.
Each sample was analyzed in triplicate. qPCR was performed using a
7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.), the expression of miR-548c-3p was normalized to
the expression of U6 small nuclear RNA, and relative expression
levels were calculated using the 2−ΔΔCq method (21). The expression level of miR-548c-3p in
the human brain was set as the wild-type control. To determine
c-Myb expression in the T98G cells (1×105), the cells
were transfected with miR-548c-3p using 1 µl
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and cultured at 37°C for 24 h. c-Myb expression
levels were then quantified by measuring cyanine dye incorporation
(SYBR Green PCR Master mix; Applied Biosystems; Thermo Fisher
Scientific, Inc.) using the 7500 Real-Time PCR system. The primer
sequences for c-Myb were as follows: Forward,
5′-ACGAGGATGATGAGGACTTTGAG-3′; and reverse,
5′-TTTTCCCCAAGTGACGCTTT-3′.
Cell proliferation and colony
formation assays. The T98G cells were plated at 3×103
cells/well in 96-well plates
All transfections were performed in triplicate.
Cells in each well were transfected with 50 nM miR-548c-3p
precursor molecule (cat. no. MC11455; Ambion; Thermo Fisher
Scientific, Inc.) or a negative control (NC) precursor miRNA (cat.
no. 17110; Ambion; Thermo Fisher Scientific, Inc.). Following 1–4
days of culture in a humidified atmosphere with 5% CO2
at 37°C, cell proliferation was assessed using a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay, a colorimetric method for determining the number of
viable cells, with a CellTiter 96® AQueous One Solution
Cell Proliferation Assay kit (Promega Corp., Madison, WI, USA),
according to the manufacturer's protocol. MTS solution was added to
each well prior to incubation at 37°C for 3 h. Cell proliferation
was assessed by measuring the absorbance at 490 nm using a
microtiter plate reader (Molecular Devices, LLC, Sunnyvale, CA,
USA). The T98G cells were transfected with 50 nM c-Myb-specific
small interfering RNA (siRNA; 50 nM; Ambion; Thermo Fisher
Scientific, Inc.) or NC siRNA (cat. no. 4392420; Ambion; Thermo
Fisher Scientific, Inc.) using Lipofectamine® 2000
reagent. The mock control group was untreated T98G cells cultured
under normal conditions. The MTS assay was performed 3 days after
transfection. To evaluate the colony formation ability, T98G cells
transfected with miR-548c-3p or NC siRNA were seeded in 3.5-cm
plates (1,000 cells/dish). The colonies were fixed with 10%
formalin at room temperature for 30 min, then stained with 0.1%
crystal violet (Sigma; Merck Millipore, Darmstadt, Germany) at room
temperature for 30 min. Colonies with >50 cells were counted
using a light microscope (Carl Zeiss, Axio Observer D1, Germany)
after 6 days.
Flow cytometric analysis of the cell
cycle
The T98G cells were plated into 60-mm dishes and
cultured at 37°C until between 50 and 70% confluence for each
transfection. Each cell line was transfected with 50 nM miR-548c-3p
precursor molecule or NC siRNA. A total of 48 h after transfection,
the cells were collected, washed with phosphate-buffered saline
(PBS) and stained with propidium iodide using the Cycletest™ Plus
DNA kit (BD Biosciences, San Jose, CA, USA) according to the
manufacturer's protocol. Stained cells (1×105) were
analyzed for DNA content using a FACSCalibur flow cytometer with
CellQuest Pro software (version 6.0) (both BD Biosciences).
Bioinformatics prediction and
luciferase reporter assays
TargetScan (www.targetscan.org) was used to predict the direct
targets of miR-548c-3p. The 3′-untranslated region (UTR) of human
c-Myb was amplified from human genomic DNA and individually cloned
into a pMIR-REPORT vector (Ambion; Thermo Fisher Scientific, Inc.)
using directional cloning. Seed regions were mutated to remove all
complementarity to nucleotides 1 to 7 of miR-548c-3p using a
QuikChange XL Site-Directed Mutagenesis kit (Agilent Technologies,
Inc., Santa Clara, CA, USA). The HEK-293 cells were co-transfected
with 0.4 µg firefly luciferase reporter vector and 0.02 µg control
vector containing Renilla luciferase (pRL-SV40 vector) (both
Promega Corp.), together with 50 nM miR-548c-3p precursor molecule
or NC precursor miRNA, using Lipofectamine® 2000 in
24-well plates. Each transfection was performed with four replicate
wells. Luciferase assays were performed 24 h after transfection
using the Dual-Luciferase Reporter Assay system (Promega Corp.)
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to Renilla luciferase activity.
Transwell migration assays
The T98G cells were transfected with 50 nM
miR-548c-3p precursor molecule or NC. After 24 h, the cells were
harvested by trypsinization and washed once with Hanks' balanced
salt solution (Invitrogen; Thermo Fisher Scientific, Inc.).
Transwell culture inserts (pore size, 8-µm; Costar; BD Biosciences)
were placed into the wells of 24-well culture plates, separating
the upper and the lower chambers. DMEM (400 µl) was added in the
lower chamber and 1×105 cells were added to the upper
chamber. A total of 24 h after incubation at 37°C and 5%
CO2, the cells that adhered to the inserts were fixed
with 70% methanol at room temperature for 30 min and then stained
with 0.1% crystal violet for 30 min. The number of cells that had
migrated through the pores was quantified by counting 10
independent visual fields using a light microscope and a ×20
objective.
Western blot analysis
The T98G cells (1×105) were transfected
with the miR-548c-3p precursor molecule or NC. A total of 24 h
after transfection, the cells were washed with ice-cold PBS and
subjected to lysis in a lysis buffer (50 mM Tris-HCl, 1 mM EDTA, 20
g/l SDS, 5 mM dithiothreitol and 10 mM phenylmethylsulfonyl
fluoride). Protein concentration of whole cell lysates was assessed
using the Pierce™ BCA Protein Assay Kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein lysates (50 µg each) were separated by
10% SDS-PAGE, then electrotransferred onto nitrocellulose
membranes. The membranes were blocked with a buffer containing 5%
skimmed milk powder in PBS with 0.05% Tween-20 for 2 h and
incubated overnight with primary antibodies (described below) at
4°C. Antibodies directed against c-Myb (1:1,000; cat. no. 12319),
protein lin-28 homolog B (Lin28b; 1:1,000; cat. no. 4196), Myc
proto-oncogene protein (Myc; 1:1,000; cat. no. 9402) and GAPDH
(1:2,000; cat. no. 2118) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Following a second wash with
PBS containing 0.05% Tween-20, the membranes were incubated at room
temperature for 1 h with horseradish peroxidase-conjugated
anti-rabbit Immunoglobulin G secondary antibody (dilution, 1:4,000;
cat. no. 7074; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
developed with an enhanced chemiluminescence detection kit (Pierce;
Thermo Fisher Scientific, Inc.). GAPDH was used as a loading
control.
Caspase activity assay
Apoptosis in the T98G cells was determined using the
Caspase-Glo® 3/7 assay kit (Promega Corp.) according to
the manufacturer's protocol. The T98G cells were plated in
triplicate in 96-well plates and transfected with miR-548c-3p as
aforementioned. Samples were then incubated at room temperature
with the caspase substrate (provided in the kit) for 2 h followed
by measurement of the optical density at 560 nm using a microtiter
plate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Wound healing assay
A total of 1.5×105 T98G cells/well were
cultured in a 12-well plate for 24 h prior to transfection with
miR-548c-3p or NC. After 2 days, the cells were scratched with a
100-µl pipette tip, washed with Hanks' balanced salt solution three
times, and cultured in serum-free DMEM at 37°C and 5%
CO2. Images were captured 0, 1 and 3 days subsequent to
the wound being made.
Statistical analysis
SPSS software (version 20.0; IBM SPSS, Inc., Armonk,
NY, USA) was used for statistical analysis. Student's t-test was
used to determine the statistical significance of differences
between groups. All results are presented as the mean ± standard
error of the mean from experiments performed at least three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
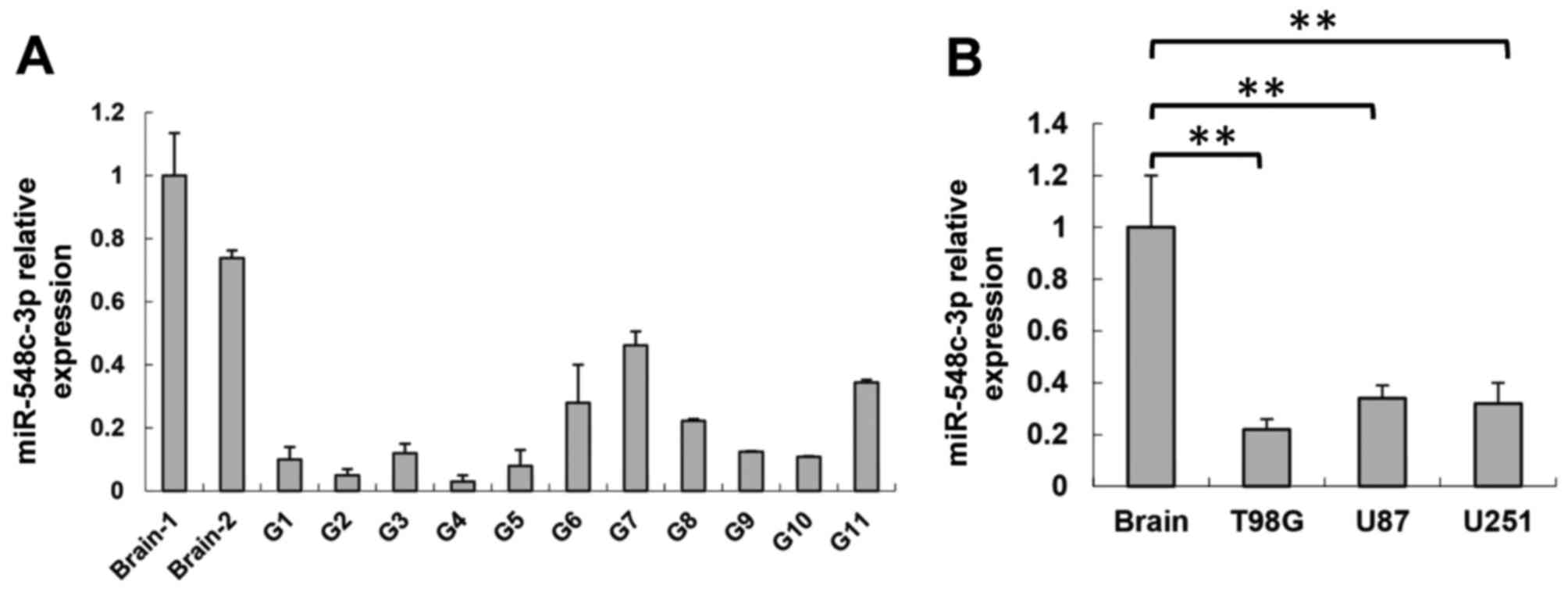
miR-548c-3p is downregulated in glioma
tissues and glioma cell lines
To determine whether miRNA was involved in the
regulation of glioma tumorigenesis, the expression of miR-548c-3p
was determined in glioma and wild-type brain tissues. Total RNA was
extracted and RT-qPCR analysis was performed. miR-548c-3p
expression was decreased significantly in all 11 glioma tissue
samples compared with two wild-type brain samples (Fig. 1A). Consistent with the data from
glioma tissues, miR-548c-3p expression was demonstrated to be
significantly downregulated in the U87, U251 and T98G glioma cell
lines compared with the brain tissue (P<0.01; Fig. 1B). These results indicated that
miR-548c-3p serves certain roles in glioma development.
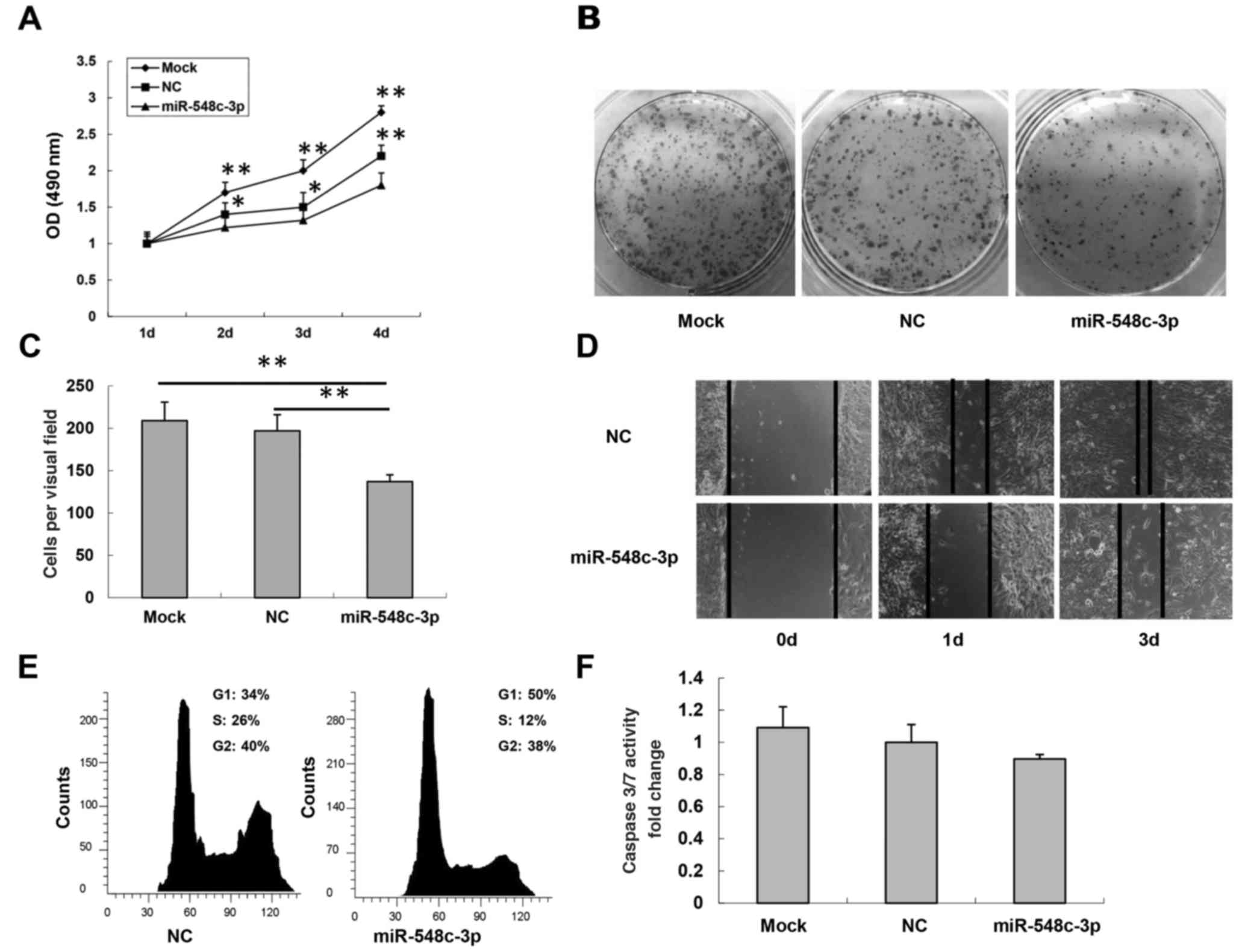
miR-548c-3p inhibits T98G cell
proliferation and migration
As miR-548c-3p expression was downregulated in
glioma, its biological effects on a glioma cell line were
investigated. The T98G cells were transfected with the miR-548c-3p
precursor molecule or NC. An MTS assay was performed to assess
growth inhibition 1, 2, 3 and 4 days after transfection. Transient
transfection of miR-548c-3p into T98G cells caused a significant
inhibition of proliferation at day 4 compared with that of the NC
and mock cells (25±6.2% inhibition; P<0.01; Fig. 2A). To further verify
miR-548c-3p-mediated inhibition of cell proliferation, a colony
formation assay was used to determine visually the effect of
miR-548c-3p transfection on T98G cells (Fig. 2B). Cell migration, a prerequisite for
malignant transformation and metastasis, was assessed using
Transwell migration and wound healing assays. In the Transwell
assay, T98G cells were transfected with either the miR-548c-3p
precursor or an NC precursor. The cells were seeded on culture
inserts, and the ability of cells to migrate to the underside of
the inserts was determined. As presented in Fig. 2C, migration of miR-548c-3p-transfected
cells was significantly decreased compared with mock- and
NC-transfected cells (mock, 205±45; NC, 197±49; miR-548c-3p,
137±38; both P<0.01). The results of the wound healing assay
were consistent with the Transwell migration assay. Migration of
miR-548c-3p-transfected T98G cells was slower than that of
NC-transfected T98G cells 1 and 3 days after transfection (Fig. 2D). Overall, the introduction of
miR-548c-3p resulted in reduced cell motility.
The cell cycle was investigated using flow
cytometry. In T98G cells transfected with miR-548c-3p, 50%
accumulated in the G1 phase compared with 34% of cells
for NC-transfected cells (Fig. 2E).
To further evaluate miR-548c-3p-mediated inhibition of cell
proliferation, caspase activity was investigated to determine the
involvement of apoptosis. No significant difference was observed in
caspase 3/7 activity between miR-548c-3p- and NC-transfected cells
(Fig. 2F). Therefore, these results
indicated that T98G cell growth was inhibited by miR-548c-3p
expression via cell cycle G1 arrest rather than the
induction of apoptosis.
c-Myb is a target of miR-548c-3p
Analysis using TargetScan (www.targetscan.org) was conducted for miR-548c-3p
target prediction to investigate the underlying molecular
mechanisms of miR-548c-3p-mediated cell proliferation and
migration. The potential targets associated with tumorigenesis were
identified as c-Myb, Myc and Lin28b. The effect of miR-548c-3p on
the expression of these genes was investigated, with alteration of
c-Myb being the most marked (Fig.
3A). The potential binding site of miR-548c-3p was predicted in
the 3′-UTR of c-Myb mRNA. Alignment between the predicted
miR-548c-3p target sites and miR-548c-3p, the conserved 7-bp seed
sequence for miR-548c-3p-mRNA pairing, is presented in Fig. 3B. To evaluate the specific regulation
of c-Myb through the predicted binding site, the c-Myb 3′-UTR
sequence was amplified and inserted downstream of the firefly
luciferase coding region of a pMIR-Luc vector. Mutants of the
putative binding site were also prepared.
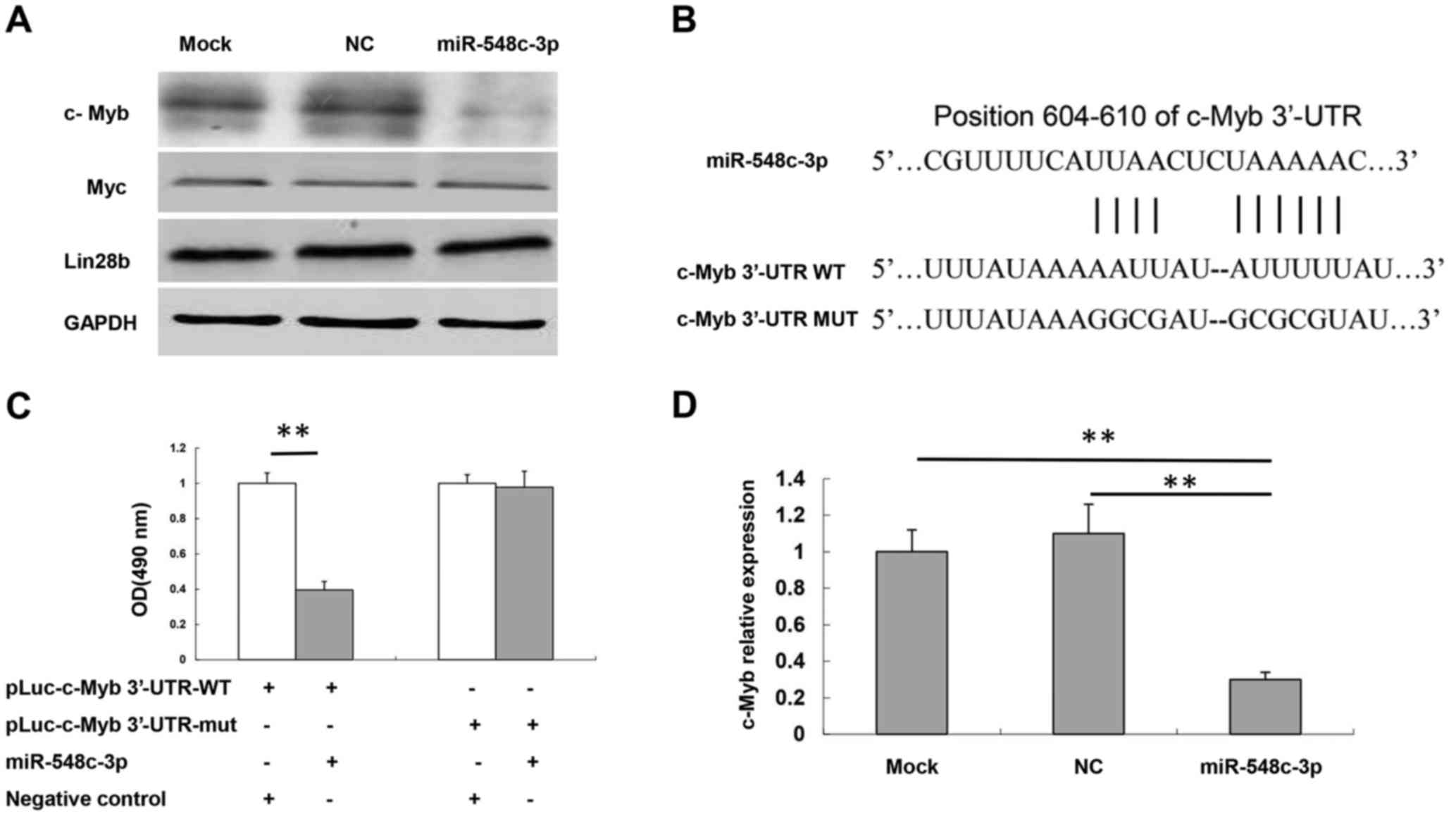 | Figure 3.c-Myb is a target of miR-548c-3p. (A)
Protein expression levels of Myc, Lin28b and c-Myb in T98G cells
following transfection with miR-548c-3p were determined using
western blotting. GAPDH was used as an internal control. (B)
Specific locations of the binding sites. Alignment between the
predicted miR-548c-3p target sites, miR-548c-3p and c-Myb 3′-UTR-WT
or 3′-UTR-mut, the conserved ‘seed’ sequence of between 7 and 8 bp,
for miR-548c-3p-mRNA pairing, is indicated. (C) Human embryonic
kidney-293 cells were co-transfected with 50 nM miR-548c-3p,
pLuc-c-Myb 3′-UTR-WT or pLuc-c-Myb 3′-UTR-mut along with a pRL-SV40
reporter plasmid. After 24 h, luciferase activity was measured. (D)
c-Myb mRNA expression levels in T98G cells following transfection
with miR-548c-3p were determined using the quantitative polymerase
chain reaction. U6 small nuclear RNA was used as an internal
control. **P<0.01. c-Myb, proto-oncogene c-Myb; miR, microRNA;
Myc, Myc proto-oncogene protein; Lin28b, protein lin-28 homolog B;
UTR, untranslated region; WT, wild-type; mut, mutant; OD, optical
density. |
As indicated, introduction of miR-548c-3p in HEK-293
cells with the wild-type 3′-UTR (pLuc-c-Myb 3′-UTR) construct
significantly inhibited the luciferase activity compared with the
NC group (P<0.01; Fig. 3C).
Mutation of the binding site, using a mutant vector (pLuc-MET
3-UTR-Mut), completely eliminated the ability of miR-548c-3p to
regulate luciferase expression. These results demonstrated that
c-Myb was a potential target of miR-548c-3p. RT-qPCR assay results
also demonstrated that miR-548c-3p was able to inhibit c-Myb mRNA
expression compared with NC and mock cells (P<0.01; Fig. 3D).
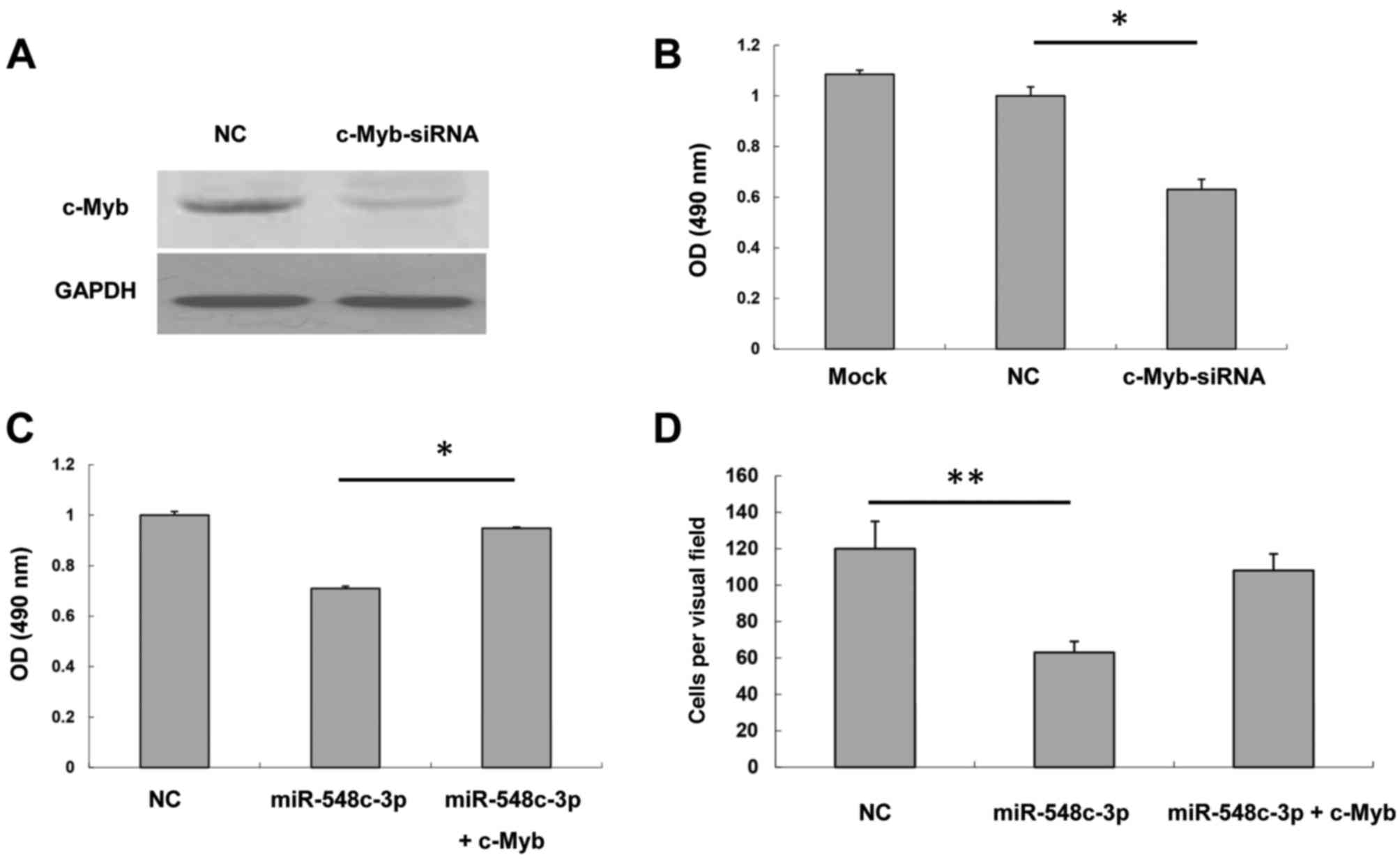
c-Myb rescues the effect of
miR-548c-3p overexpression
To confirm that c-Myb was responsible for
miR-548c-3p inhibition of T98G proliferation and migration, c-Myb
was knocked down and the knockdown efficiency of c-Myb siRNA was
analyzed. A western blot assay demonstrated that c-Myb siRNA was
able to inhibit c-Myb expression to a marked extent (Fig. 4A). An MTS assay was performed to test
the effect of c-Myb siRNA on T98G proliferation. The proliferation
of T98G cells transfected with c-Myb siRNA was inhibited compared
with the NC (Fig. 4B). Furthermore,
cells were co-transfected with miR-548c-3p and c-Myb to evaluate
the effects of c-Myb on miR-548c-3p-overexpressed cells. As
presented in Fig. 4C, miR-548c-3p
inhibited T98G cell proliferation; however, cells co-transfected
with miR-548c-3p and c-Myb rescued proliferation markedly compared
with NC. This suggested that miR-548c-3p may exert its effect
through the inhibition of c-Myb. The Transwell assay demonstrated
that cells co-transfected with miR-548c-3p and c-Myb rescued the
inhibitory effect of miR-548c-3p on T98G migration (Fig. 4D). Therefore, these data indicated
that c-Myb is responsible for the inhibition by miR-548c-3p of the
proliferation and migration of T98G cells.
Discussion
Following the identification of the miRNA let-7, a
number of miRNAs have been linked to oncogenes and tumor suppressor
genes, including the Ras proto-oncogene, the anti-apoptotic gene
BCL2 and the potent p53 tumor suppressor gene (22,23). As
downregulation of miRNA has been observed in various types of
tumor, miRNAs function as tumor suppressors or oncogenes by
interacting with their corresponding targets. However, it was
previously demonstrated that upregulation of miRNA occurs in
certain types of tumor (24). It was
hypothesized that miRNAs are able to act as oncogenes or as tumor
suppressors, depending on the type of tissue and the context in
which they are expressed (25,26).
Knowledge of the underlying molecular mechanism of miRNAs in cancer
remains limited. miR-548c-3p has been investigated in certain types
of cancer, including breast, prostate and H. pylori-negative
gastric cancer (13,15,27).
Mutation of miR-548c-3p may be an important driving force in
tumorigenesis. Furthermore, it was reported that downregulation of
miR-34b and miR-548c-3p, which target high-mobility group protein
A1 (HMGA1), may account for the overexpression of HMGA1 protein
detected in the majority of human pituitary adenomas (28). In the present study, the expression
level of miR-548c-3p was investigated, and downregulation of
miR-548c-3p in glioma tissues and the T98G cell line was
demonstrated. Furthermore, miR-548c-3p inhibited T98G cell
proliferation and migration. These results suggested that
miR-548c-3p serves an important role in glioma.
To identify the potential targets of miR-548c-3p,
candidate genes identified using TargetScan were screened. c-Myb
was selected due to its involvement in numerous cancer types
(29,30). c-Myb has the potential to induce cell
proliferation and is likely to serve a role in stimulating
progression through the cell cycle in wild-type cells. Furthermore,
interactions with cell cycle regulators, including cyclin D1, may
be important for its oncogenic activity (31). In the present study, the c-Myb 3′-UTR
was inserted downstream of the firefly luciferase coding region of
a pMIR-Luc vector. Using western blot analysis, it was demonstrated
that c-Myb expression was markedly reduced when transfected with
miR-548c-3p. These results identified that c-Myb was the target of
miR-548c-3p. miR-548c-3p induced T98G arrest at G1
phase, which was possibly associated with the activation of genes
required for the G1/S phase transition by c-Myb.
However, elucidation of the signaling pathways involved requires
further study.
It was previously demonstrated that the modulation
of c-Myb levels in a number of tumor lines was frequently
associated with increased migration and invasion capabilities
(32). Tanno et al (33) demonstrated that the expression of
neuronal cadherin was increased in c-Myb-expressing HEK-293 and
LAN-5 cells, and may be responsible for the biological function of
c-Myb. In the present study, bioinformatic prediction and
experimental data indicated that miR-548c-3p is able to target
c-Myb. Overexpression of c-Myb rescued the effect of miR-548c-3p.
Therefore, this revealed that miR-548c-3p exerts its effect on cell
proliferation and migration though downregulation of c-Myb;
however, the underlying molecular mechanism remains unclear.
In summary, miR-548c-3p was identified as one of the
key regulators of c-Myb involved in the tumorigenesis of glioma.
Reconstruction of miR-548c-3p or inhibition of c-Myb function may
be a promising therapeutic strategy for the treatment of
glioma.
Acknowledgements
The authors would like to thank Dr Yihong Wang (Sir
Run Run Shaw Hospital, Zhejiang University College of Medicine,
Hangzhou, Zhejiang, China) for providing the clinical diagnosis of
the glioma tissues. The present study was partially supported by
the Natural Science Foundation of China (grant nos. NSFC81201588)
the Key Projects in the National Science & Technology Pillar
Program (No. 2015BAI09B01) and the Natural Science Foundation of
Zhejiang Province (grant no. LQ12H16003).
References
|
1
|
Stupp R, Tonn JC, Brada M and
Pentheroudakis G: High-grade malignant glioma: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21:(Suppl 5). v190–v193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleihues P and Ohgaki H: Primary and
secondary glioblastomas: From concept to clinical diagnosis. Neuro
Oncol. 1:44–51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher JL, Schwartzbaum JA, Wrensch M and
Wiemels JL: Epidemiology of brain tumors. Neurol Clin. 25:867–890,
vii. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McNeill KA: Epidemiology of brain tumors.
Neurol Clin. 34:981–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The C.
Elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanno B, Cesi V, Vitali R, Sesti F,
Giuffrida ML, Mancini C, Calabretta B and Raschellà G: Silencing of
endogenous IGFBP-5 by micro RNA interference affects proliferation,
apoptosis and differentiation of neuroblastoma cells. Cell Death
Differ. 12:213–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang T, Guo L and Liu C: Genome-wide
analysis of mir-548 gene family reveals evolutionary and functional
implications. J Biomed Biotechnol. 2012:6795632012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rane JK, Scaravilli M, Ylipää A, Pellacani
D, Mann VM, Simms MS, Nykter M, Collins AT, Visakorpi T and
Maitland NJ: MicroRNA expression profile of primary prostate cancer
stem cells as a source of biomarkers and therapeutic targets. Eur
Urol. 67:7–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang H, Kim N, Park JH, Nam RH, Choi YJ,
Lee HS, Yoon H, Shin CM, Park YS, Kim JM and Lee DH: Different
microRNA expression levels in gastric cancer depending on
Helicobacter pylori infection. Gut Liver. 9:188–196. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rushton JJ, Davis LM, Lei W, Mo X, Leutz A
and Ness SA: Distinct changes in gene expression induced by A-Myb,
B-Myb and c-Myb proteins. Oncogene. 22:308–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rushton JJ and Ness SA: The conserved DNA
binding domain mediates similar regulatory interactions for A-Myb,
B-Myb and c-Myb transcription factors. Blood Cells Mol Dis.
27:459–463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramsay RG and Gonda TJ: MYB function in
normal and cancer cells. Nat Rev Cancer. 8:523–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y and Ness SA: Myb proteins: Angels
and demons in normal and transformed cells. Front Biosci (Landmark
Ed). 16:1109–1131. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramkissoon LA, Horowitz PM, Craig JM,
Ramkissoon SH, Rich BE, Schumacher SE, McKenna A, Lawrence MS,
Bergthold G, Brastianos PK, et al: Genomic analysis of diffuse
pediatric low-grade gliomas identifies recurrent oncogenic
truncating rearrangements in the transcription factor MYBL1. Proc
Natl Acad Sci USA. 110:8188–8193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dobson JR, Taipaleenmäki H, Hu YJ, Hong D,
van Wijnen AJ, Stein JL, Stein GS, Lian JB and Pratap J:
hsa-mir-30c promotes the invasive phenotype of metastatic breast
cancer cells by targeting NOV/CCN3. Cancer Cell Int. 14:732014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim BH, Hong SW, Kim A, Choi SH and Yoon
SO: Prognostic implications for high expression of oncogenic
microRNAs in advanced gastric carcinoma. J Surg Oncol. 107:505–510.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stephens PJ, Tarpey PS, Davies H, Van Loo
P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell
GR, et al: The landscape of cancer genes and mutational processes
in breast cancer. Nature. 486:400–404. 2012.PubMed/NCBI
|
|
28
|
Fedele M, Visone R, De Martino I, Troncone
G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C,
Melillo RM, et al: HMGA2 induces pituitary tumorigenesis by
enhancing E2F1 activity. Cancer Cell. 9:459–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou YE, O'Rourke JP, Edwards JS and Ness
SA: Single molecule analysis of c-myb alternative splicing reveals
novel classifiers for precursor B-ALL. PLoS One. 6:e228802011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brill LB II, Kanner WA, Fehr A, Andrén Y,
Moskaluk CA, Löning T, Stenman G and Frierson HF Jr: Analysis of
MYB expression and MYB-NFIB gene fusions in adenoid cystic
carcinoma and other salivary neoplasms. Mod Pathol. 24:1169–1176.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganter B, Fu S and Lipsick JS: D-type
cyclins repress transcriptional activation by the v-Myb but not the
c-Myb DNA-binding domain. EMBO J. 17:255–268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ameh EA, Mshelbwala PM, Nasir AA, Lukong
CS, Jabo BA, Anumah MA and Nmadu PT: Surgical site infection in
children: Prospective analysis of the burden and risk factors in a
sub-Saharan African setting. Surg Infect (Larchmt). 10:105–109.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanno B, Sesti F, Cesi V, Bossi G,
Ferrari-Amorotti G, Bussolari R, Tirindelli D, Calabretta B and
Raschellà G: Expression of Slug is regulated by c-Myb and is
required for invasion and bone marrow homing of cancer cells of
different origin. J Biol Chem. 285:29434–29445. 2010. View Article : Google Scholar : PubMed/NCBI
|