Introduction
Colorectal cancer is a common type of malignancy and
approximately 25% of individuals will have liver metastases at the
time of the initial diagnosis. Furthermore, 40–50% of patients
develop colorectal liver metastasis (CRLM) within three years of
resection of the primary tumor (1).
Hepatic resection is the most effective and potentially curative
therapy for CRLM, with a reported five-year survival rate of 30–50%
(2–4);
the recent development of chemotherapeutic agents has further
improved the outcome of patients with CRLM (5,6).
Therefore, assessment of prognostic predictors is important for the
management of patients with CRLM.
Previously, several studies have indicated that
systemic inflammatory response predicts cancer-specific survival in
patients with cancer. The Glasgow prognostic score (GPS), which is
calculated by the combination of serum C-reactive protein (CRP) and
albumin concentrations, and the elevated preoperative
neutrophil-to-lymphocyte ratio (NLR), have been revealed to predict
cancer-specific survival (7–12). The previous study reported negative
impact of GPS on post-operative complications following hepatic
resection (13,14), and the association between
perioperative immunological response and prognosis subsequent to
hepatic resection for HCC (15,16) and
CRLM (17). In the present study, the
association between preoperative peripheral blood neutrophil count
and disease-free, as well as overall survival, following elective
hepatic resection for patients with CRLM was retrospectively
investigated.
Materials and methods
Between January 2000 and December 2010, 96 patients
with CRLM underwent hepatic resection at the Department of Surgery,
Jikei University Hospital (Tokyo, Japan). Of these, seven patients
were excluded, one patient for mortality due to a cardiovascular
event, two patients due to lack of data and four patients who were
lost to follow up, leaving the remaining 89 patients for this
study. All patients underwent macroscopic curative resection for
liver, lung and lymph node metastases. Liver resection was carried
out prior to any other therapy treatments in order to avoid the
possibility of liver failure. Neoadjuvant chemotherapy was
administered when liver metastases were identified as unresectable
or borderline resectable. Pre-operative chemotherapy was
discontinued >6 weeks prior to hepatic resection in order to
reduce liver injury and bone marrow suppression by chemotherapy.
Generally, the extent of hepatic resection was determined based on
the retention rate of indocyanine green at 15 min
(ICGR15) prior to surgery and hepatic reserve, as
previously described by Miyagawa et al (18). A percutaneous transhepatic portal
embolization was performed for patients with an estimated residual
hepatic volume of <30%. Nomenclature of segments and types of
operations follow the Brisbane 2000 terminology (19). The type of resection was classified
into two groups: Major resection (resection of ≥3 couinaud sub
segments) and minor resection (resection <3 sub segments, or
partial resection). The present study was approved by the Ethics
Committee of The Jikei University School of Medicine (Tokyo,
Japan).
Patient characteristics were classified into two
groups for the Log-rank test and the Cox proportional hazards
regression model as follows: Age <65 or ≥65 years, number of
regional lymph node metastases <4 or ≥4, size of largest tumor
<50 or ≥50 mm, duration of operation <300 or ≥300 min and
intraoperative blood loss <1,000 or ≥1,000 g, according to
previous studies (10–14). Using the mean or median of
preoperative white blood cell subsets counts, they were classified
as follows: Neutrophil <3,500 or ≥3,500/µl, lymphocyte <1,500
or ≥1,500/µl, monocyte counts <500 or ≥500/µl.
Firstly, the association between clinical variables
and disease-free or overall survival following hepatic resection by
univariate and multivariate analysis was investigated. The
following 13 variables were evaluated: Age, gender, number of
regional lymph node metastases of primary colorectal cancer,
synchronous or metachronous CRLM, status of neoadjuvant
chemotherapy, tumor distribution, diameter of the largest tumor,
type of resection, duration of operation, intraoperative blood loss
and the neutrophil, lymphocyte, and monocyte count.
Subsequently, the correlation between neutrophil
count and the patient characteristics was analyzed using the
following 12 factors: Age, gender, number of regional lymph node
metastases of primary colorectal cancer, synchronous or
metachronous CRLM, status of neoadjuvant chemotherapy, tumor
distribution, diameter of the largest tumor, type of resection,
duration of operation, intraoperative blood loss and the lymphocyte
and monocyte count.
Recurrence of colorectal cancer was defined as newly
detected local, hepatic, lung or extrahepatic tumors by
ultrasonography, computed tomography, or magnetic resonance
imaging, with or without an increase in serum carcinoembryonic
antigen or carbohydrate antigen 19–9 (CA 19-9). For recurrent liver
metastasis, repeated hepatic resection, local ablation therapy or
systemic chemotherapy was performed based primarily on the number,
size and location of the recurrent liver tumors, in addition to
hepatic functional reserve, including ICGR15, and
remnant liver volume. For lung metastasis, limited partial lung
resection or systemic chemotherapy was performed. For local
recurrence, tumor resection, radiotherapy or systemic chemotherapy
was performed. With regards to chemotherapy, 5-fluorouracil
(5-Fu)-based chemotherapy was selected as adjuvant and/or
neoadjuvant chemotherapy prior to 2003. Following 2004, the
patients received infusional 5-Fu/1-leucovorin with oxaliplatin
and/or infusional 5-Fu/1-leucovorin with irinotecan.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Analysis of disease-free and overall survival was performed
using the Log-rank test. Univariate analysis was performed using
the Mann-Whitney U-test and χ2 test. Multivariate
analysis was performed using the Cox proportional regression model,
incorporating all variables with P<0.05 in the univariate
analysis. These analyses were conducted using IBM® SPSS
statistics version 20.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Patient characteristics are presented in Table I as the mean ± SD, range or ratio.
Preoperative neutrophil counts were 3,466.3±1,206.6/µl (mean ± SD).
Certain patients received neoadjuvant chemotherapy for liver
resection (7/89).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Factor | Mean ± SD or
ratio | Range |
|---|
| Age (years) | 64.0±9.8 | 39–85 |
| Gender
(male:female) | 62:27 |
|
| No. of lymph node
metastases (<4:≥4) | 67:22 |
|
| Timing of tumor
(synchronous:metachronous) | 41:48 |
|
| Neoadjuvant
chemotherapy (yes:no) | 7:82 |
|
| Tumor distribution
(unilobar:bilobar) | 22:67 |
|
| Tumor size (mm) | 43.4±31.8 |
10–200 |
| Type of resection
(major:minor) | 33:56 |
|
| Duration of operation
(min) | 349.8±144.9 |
85–867 |
| Intraoperative blood
loss (g) | 1,132.8±1,088.6 |
25-5,485 |
| Neutrophil count
(µl) | 3,466.3±1,206.6 | 1,300-8,000 |
| Lymphocyte count
(µl) | 1,514.6±443.0 |
700-2,700 |
| Monocyte count
(µl) | 294.4±113.2 | 0–600 |
Univariate and multivariate analysis
of disease-free survival following hepatic resection and clinical
variables
Table II presents the
association between the clinical variables and disease-free
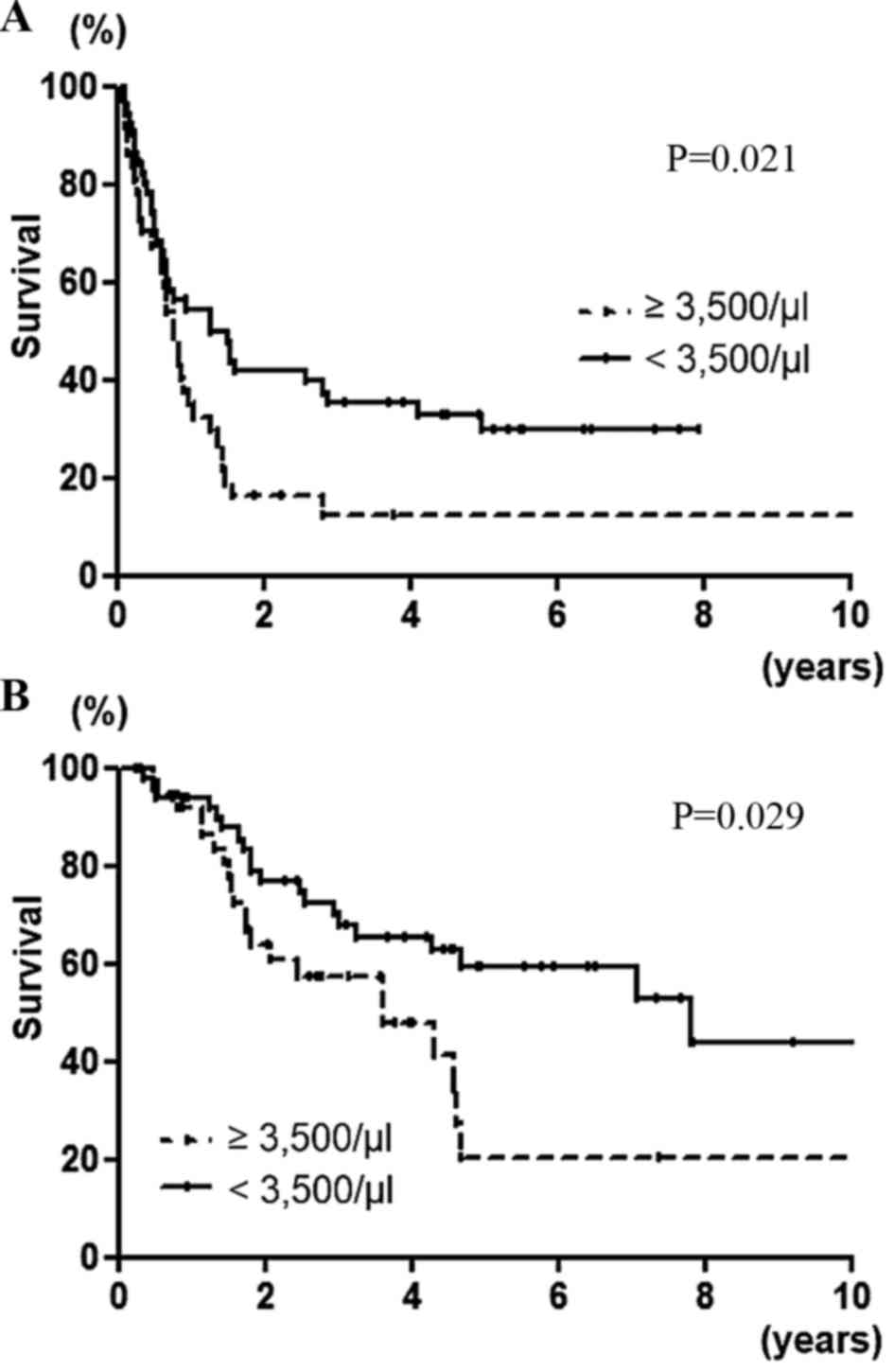
survival following hepatic resection. In univariate analysis,
disease-free survival was significantly poorer in patients with
>4 lymph node metastases (P=0.018), presence of neoadjuvant
chemotherapy (P=0.026), bilobar distribution (P=0.002) and
neutrophil count ≥3,500/µl (P=0.021; Fig.
1A). In multivariate analysis, the presence of neoadjuvant
chemotherapy (P=0.015), bilobar distribution (P=0.015) and
neutrophil count ≥3,500/µl (P=0.025), were independent and
significant predictors of disease-free survival.
 | Table II.Univariate and multivariate analysis
of clinical variables in association with disease-free survival
following hepatic resection. |
Table II.
Univariate and multivariate analysis
of clinical variables in association with disease-free survival
following hepatic resection.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | N | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (years) |
|
| 0.192 |
|
|
| ≥65 | 46 | 1.382 |
|
|
|
|
<65 | 43 | (0.850–2.850) |
|
|
|
| Gender |
|
| 0.913 |
|
|
| Male | 62 | 1.030 |
|
|
|
|
Female | 27 | (0.606–1.606) |
|
|
|
| No. of lymph node
metastases |
|
| 0.018a |
| 0.079 |
| ≥4 | 22 | 2.107 |
| 1.656 |
|
|
<4 | 67 | (1.133–3.133) |
| (0.947- 2,895) |
|
| Timing of
tumor |
|
| 0.200 |
|
|
|
Synchronous | 41 | 1.381 |
|
|
|
|
Metachronous | 48 | (0.843–2.843) |
|
|
|
| Neoadjuvant
chemotherapy |
|
| 0.026a |
| 0.015a |
|
Yes | 7 | 4.170 |
| 3.155 |
|
| No | 82 | (1.182–14.182) |
| (1.249–7.249) |
|
| Tumor
distribution |
|
| 0.002a |
| 0.015a |
|
Bilobar | 22 | 2.770 |
| 1.961 |
|
|
Unilobar | 67 | (1.458–5.458) |
| (1.143–3.143) |
|
| Tumor size
(mm) |
|
| 0.402 |
|
|
|
≥50 | 24 | 1.272 |
|
|
|
|
<50 | 65 | (0.724–2.724) |
|
|
|
| Type of
resection |
|
| 0.818 |
|
|
|
Major | 33 | 1.061 |
|
|
|
|
Minor | 56 | (0.639–1.639) |
|
|
|
| Duration of
operation (min) |
|
| 0.397 |
|
|
|
≥300 | 55 | 1.238 |
|
|
|
|
<300 | 34 | (0.756–2.756) |
|
|
|
| Intraoperative
blood loss (g) |
|
| 0.875 |
|
|
|
≥1,000 | 39 | 1.040 |
|
|
|
|
<1,000 | 50 | (0.639–1.639) |
|
|
|
| Neutrophil count
(µl) |
|
| 0.021a |
| 0.025a |
|
≥3,500 | 37 | 1.827 |
| 1.838 |
|
|
<3,500 | 52 | (1.093–3.093) |
| (1.087–3.087) |
|
| Lymphocyte count
(µl) |
|
| 0.805 |
|
|
|
≥1,500 | 49 | 1.063 |
|
|
|
|
<1,500 | 40 | (0.653–1.653) |
|
|
|
| Monocyte count
(µl) |
|
| 0.899 |
|
|
|
≥300 | 57 | 1.143 |
|
|
|
|
<300 | 32 | (0.694–1.694) |
|
|
|
Univariate and multivariate analysis
of overall survival following hepatic resection and clinical
variables
Table III presents
the association between the clinical variables and overall survival
following hepatic resection. In univariate analysis, overall
survival was significantly poorer in patients with >4 lymph node
metastases (P<0.001), the presence of neoadjuvant chemotherapy
(P=0.003), bilobar distribution (P=0.0007) and neutrophil count
≥3,500/µl (P=0.029; Fig. 1B). In
multivariate analysis, >4 lymph node metastases (P=0.001),
presence of neoadjuvant chemotherapy (P=0.003), bilobar
distribution (P=0.039) and neutrophil count ≥3,500/µl (P=0.040),
were independent and significant predictors of overall
survival.
 | Table III.Univariate and multivariate analysis
of clinical variables in association with overall survival
following hepatic resection. |
Table III.
Univariate and multivariate analysis
of clinical variables in association with overall survival
following hepatic resection.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | N | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (years) |
|
| 0.947 |
|
|
|
≥65 | 46 | 1.021 |
|
|
|
|
<65 | 43 | (0.552–1.552) |
|
|
|
| Gender |
|
| 0.885 |
|
|
|
Male | 62 | 0.952 | |
|
|
|
Female | 27 | (0.488–1.488) |
|
|
|
| No. of lymph node
metastases |
|
|
<0.001a |
| 0.001a |
| ≥4 | 22 | 4.311 |
| 3.023 |
|
|
<4 | 67 | (1.968–9.968) |
| (1.585–5.585) |
|
| Timing of
tumor |
|
| 0.423 |
|
|
|
Synchronous | 41 | 1.287 |
|
|
|
|
Metachronous | 48 | (0.694–2.694) |
|
|
|
| Neoadjuvant
chemotherapy |
|
| 0.003a |
| 0.003a |
|
Yes | 7 | 12.46 | | 5.058 |
|
| No | 82 | (2.370–65.370) |
|
(1.748–14.748) |
|
| Tumor
distribution |
|
| 0.007a |
| 0.039a |
|
Bilobar | 22 | 2.093 | | 2.021 |
|
|
Unilobar | 67 | (1.337–6.337) |
| (1.035–3.035) |
|
| Tumor size
(mm) |
|
| 0.275 |
|
|
|
≥50 | 24 | 1.510 | |
|
|
|
<50 | 65 | (0.721–3.721) |
|
|
|
| Type of
resection |
|
| 0.884 |
|
|
|
Major | 33 | 1.049 | |
|
|
|
Minor | 56 | (0.556–1.556) |
|
|
|
| Duration of
operation (min) |
|
| 0.373 |
|
|
|
≥300 | 55 | 1.335 | |
|
|
|
<300 | 34 | (0.707–2.707) |
|
|
|
| Intraoperative
blood loss (g) |
|
| 0.185 |
|
|
|
≥1,000 | 39 | 1.527 | |
|
|
|
<1,000 | 50 | (0.817–2.817) |
|
|
|
| Neutrophil count
(µl) |
|
| 0.029a |
| 0.040a |
|
≥3,500 | 37 | 2.066 | | 2.016 |
|
|
<3,500 | 52 | (1.078–3.078) |
| (1.031–3.031) |
|
| Lymphocyte count
(µl) |
|
| 0.660 |
|
|
|
≥1,500 | 49 | 1.149 |
|
|
|
|
<1,500 | 40 | (0.620–2.620) |
|
|
|
| Monocyte count
(µl) |
|
| 0.822 |
|
|
|
≥300 | 57 | 0.930 | |
|
|
|
<300 | 32 | (0.492–1.492) |
|
|
|
Univariate analysis of clinical
variables in association with the neutrophil count
Table IV presents the
association between clinical variables and the neutrophil count. In
univariate analysis, tumor diameter (P=0.021) and monocyte count
(P<0.001) were significantly greater in the group of patients
with an elevated neutrophil count. Synchronous CRLM (P=0.088) and
intraoperative blood loss (P=0.065) tended to be greater in
patients with elevated neutrophil count group; however, this was
not statistically significant.
 | Table IV.Univariate analysis of clinical
variables in association with preoperative neutrophil counts. |
Table IV.
Univariate analysis of clinical
variables in association with preoperative neutrophil counts.
|
| Neutrophil
count |
|---|
|
|
|
|---|
| Factor | <3,500/µl
(n=52) | ≥3,500/µl
(n=37) | P-value |
|---|
| Age (years) | 63.8±8.9 |
64.2±11.1 | 0.723 |
| Gender
(male:female) | 34:18 | 28:9 | 0.298 |
| No. of lymph node
metastases (<4:≥4) | 42:10 |
27:10 | 0.155 |
| Timing of tumor
(synchronous:metachronous) | 20:32 |
21:16 | 0.088 |
| Neoadjuvant
chemotherapy (yes:no) |
6:46 |
1:36 | 0.127 |
| Tumor distribution
(unilobar:bilobar) | 40:12 |
27:10 | 0.670 |
| Tumor size
(mm) |
35.4±19.4 |
54.5±41.6 | 0.021 |
| Type of resection
(major:minor) | 20:32 |
13:24 | 0.749 |
| Duration of
operation (min) |
341.3±142.7 |
361.8±149.0 | 0.524 |
| Intraoperative
blood loss (g) |
939.1±962.3 |
1,405.0±1,206.1 | 0.065 |
| Lymphocyte count
(/µl) | 1,526.9±422.0 | 1,497.3±476.4 | 0.789 |
| Monocyte count
(/µl) |
251.9±101.9 |
354.1±101.6 |
<0.001a |
Discussion
Systemic inflammation has been reported to correlate
with poorer cancer-specific survival in numerous types of cancer
(7–12,20).
Previous studies have demonstrated that the host inflammatory
response to cancer and/or the systemic effects exerted by cancer
cells leads to the upregulation of the inflammatory process,
predisposing the cancer to proliferation and metastasis by the
inhibition of apoptosis, promotion of angiogenesis and repair of
DNA damage (21,22). The presence of a systemic inflammatory
response may be detected by the elevation of the CRP level and
neutrophil count (7–12). Using these parameters, prognostic
markers, including GPS and NLR, were reported to be associated with
poor survival following hepatic resection for CRLM (9–11,23). In the present study disease-free and
overall survival of patients with preoperative high neutrophil
counts following elective hepatic resection for CRLM, was revealed
to be significantly poorer by statistical analyses. The prognostic
value of NLR in the present study cohort was also investigated;
however, NLR was not a significant predictor of the overall
survival (P=0.193, data not presented). This result indicates that
neutrophil counts may themselves be an inflammatory and prognostic
marker. Neal et al (24)
demonstrated that the neutrophil count had a greater predictive
value on long-term outcomes compared with NLR, when the
multivariate Cox proportional regression model analyzed
factors.
Inflammatory status represents a response process to
detection of CRLM (25). In the
present study, tumor size was larger in patients with higher
neutrophil counts. These results indicate that tumor invasion or
expansion elicits inflammation in the microenvironment. Neutrophils
contribute to continuous angiogenic stimulation, including the
release of endothelial growth factor (26). This condition may accelerate the
growth of cancer cells or micro-metastases (27). Additionally, systemic inflammation
also induces the suppression of antitumor immunity by recruitment
of regulatory T cells and activation of cytokines (25). Preoperative systemic inflammation and
an immunosuppressive state may increase the risk of postoperative
infectious complications, which influence long-term outcomes in
patients with CRLM (24,28).
In the present study, neoadjuvant chemotherapy
demonstrated a negative impact on long-term outcomes following
hepatic resection for CRLM. Neoadjuvant chemotherapy prior to
hepatic resection in patients with resectable CRLM may facilitate
the resectability of liver lesions and treat occult metastasis;
however, it may also lead to hepatic parenchymal injury, which may
increase morbidity and mortality following surgery (29,30). There
are conflicting opinions over the oncological benefit of this
practice in patients who may already be suitable for a curative
hepatic resection (31,32). Additionally, in the present study
patients treated with neoadjuvant chemotherapy were limited and had
a more aggressive disease, including bilobar distribution (P=0.038,
data not presented), compared with patients without neo-adjuvant
chemotherapy on initial presentation, as liver resection had
priority over other therapies at Jikei University Hospital.
In summary, preoperative elevation of the peripheral
neutrophil count is an independent risk factor for disease-free as
well as overall survival. Prevention of systemic inflammatory
response may improve perioperative outcomes and long-term survival
following resection of malignant tumors. Several therapeutic agents
targeting the inflammatory response are undergoing clinical trials
(33). Further investigation to
clarify the association between the immunosuppressive mechanisms
induced by systemic inflammation and tumor progression is important
in order to improve the therapeutic outcome of oncological surgery.
In conclusion, preoperative peripheral blood neutrophil count is an
independent and significant indicator of long-term outcomes in
patients with CRLM following hepatic resection.
References
|
1
|
O'Reilly DA and Poston GJ: Colorectal
liver metastases: Current and future perspectives. Future Oncol.
2:525–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fong Y, Fortner J, Sun RL, Brennan MF and
Blumgart LH: Clinical score for predicting recurrence after hepatic
resection for metastatic colorectal cancer: Analysis of 1001
consecutive cases. Ann Surg. 230:309–321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malik HZ, Prasad KR, Halazun KJ, Aldoori
A, Al-Mukhtar A, Gomez D, Lodge JP and Toogood GJ: Preoperative
prognostic score for predicting survival after hepatic resection
for colorectal liver metastases. Ann Surg. 246:806–814. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simmonds PC, Primrose JN, Colquitt JL,
Garden OJ, Poston GJ and Rees M: Surgical resection of hepatic
metastases from colorectal cancer: A systematic review of published
studies. Br J Cancer. 94:982–999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
Colorectal Cancer Group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumor site: A Glasow inflammation outcome study. Br
J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:257–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halazun KJ, Aldoori A, Malik HZ,
Al-Mukhtar A, Prasad KR, Toogood GJ and Lodge JP: Elevated
preoperative neutrophil to lymphocyte ratio predicts survival
following hepatic resection for colorectal liver metastases. Eur J
Surg Oncol. 34:55–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giakoustidis A, Neofytou K, Khan AZ and
Mudan S: Neutrophil to lymphocyte ratio predicts pattern of
recurrence in patients undergoing liver resection for colorectal
liver metastasis and thus the overall survival. J Surg Oncol.
111:445–450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomez D, Morris-Stiff G, Wyatt J, Toogood
GJ, Lodge JP and Prasad KR: Surgical technique and systemic
inflammation influences long-term disease-free survival following
hepatic resection for colorectal metastasis. J Surg Oncol.
98:371–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mano Y, Shirabe K, Yamashita Y, Harimoto
N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T,
Yamanaka T and Maehara Y: Preoperative neutrophil-to-lymphocyte
ratio is a predictor of survival after hepatectomy for
hepatocellular carcinoma: A retrospective analysis. Ann Surg.
258:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujiwara Y, Shiba H, Furukawa K, Iida T,
Haruki K, Gocho T, Wakiyama S, Hirohara S, Ishida Y, Misawa T, et
al: Glasgow prognostic score is related to blood transfusion
requirements and post-operative complications in hepatic resection
for hepatocellular carcinoma. Anticancer Res. 30:5129–5136.
2010.PubMed/NCBI
|
|
14
|
Haruki K, Shiba H, Fujiwara Y, Furukawa K,
Wakiyama S, Ogawa M, Ishida Y, Misawa T and Yanaga K: Negative
impact of surgical site infection on long-term outcomes after
hepatic resection for colorectal liver metastases. Anticancer Res.
33:1697–1703. 2013.PubMed/NCBI
|
|
15
|
Fujiwara Y, Shiba H, Furukawa K, Iida T,
Sakamoto T, Gocho T, Wakiyama S, Hirohara S, Ishida Y, Misawa T, et
al: Perioperative change in white blood cell count predicts outcome
of hepatic resection for hepatocellular carcinoma. J Hepatobiliary
Pancreat Sci. 17:892–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiba H, Furukawa K, Fujiwara Y, Futagawa
Y, Haruki K, Wakiyama S, Ishida Y, Misawa T and Yanaga K:
Postoperative peak serum C-reactive protein predicts outcome of
hepatic resection for hepatocellular carcinoma. Anticancer Res.
33:705–709. 2013.PubMed/NCBI
|
|
17
|
Haruki K, Shiba H, Fujiwara Y, Furukawa K,
Wakiyama S, Ogawa M, Ishida Y, Misawa T and Yanaga K: Perioperative
change in peripheral blood monocyte count may predict prognosis in
patients with colorectal liver metastasis after hepatic resection.
J Surg Oncol. 106:31–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyagawa S, Makuuchi M, Kawasaki S and
Kakazu T: Criteria for safe hepatic resection. Am J Surg.
169:589–594. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strasberg SM: Nomenclature of hepatic
anatomy and resections: A review of the Brisbane 2000 system. J
Hepatobiliary Pancreat Surg. 12:351–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaiswal M, LaRusso NF, Burgart LJ and
Gores GJ: Inflammatory cytokines induce DNA repair in
cholangiocarcinoma cells by a nitricoxide-dependent mechanism.
Cancer Res. 60:184–190. 2000.PubMed/NCBI
|
|
22
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection of colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi T, Teruya M, Kishiki T, Endo D,
Takenaka Y, Miki K, Kobayashi K and Morita K: Elevated C-reactive
protein and hypoalbuminemia measured before resection of colorectal
live rmetastases predict postoperative survival. Dig Surg.
27:285–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neal CP, Mann CD, Garcea G, Briggs CD,
Dennison AR and Berry DP: Preoperative systemic inflammation and
infectious complications after resection of colorectal liver
metastases. Arch Surg. 146:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taichman NS, Young S, Cruchley AT, Taylor
P and Paleolog E: Human neutrophils secrete vascular endothelial
growth factor. J Leukoc Biol. 62:397–400. 1997.PubMed/NCBI
|
|
27
|
Maniwa Y, Okada M, Ishii N and Kiyooka K:
Vascular endothelial growth factor increased by pulmonary surgery
accelerates the growth of micrometastases in metastatic lung
cancer. Chest. 114:1668–1675. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farid SG, Aldouri A, Morris-Stiff G, Khan
AZ, Toogood GJ, Lodge JP and Prasad KR: Correlation between
postoperative infective complications and long-term outcomes after
hepatic resection for colorectal liver metastasis. Ann Surg.
251:91–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakano H, Oussoultzoglou E, Rosso E,
Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P and Jaeck D:
Sinusoidal injury increases morbidity after major hepatectomy in
patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vauthey JN, Pawlik TM, Ribero D, Wu TT,
Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et
al: Chemotherapy regimen predicts steatohepatitis and an increase
in 90-day mortality after surgery for hepatic colorectal
metastases. J Clin Oncol. 24:2065–2072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Viganò L, Capussotti L, De Rosa G, De
Saussure WO, Mentha G and Rubbia-Brandt L: Liver resection for
colorectal metastases after chemotherapy: Impact of
chemotherapy-related liver injuries, pathological tumor response,
and micrometastases on long-term survival. Ann Surg. 258:731–742.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leal JN, Bressan AK, Vachharajani N, Gonen
M, Kingham TP, D'Angelica MI, Allen PJ, DeMatteo RP, Doyle MB,
Bathe OF, et al: Time-to-surgery and survival outcomes in
resectable colorectal liver metastases: A multi-institutional
evaluation. J Am Coll Surg. 222:766–779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balkwill F and Mantovani A: Cancer and
inflammation: Implications for pharmacology and therapeutics. Clin
Pharmacol Ther. 87:401–406. 2010. View Article : Google Scholar : PubMed/NCBI
|