Introduction
Gastric cancer is the fifth most common malignancy
and the third leading cause of cancer-associated mortality
(1), and still poses a considerable
global health burden, despite a substantial decrease in the
incidence of cancer for the majority of the world (2). In China, gastric cancer is the third
most frequently occurring type of cancer and cause of
cancer-associated mortality (3).
Approximately 405,000 novel cases are diagnosed every year in
China, accounting for 42.5% of the worldwide total (4). Gastric cancer is often asymptomatic or
induces only nonspecific symptoms in its early stages (5). Consequently, it is often diagnosed at
the advanced stages and is associated with a poor prognosis
(5). According to a statistical
study, ~70% of patients with gastric cancer have lymph node
metastasis at the time of diagnosis, leading to a median overall
survival time of 16.7 months (6).
Complete resection of the primary tumor with D2 lymphadenectomy is
the only method of curing the disease in the early stages (6). Early detection as well as the
availability and reliability of appropriate biomarkers may
contribute towards the effective treatment of gastric cancer
(7).
At present, the molecular mechanisms underlying
gastric cancer have not been well elucidated, owing to the
currently limited knowledge of germline susceptibility traits for
risk and somatic drivers of progression (8). The presence of cancer stem cells has
been demonstrated to be associated with the initiation, metastasis,
chemoresistance and rapid recurrence of various types of tumor
(9). Musashi-1, a highly conserved
RNA-binding protein, has been characterized as a putative stem or
progenitor cell marker (10). It
serves important roles in cell fate decision, including the
maintenance of the stem cell state, differentiation and
tumorigenesis (10).
Musashi-1-mediated translational control has been implicated to
promote pathological and physiological stem cell proliferation
(11). Loss of Musashi-1 function
disrupts the balance between germ-line stem cell differentiation
and renewal, leading to premature germ-line stem cell
differentiation (12). The Musashi-1
signaling pathway has previously been reported to be upregulated in
numerous types of tumor, including glioma (13), esophageal adenocarcinoma (14), colorectal cancer (15,16),
gallbladder adenocarcinoma (17),
endometrial carcinoma (18) and small
intestinal adenocarcinoma (19).
Furthermore, Musashi-1 has been identified as a biomarker
associated with cancer progression and poor prognosis in patients
with breast cancer (20), ovarian
adenocarcinoma (21) and oral
squamous cell carcinoma (22).
Musashi-1 is a candidate stem cell marker in the
human stomach and mouse intestine, and a marker for progenitor
cells in the human stomach (23).
Musashi-1-positive cells may serve a key role in the early events
occurring during carcinogenesis, and may be involved in the
progression of gastric cancer (24).
It was previously revealed that the expression levels of Musashi-1
were significantly elevated in gastric cancer and precancerous
lesions, including intestinal metaplasia and dysplasia (24,25). By
contrast, an immunohistochemistry study demonstrated that Musashi-1
expression in the gastric glands with intestinal metaplasia was
lower compared with that in glands without intestinal metaplasia
(26). Currently, the expression
pattern of Musashi-1 protein and its impact on the progression and
prognosis of gastric cancer has not yet been elucidated.
With an aim to evaluate the clinicopathological
implications of Musashi-1 in the progression and prognosis of
gastric cancer, the present study detected the expression of
Musashi-1 protein in gastric cancer tissues by western blotting and
immunohistochemistry, and compared Musashi-1 expression levels with
the clinicopathological parameters and survival rates of 436
patients with gastric cancer. The present study revealed that
Musashi-1 protein was significantly upregulated in gastric cancer
tissues and was associated with the progression and poor prognosis
of gastric cancer.
Materials and methods
Patients and tissue specimens
The present study was approved by the Institutional
Review Board of Zhejiang Provincial People's Hospital (Hangzhou,
China). Written informed consent was obtained from all patients
prior to enrollment in the present study. All specimens were
anonymously handled in accordance with the Declaration of Helsinki
and legal standards.
For western blotting, 36 patients who underwent
gastrectomy for gastric cancer at Zhejiang Provincial People's
Hospital were recruited between July 2013 and February 2014. All
cases were diagnosed clinically at the Department of
Gastrointestinal Surgery and histopathologically at the Department
of Pathology (Zhejiang Provincial People's Hospital). These
patients consisted of 19 males and 17 females, with a mean age of
66.7 years (range, 47–78 years) at the time of surgery. According
to the Lauren classification (27),
there were 18 diffuse-type and 20 intestinal-type gastric cancer
tissues. Following gastric resection, fresh specimens of cancerous
and matched non-cancerous tissues (adjacent gastric cancer margins
≥5 cm) were obtained immediately, dissected, snap-frozen in liquid
nitrogen in separate vials and stored at −80°C for further
analysis.
For immunohistochemistry, 436 patients who underwent
gastrectomy for gastric cancer at Zhejiang Provincial People's
Hospital between January 1998 and January 2004 were included in the
current study. All cases were diagnosed clinically and
histopathologically. The patient cohort consisted of 311 males and
125 females, with a median age of 64 years (range, 30–91 years).
All patients had follow-up records for ≥5 years. The follow-up
deadline was December 2008. The survival time was determined from
the date of surgery to the follow-up deadline or date of mortality.
Among the 436 gastric cancer tissues, 55 were from the cardia, 163
from the body and 218 from the gastric antrum. According to the
World Health Organization histological classification (28) of gastric carcinoma, 16 cases were
identified as papillary, 326 tubular, 29 mucinous and 65
signet-ring cell adenocarcinomas; 13 were highly differentiated,
128 well or moderately differentiated, 293 poorly differentiated
and 2 were undifferentiated adenocarcinomas. On the basis of the
Lauren classification of gastric cancer, 223 cases were
intestinal-type and 213 were diffuse-type. There were 61 cases with
distant metastasis and 270 cases with lymph node metastasis. In
terms of the 7th edition of the Union for International Cancer
Control Tumor-Node-Metastasis (TNM) classification system for
gastric cancer (29); 90 cases were
categorized as stage I, 104 as stage II, 173 as stage III and 69 as
stage IV. A total of 436 gastric cancer tissues and 92 adjacent
non-cancerous gastric mucosae were collected following gastrectomy
and formalin-fixed and paraffin-embedded (FFPE) for further study.
Following surgery, routine chemotherapy was administered to
patients with advanced disease, and no radiation treatment was
administered to any of the patients.
Evaluation of Musashi-1 protein
expression level by western blotting
The Musashi-1 protein expression level was
determined by western blotting in extracts of 36 gastric cancer
tissues and matched non-cancerous gastric mucosae. Total protein
was extracted using the KC™ Cell and Tissue Total Protein
Extraction kit (KC-415; KangChen Bio-tech Inc., Shanghai, China)
containing protease inhibitors (1 ml/250 mg specimen, 1 ml
extraction reagent suppleented with 10 µl protease inhibitor
mixture, 10 µl PMSF and 10 µl phosphatase mixture). The protein
concentration was determined using the KC™ bicinchoninic acid assay
protein quantification kit (KC-430; KangChen Bio-tech Inc.). A
total of 50 µg total protein was separated on 10% polyacrylamide
(acrylamide: bisacrylamide, 30:0.8%, w/v) SDS gel. The protein was
then transferred onto a polyvinylidine fluoride membrane. The
membrane was blocked at room temperature for 1 h with 5% bovine
serum albumin (Amresco, LLC, Solon, OH, USA), followed by
incubation at 4°C overnight with primary antibody (rabbit
monoclonal antibody to human Musashi-1; cat. no. 1877-1; dilution,
1:2,000; Epitomics, Burlingame, CA, USA). Following washing in TBST
(Tween-20 0.05%, v/v; TBS 10 mM, pH=7.5) for 5 min three times, the
membrane was incubated with secondary antibody (horseradish
peroxidase-conjugated anti-rabbit immunoglobulin; catalog no.
ab205718; dilution, 1:5,000; Epitomics) at room temperature for 1
h. Following three additional rinses with TBST, immunocomplexes
were revealed using the KC™ chemiluminescence kit (KC-420, KangChen
Bio-tech Inc.). Protein bands were scanned (Tanon 5,200 Multi;
Tanon Science and Technology Co., Ltd., Shanghai, China) and
quantified using ImageJ software (version 2.0; National Institutes
of Health, Bethesda, MD, USA). Analysis of
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels
was carried out as the control for western blotting using mouse
monoclonal anti-GAPDH antibody (catalog no. KC-5G4; dilution,
1:10,000; KangChen Bio-tech Inc.).
Tissue microarray (TMA)
construction
For diagnostic confirmation and establishing the
representative area, 4 µm sections were cut from each FFPE tissue
specimen and stained with hematoxylin and eosin (H&E) prior to
TMA construction. Subsequently, TMA blocks containing gastric
cancer tissues and non-cancerous gastric mucosae were prepared
using the method, as described previously (30). Briefly, tissue cylinders 2 mm in
diameter were punched from the targeted area of each donor block
and precisely arrayed into a recipient block using a TMA instrument
(no. HM315R; GMI, Inc., Ramsey, MN, USA). Each TMA block contained
six non-cancerous gastric mucosae as the controls. Consecutive 4 µm
thick sections were cut from each of the resulting TMA blocks, and
one section from each block was H&E stained for histological
verification of the adequacy of the arrayed tumor tissues. Eligible
sections were those in which the tumor tissue occupied >10% of
the core area. Sections were then placed on microscope slides for
further analysis.
Immunohistochemistry
Immunohistochemical staining was performed on the
TMA slides, as described previously (30). Briefly, the TMA slides were heated to
60°C for 2 h, de-waxed with xylene and rehydrated in graded ethanol
sequentially (100, 95 and 80%, v/v). Following antigen retrieval
[0.01 M citrate buffer (Beijing Solarbio Science and Technology
Co., Ltd., Beijing, China; pH, 6.0), 5 min, pressure cooker] and
endogenous peroxidase blockade [3% (w/v) H2O2
in pure methanol], the slides were incubated with 10% normal goat
serum (Beijing Solarbio Science & Technology Co., Ltd.,) at
room temperature for 10 min to reduce nonspecific reactions.
Incubation with the primary antibody (rabbit monoclonal antibody to
human Musashi-1; cat. no. 1877-1; dilution, 1:100; Epitomics) was
performed in a moist chamber at 4°C overnight. Following washing
three times with 0.01 M phosphate buffer (Beijing Solarbio Science
& Technology Co., Ltd.; pH, 7.2), the slides were incubated
with secondary antibody (horseradish peroxidase-conjugated mouse
monoclonal anti-rabbit immunoglobulin; cat. no. M0737; dilution,
1:1; Dako; Agilent Technologies Inc., Santa Clara, CA, USA) for 20
min at room temperature and stained with
diaminobenzidine-H2O2. Finally, the TMA
slides were counterstained with hematoxylin (0.5%, w/v), dehydrated
and mounted on a coverslip using neutral balsam (Shanghai Specimen
and Model Factory, Shanghai, China) and subsequently viewed under
an optical microscope. Omission of primary antibody served as the
negative control.
Evaluation of immunoreactivity
The Musashi-1 protein was immunohistochemically
stained and independently examined under a light microscope by two
pathologists who were blinded to the clinical data. The
immunoreactivity was evaluated by applying a scoring system
combining the intensity of immunostaining with the proportion of
immunoreactive cells (30). In brief,
the intensity of immunostaining was scored as 0 (no staining), 1
(weak staining, light yellow), 2 (moderate staining, yellow brown)
and 3 (intense staining, brown), and the proportion of
immunoreactive cells was scored as 0 (≤5% positive cells), 1 (6–25%
positive cells), 2 (26–50% positive cells) and 3 (≥51% positive
cells). In the case of a discrepancy, a consensus score was
selected. The product of the scores for intensity and proportion
was used to signify the level of protein expression. The expression
level of Musashi-1 was considered low if the product was ≤3 and
high if the product was ≥4.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation. Data were analyzed using the Student's t-test,
whereas categorical data were assessed using the χ2 test
or Fisher's exact test. The correlation coefficients between
protein expression and clinicopathological parameters were
estimated using the Spearman correlation method. The Kaplan-Meier
method was used to plot the survival curve and extract the
cumulative survival rate and mean survival time. The difference
between groups was compared with the log-rank test. Multivariate
survival analysis was carried out using the Cox proportional
hazards model, and variables that were significant in the
univariate analysis were included in the model with the Enter
method. All statistical analyses were performed using SPSS 16.0 for
Windows (SPSS, Inc., Chicago, IL, USA). All P-values were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of Musashi-1 protein
in gastric cancer and non-cancerous gastric mucosae
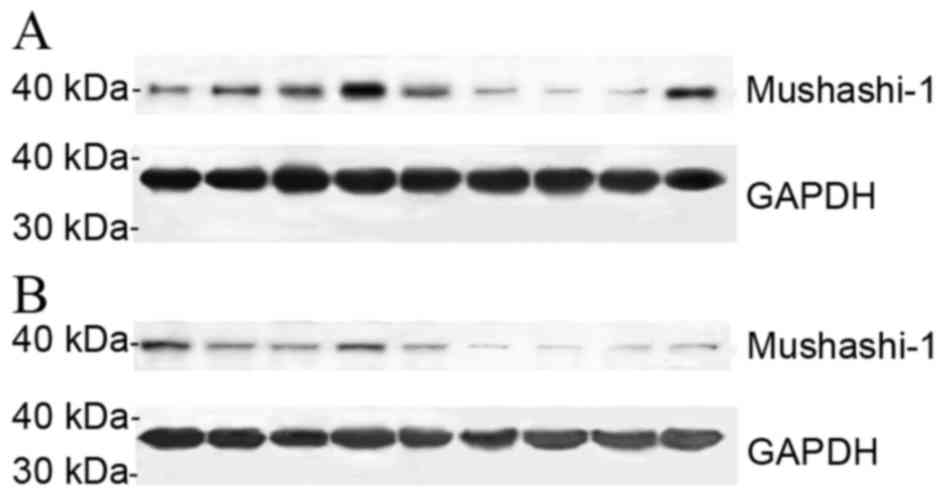
The expression levels of Musashi-1 protein in 36
frozen gastric cancer tissues and the corresponding adjacent
non-cancerous gastric mucosae were determined by western blotting.
The relative expression levels of Musashi-1 protein in gastric
cancer tissues were significantly higher compared with those in
non-cancerous gastric mucosae (0.317±0.045 vs. 0.203±0.030;
P<0.05), there were no significant differences identified in
Musashi-1 protein expression between intestinal-type and
diffuse-type gastric cancer (0.322±0.075 vs. 0.312±0.051,
P>0.05), as presented in Fig.
1.
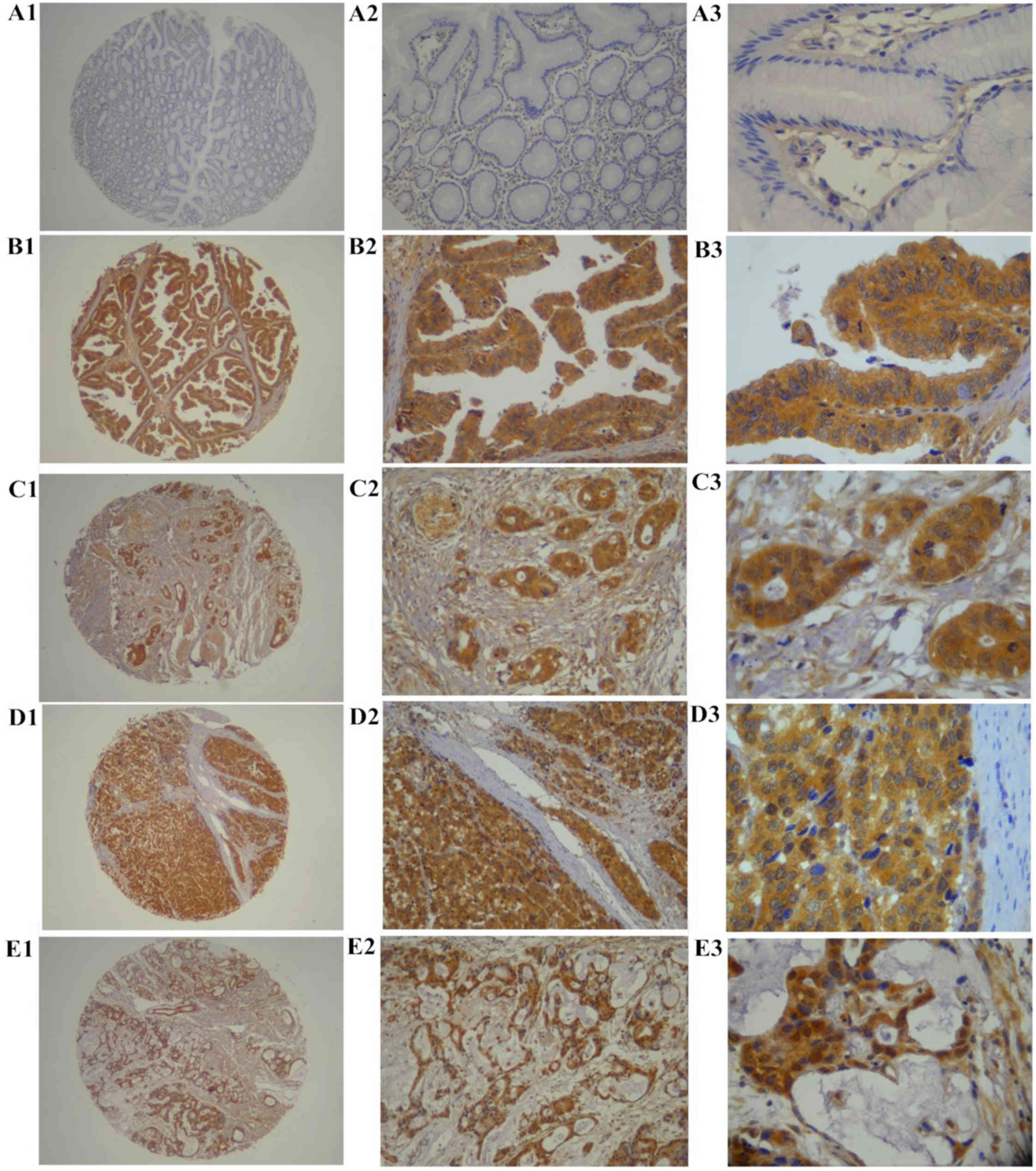
The expression of Musashi-1 protein in archived
specimens of 436 gastric cancer tissues and 92 non-cancerous
gastric mucosae was assessed by immunohistochemistry. Musashi-1
protein was predominantly expressed in the cytoplasm and on the
membrane of epithelial cells (Fig.
2). Musashi-1 protein expression was detected in 215/436
(49.3%) patients with gastric cancer, including high expression
levels in 154 (35.3%) cases, and low expression levels in 61
(14.0%) patients, whereas Musashi-1 protein was weakly (12/92
cases) or not expressed in non-cancerous gastric mucosae tissues.
The percentage of tissues with high Musashi-1 protein expression
level was significantly higher (P<0.0001) in gastric cancer
tissues compared with adjacent non-cancerous gastric mucosae
tissues.
Correlation of Musashi-1 protein
expression with clinicopathological parameters
The correlation was evaluated between Musashi-1
protein expression and clinicopathological parameters of patients
with gastric cancer. The expression level of Musashi-1 protein in
gastric cancer was associated with age, location, size, TNM stage,
depth of invasion, vessel invasion, Lauren classification, lymph
node metastasis and distant metastasis of the tumor, whereas it was
not associated with gender, differentiation and the histological
type of the tumor. Gastric cancer tissues from patients with with
deep tumor invasion (T3 and T4), high TNM stage (stage III and IV),
vessel invasion, lymph node metastasis and distant metastasis had
significantly higher expression levels of Musashi-1 compared with
those with superficial tumor invasion (T1 and T2), low TNM stage
(stage I and II) and without vessel invasion or lymph node and
distant metastasis (Table I). The
Spearman's correlation coefficients of Musashi-1 expression level
with depth of invasion, TNM stage, vessel invasion, lymph node
metastasis and distant metastasis of tumor were 0.287, 0.465,
0.337, 0.382 and 0.297, respectively.
 | Table I.Association of Musashi-1 protein
expression with clinicopathological parameters of patients with
gastric cancer. |
Table I.
Association of Musashi-1 protein
expression with clinicopathological parameters of patients with
gastric cancer.
|
|
| Musashi-1 protein
expression level |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Total no.
patients | Low (n, %) | High (n, %) | χ2 | P-value | r |
|---|
| Gender |
|
|
| 0.168 | 0.682 | 0.020 |
|
Male | 311 | 203 (65.3) | 108 (34.7) |
|
|
|
|
Female | 125 | 79 (63.2) | 46 (36.8) |
|
|
|
| Age range |
|
|
| 7.607 | 0.006 | 0.132 |
| ≤60
years | 237 | 167 (70.5) | 70 (29.5) |
|
|
|
| >60
years | 199 | 115 (57.8) | 84 (42.2) |
|
|
|
| Location of
tumor |
|
|
| 11.332 | 0.003 | −0.137 |
|
Cardia | 55 | 25 (45.5) | 30 (54.5) |
|
|
|
|
Body | 163 | 105 (64.4) | 58 (35.6) |
|
|
|
|
Antrum | 218 | 152 (69.7) | 66 (30.3) |
|
|
|
| Tumor size |
|
|
| 22.721 | <0.0001 | 0.228 |
| <5
cm | 256 | 189 (73.8) | 67 (26.2) |
|
|
|
| ≥5
cm | 180 | 93 (51.7) | 87 (48.3) |
|
|
|
| Depth of
invasion |
|
|
| 35.923 | <0.0001 | 0.287 |
| T1 | 57 | 50 (87.7) | 7 (12.3) |
|
|
|
| T2 | 109 | 85 (78.0) | 24 (22.0) |
|
|
|
| T3 | 244 | 136 (55.7) | 108 (44.3) |
|
|
|
| T4 | 26 | 11 (42.3) | 15 (57.7) |
|
|
|
| Vessel
invasion |
|
|
| 49.455 | <0.0001 | 0.337 |
|
Negative | 183 | 153 (83.6) | 30 (16.4) |
|
|
|
|
Positive | 253 | 129 (51.0) | 124 (49.0) |
|
|
|
| TNM stage |
|
|
| 96.863 | <0.0001 | 0.465 |
| I | 90 | 83 (92.2) | 7 (7.8) |
|
|
|
| II | 104 | 86 (82.7) | 18 (17.3) |
|
|
|
|
III | 173 | 95 (54.9) | 78 (45.1) |
|
|
|
| IV | 69 | 18 (26.1) | 51 (73.9) |
|
|
|
| Distant
metastasis |
|
|
| 38.402 | <0.0001 | 0.297 |
|
Negative | 375 | 264 (70.4) | 111 (29.6) |
|
|
|
|
Positive | 61 | 18 (29.5) | 43 (70.5) |
|
|
|
| Lymph node
metastasis |
|
|
| 63.553 | <0.0001 | 0.382 |
|
Negative | 166 | 146 (88.0) | 20 (12.0) |
|
|
|
|
Positive | 270 | 136 (50.4) | 134 (49.6) |
|
|
|
| Lauren
classification |
|
|
| 148.400 | <0.0001 | 0.583 |
|
Intestinal | 223 | 205 (91.9) | 18 (8.1) |
|
|
|
|
Diffuse | 213 | 77 (36.2) | 136 (63.8) |
|
|
|
| Grade of
differentiation |
|
|
| 0.120 | 0.913 | 0.005 |
| Well
and moderate | 143 | 93 (65.0) | 50 (35.0) |
|
|
|
| Poor
and not | 293 | 189 (64.5) | 104 (35.5) |
|
|
|
| Histological
type |
|
|
| 0.958 | 0.811 | 0.047 |
|
Papillary | 16 | 11 (68.8) | 5 (31.2) |
|
|
|
|
Tubular | 326 | 214 (65.6) | 112 (34.4) |
|
|
|
|
Mucinous | 29 | 18 (62.1) | 11 (37.9) |
|
|
|
|
Signet-ring cell | 65 | 39 (60.0) | 26 (40.0) |
|
|
|
Correlation between Musashi-1 protein
expression level and prognosis of patients with gastric cancer
Univariate survival analysis indicated that high
expression levels of Musashi-1 protein were associated with poor
prognosis of patients with gastric cancer (log-rank=236.846;
P<0.0001). The 1-, 3- and 5-year cumulative survival rates were
97.2, 86.9 and 48.9%, for patients with low Musashi-1 expression
level, and 83.1, 22.1 and 3.1% for patients with high Musashi-1
expression levels, respectively. The mean survival time for
patients with low expression levels of Musashi-1 was 51.1 months,
which was significantly higher (P<0.0001) compared with 28.1
months for patients with high expression levels of Musashi-1. It
was also revealed that age, tumor location, size, depth of
invasion, TNM stage, Lauren classification, vessel invasion, lymph
node metastasis and distant metastasis were significantly
associated with the survival of patients with gastric cancer,
whereas histological type and grade of differentiation were not
significantly associated with survival (Table II).
 | Table II.Univariate analysis of the
correlation between clinicopathological parameters and the survival
rate of patients with gastric cancer. |
Table II.
Univariate analysis of the
correlation between clinicopathological parameters and the survival
rate of patients with gastric cancer.
|
| Cumulative survival
(%) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | 1-year | 3-year | 5-year | Mean survival time
(months, 95% CI) | Log-rank | P-value |
|---|
| Age range |
|
|
|
| 14.745 | <0.001 |
| ≤60
years | 95.4 | 69.6 | 40.6 | 45.8
(43.7–48.7) |
|
|
| >60
years | 88.4 | 57.3 | 23.1 | 39.6
(37.0–42.0) |
|
|
| Tumor location |
|
|
|
| 7.849 | 0.020 |
|
Cardia | 89.1 | 49.1 | 21.3 | 37.8
(33.3–42.3) |
|
|
|
Body | 90.8 | 62.0 | 30.6 | 43.2
(40.4–46.4) |
|
|
|
Antrum | 94.0 | 69.3 | 36.2 | 44.1
(41.8–46.8) |
|
|
| Tumor size |
|
|
|
| 49.579 | <0.0001 |
| <5
cm | 94.9 | 74.2 | 45.3 | 47.5
(45.5–49.5) |
|
|
| ≥5
cm | 88.3 | 49.4 | 15.1 | 36.6
(33.9–39.9) |
|
|
| Histological
type |
|
|
|
| 0.934 | 0.817 |
|
Papillary | 93.8 | 62.5 | 23.4 | 41.9
(34.7–49.7) |
|
|
|
Tubular | 93.3 | 63.8 | 33.7 | 43.3
(41.3–45.3) |
|
|
|
Mucinous | 89.7 | 72.4 | 18.6 | 44.3
(38.0–50.0) |
|
|
|
Signet-ring cell | 87.7 | 61.5 | 35.4 | 41.5
(36.8–46.8) |
|
|
| Grade of
differentiation |
|
|
|
| 0.617 | 0.432 |
| Well
and moderate | 93.0 | 68.5 | 34.8 | 44.1
(41.2–47.2) |
|
|
| Poor
and not | 91.8 | 61.8 | 31.2 | 42.4
(40.4–44.4) |
|
|
| TNM stage |
|
|
|
| 370.398 | <0.0001 |
| I | 100.0 | 95.6 | 92.4 | 58.1
(56.2–60.2) |
|
|
| II | 96.2 | 83.7 | 72.5 | 53.0
(50.1–55.1) |
|
|
|
III | 91.3 | 57.2 | 1.2 | 37.7
(35.4–40.4) |
|
|
| IV | 78.3 | 10.1 | 0.0 | 23.3
(20.4–26.4) |
|
|
| Depth of
invasion |
|
|
|
| 135.118 | <0.0001 |
| T1 | 100.0 | 93.0 | 90.9 | 57.2
(54.7–59.7) |
|
|
| T2 | 93.6 | 78.9 | 53.9 | 50.0
(46.9–53.9) |
|
|
| T3 | 90.6 | 55.3 | 14.6 | 38.4
(36.2–40.2) |
|
|
| T4 | 84.6 | 19.2 | 0.0 | 26.8
(21.0–32.0) |
|
|
| Lymph node
metastasis |
|
|
|
| 176.051 | <0.0001 |
|
Negative | 97.6 | 86.1 | 78.4 | 54.2
(52.1–56.1) |
|
|
|
Positive | 88.9 | 50.4 | 6.8 | 36.3
(34.3–38.3) |
|
|
| Distant
metastasis |
|
|
|
| 141.372 | <0.0001 |
|
Negative | 95.2 | 72.3 | 37.5 | 46.2
(44.6–47.6) |
|
|
|
Positive | 73.8 | 13.1 | 1.6 | 23.2
(19.7–26.7) |
|
|
| Vessel
invasion |
|
|
|
| 127.410 | <0.0001 |
|
Negative | 97.8 | 86.3 | 67 | 52.6
(50.5–54.5) |
|
|
|
Positive | 88.1 | 47.8 | 10.4 | 36.2
(34.1–38.1) |
|
|
| Lauren
classification |
|
|
|
| 239.586 | <0.0001 |
|
Intestinal | 97.8 | 92.8 | 61.8 | 54.1
(52.5–55.5) |
|
|
|
Diffuse | 86.4 | 33.8 | 4.4 | 31.5
(29.4–33.4) |
|
|
| Musashi-1
expression |
|
|
|
| 236.846 | <0.0001 |
|
Low | 97.2 | 86.9 | 48.9 | 51.1
(49.5–52.5) |
|
|
|
High | 83.1 | 22.1 | 3.1 | 28.1
(25.8–30.8) |
|
|
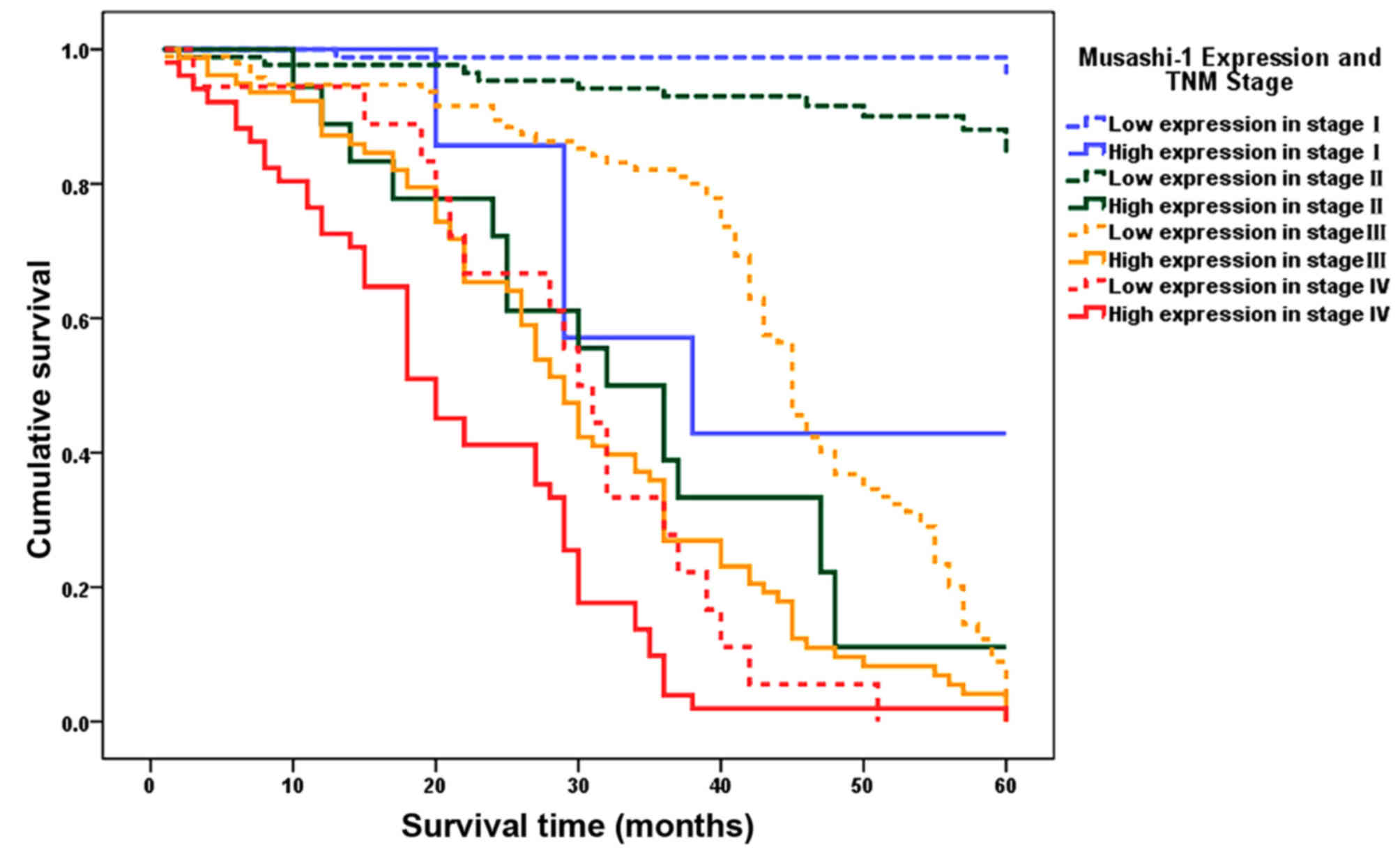
Upon stratification by TNM stage, the mean survival
time of patients with low Musashi-1 expression level was
significantly longer compared with that of patients with high
Musashi-1 expression level in TNM stage I (59.4 vs. 42.3 months;
P<0.0001), stage II (56.8 vs. 33.8 months; P<0.0001), stage
III (44.0 vs. 30.0 months; P<0.0001) and stage IV (29.3 vs. 21.1
months; P=0.018). Notably, patients with TNM II gastric cancer and
low expression levels of Musashi-1 had a longer mean survival time
compared with TNM stage I patients with high Musashi-1 expression
levels (56.8 vs. 42.3 months; P<0.001), and TNM stage III
patients with low expression levels of Musashi-1 had a longer mean
survival time compared with TNM stage II patients with high
Musashi-1 expression levels (44.0 vs. 33.8 months, P=0.034), as
presented in Table III and Fig. 3.
 | Table III.Correlation between Musashi-1 protein
expression level and mean survival time of 436 patients with
gastric cancer as stratified by TNM stage. |
Table III.
Correlation between Musashi-1 protein
expression level and mean survival time of 436 patients with
gastric cancer as stratified by TNM stage.
|
| Mean survival time
(month, 95% CI) |
|
|
|---|
|
|
|
|
|
|---|
| TNM stage | Musashi-1 low
expression level | Musashi-1 high
expression level | Log-rank | P-value |
|---|
| I | 59.4
(57.9–61.9) | 42.3
(30.4–54.4) | 34.501 | <0.0001 |
| II | 56.8a (54.4–59.4) | 33.8
(26.6–41.6) | 56.560 | <0.0001 |
| III | 44.0b (41.2–46.2) | 30.0
(26.9–33.9) | 32.321 | <0.0001 |
| IV | 29.3
(24.0–34.0) | 21.1
(17.9–24.9) | 5.557 | 0.018 |
Clinicopathological factors that were associated
with the survival of patients with gastric cancer in the univariate
survival analysis were included as covariates in the Cox regression
analysis. It was revealed that Musashi-1 protein expression level,
Lauren classification, distant metastasis, TNM stage and depth of
invasion were independent prognostic indicators for the survival of
patients with gastric cancer, whereas age, tumor location, size,
lymph node metastasis and vessel invasion were not (Table IV).
 | Table IV.Multivariate analysis of the
correlation between clinicopathological parameters and the survival
rate of 436 patients with gastric cancer. |
Table IV.
Multivariate analysis of the
correlation between clinicopathological parameters and the survival
rate of 436 patients with gastric cancer.
| Covariates | Coefficient | SE | HR (95% CI) | P-value |
|---|
| Age range (>60
vs. ≤60) | 0.194 | 0.130 | 1.215
(0.942–1.942) | 0.135 |
| Tumor location
(cardia vs. other locations) | −0.168 | 0.180 | 0.846
(0.595–1.595) | 0.350 |
| Tumor size (≥5 cm
vs. <5 cm) | 0.061 | 0.131 | 1.062
(0.822–1.822) | 0.644 |
| Lauren
classification (diffuse vs. intestinal) | 0.673 | 0.170 | 1.960
(1.404–2.404) | <0.0001 |
| Lymph node
metastasis (positive vs. negative) | 0.203 | 0.335 | 1.225
(0.635–2.635) | 0.545 |
| Vessel invasion
(positive vs. negative) | −0.098 | 0.189 | 0.907
(0.626–1.626) | 0.606 |
| Distant metastasis
(positive vs. negative) | 0.707 | 0.272 | 2.028
(1.189–3.189) | 0.009 |
| Musashi-1 protein
expression (high vs. low) | 0.789 | 0.140 | 2.201
(1.673–2.673) | <0.0001 |
| Depth of
invasion |
|
|
| 0.007 |
| T2 vs.
T1 | 0.482 | 0.531 | 1.620
(0.572–4.572) | 0.364 |
| T3 vs.
T1 | 0.989 | 0.535 | 2.687
(0.942–7.942) | 0.065 |
| T4 vs.
T1 | 0.411 | 0.573 | 1.508
(0.490–4.490) | 0.474 |
| TNM stage |
|
|
| <0.001 |
| Stage
II vs. stage I | 0.810 | 0.520 | 2.247
(0.811–6.811) | 0.119 |
| Stage
III vs. stage I | 2.088 | 0.604 | 8.069
(2.472–26.472) | <0.001 |
| Stage
IV vs. stage I | 2.594 | 0.654 | 13.380
(3.715–48.715) | <0.0001 |
Discussion
Musashi-1 expression has been identified to be
restricted to the isthmus neck region (the putative position of
stem cells) of normal gastric glands (26). Upregulation of Musashi-1 has
previously been revealed to occur in rat gastric corpus mucosa,
following ethanol-induced mucosal injury (31), leading to the suggestion that a
subpopulation of parietal cells are a source of Musashi-1, which
contributes to rapid re-epithelization by restoration of stem cells
and regulation of cell differentiation (31). The Musashi-1 expression level has also
been demonstrated to be associated with Helicobacter pylori
infection (32). Increased expression
levels of Musashi-1 in gastric precancerous lesions, including
intestinal metaplasia and dysplasia, suggested that Musashi-1 may
serve a crucial role in the carcinogenesis of gastric cancer
(25).
In the present study, the results from western
blotting and immunohistochemistry revealed that the expression
levels of Musashi-1 protein in gastric cancer tissues were
significantly higher compared with those in adjacent non-cancerous
gastric mucosae. In discordance with a study by Choi et al
(33), which demonstrated that
Musashi-1 protein was more frequently overexpressed in young
patients (≤30 years) compared with in patients >60 years, the
immunohistochemical assay of the present study demonstrated that
Musashi-1 protein expression levels were significantly upregulated
in patients aged >60 years compared with those aged ≤60 years.
There was a difference in age categorization between the current
study and this previous study, and the cohort of the present study
recruited only one patient aged ≤30 years (17 years), making
further analysis and comparison unattainable. The present study
also indicated that the expression level of Musashi-1 in gastric
cancer tissues was significantly associated with location, size,
depth of invasion, vessel invasion, TNM stage, Lauren
classification and lymph node and distant metastasis of the tumors.
High Musashi-1 expression level was more frequently observed in
tumors at high TNM stages (stages III and IV), with deep invasion
(T3 and T4), presence of vessel invasion, lymph node metastasis and
distant metastasis, compared with tumors at low TNM stages (stages
I and II), with superficial invasion (T1 and T2), absence of vessel
invasion, lymph node metastasis and distant metastasis. The results
indicate that Musashi-1 may be involved in the invasion and
metastasis of gastric cancer. Finally, it is of note that the
immunohistochemistry assay demonstrated an association between high
Musashi-1 protein expression levels and diffuse-type tumors, which
was similar to the results of a previous study by Choi et al
(33); however, the western blot
analysis of the present study did not identify a difference in
Musashi-1 expression level between diffuse-type and intestinal-type
gastric cancer.
As a well-established stem/progenitor cell marker in
both normal and cancer cells, Musashi-1 protein has been documented
to be overexpressed in numerous types of cancer (34). The molecular mechanisms underlying the
functions and regulation of Musashi-1 are not currently well known.
Musashi-1 serves roles in the maintenance of the stem-cell state,
differentiation and tumorigenesis as a translational repressor of
target mRNAs (35). Musashi-1 protein
upregulates the Notch signaling pathway by translationally
suppressing Numb mRNA, a Notch pathway repressor (36). The Notch signaling pathway is
established to control cell fate decisions and the stem cell
phenotype (37), and is associated
with the growth (38,39) and progression (40,41) of a
wide spectrum of tumor types. Currently, the Musashi/Numb/Notch
signaling pathway cascade is considered to be associated with
numerous adult malignancies (42).
The cyclin-dependent kinase inhibitor p21WAF-1 is another Musashi-1
target (43). It was revealed that
Musashi-1 modulates endometrial carcinoma cell cycle progression
and apoptosis via the stemness-associated factors Notch-1, Hes-1
and p21WAF-1 (44), thereby inducing
crosstalk between a number of signal systems involved in the
self-renewal of stem cells (10).
Musashi-1 modulates cancer cell growth by the post-transcriptional
regulation of phosphoinositide 3-kinase/protein kinase B signaling
pathways (45). By contrast, within
the context of a primary mammalian neural stem/progenitor cell,
Musashi-1 may be converted from a repressor to an activator of mRNA
translation in response to extracellular stimuli (11). Musashi-1 protein was also identified
to serve an oncogenic role in hepatocellular carcinoma by
activating the Wnt signaling pathway via direct downregulation of
the tumor suppressor protein, Dickkopf-1 (46). In addition, tumor suppressor microRNAs
(miRNAs) are known to target genes with oncogenic properties,
including Musashi-1, and for being downregulated or deleted in
tumor tissue (34). The long 3′
untranslated region of Musashi-1 is potentially targeted by tumor
suppressor miRNAs, thereby affecting its expression pattern during
tumorigenesis of malignancies (34).
miR-34a, −101, −128, −137 and −138 were revealed to function as
tumor-suppressive miRNAs and negatively regulated Musashi-1
(47). Finally, Musashi-1 is also
regulated by human antigen R via mRNA translation and stability in
glioblastoma cells, which resulted in a positive regulation of
Musashi-1 expression level (48).
As a result of its involvement in the invasion and
metastasis of tumors, Musashi-1 had been proposed to be a biomarker
associated with poor prognosis for a number of cancer subtypes
(20–22). The present study demonstrated that the
cumulative survival rates and mean survival time for patients with
low Musashi-1 expression levels were significantly higher compared
with those for patients with high Musashi-1 expression levels.
Furthermore, it was revealed that a high expression level of
Musashi-1 was an independent prognostic factor for patients with
gastric cancer. Other factors correlated with the survival rate of
patients included age, tumor location, size, depth of invasion, TNM
stage, Lauren classification, vessel invasion, lymph node
metastasis and distant metastasis. In addition, Lauren
classification, distant metastasis, TNM stage and depth of invasion
are independent prognostic indicators for the survival rate of
patients with gastric cancer. As stratified by TNM stage, the mean
survival time for patients with low Musashi-1 expression levels
were significantly longer compared with that for patients with high
Musashi-1 expression level in each TNM stage. Of note, patients
with TNM stage II gastric cancer and low expression levels of
Musashi-1 demonstrated a longer mean survival time compared with
patients with TNM stage I and high Musashi-1 expression levels, and
patients with TNM stage III gastric cancer and low expression
levels of Musashi-1 revealed a longer mean survival time compared
with patients with TNM stage II and high Musashi-1 expression
levels. The results of the present study from univariate and
multivariate survival analysis highlighted the prognostic relevance
of Musashi-1 protein in patients with gastric cancer. Therefore, it
was suggested that the expression level of Musashi-1 protein may be
used on the basis of TNM stage to redefine the prognosis of
patients with gastric cancer, contributing to developing a
chemotherapeutic strategy for the effective treatment of gastric
cancer.
In addition to being a putative prognostic factor
for numerous malignancies, Musashi-1 has also received considerable
attention as a potential target for cancer therapy (16,49).
Musashi-1-overexpressing cells exhibit tumorigenic properties in
tumor graft experiments (50),
whereas knockdown of Musashi-1 resulted in mitotic catastrophe,
reduced cell proliferation and survival rate (45,49),
increased apoptosis in tumor cells and tumor growth arrest in
grafts (51). A natural product
(−)-gossypol was identified to inhibit colon cancer cell growth by
targeting Musashi-1 protein (52).
Further investigation revealed that Musashi-1 silencing
significantly inhibited proliferative ability and attenuated the
migration and invasion activity of colon cancer cells (16). The aforementioned observations
suggested that the inhibition of Musashi-1′s RNA binding activity
may be an effective anticancer strategy and that Musashi-1
represents a promising target for anticancer agent discovery.
The limitation of the present study is the small
size of tissue samples used in western blotting, which may
partially account for the discordance in results regarding the
association between Musashi-1 protein expression level and Lauren
classification from immunohistochemistry and western blotting.
Further studies are required to dissect the association between
Musashi-1 protein expression level and Lauren classification for
gastric cancer.
In conclusion, Musashi-1 protein serves an important
role in the progression of gastric cancer. The detection of
Musashi-1 protein expression level alone or in combination with TNM
staging is useful for predicting the prognosis of patients with
gastric cancer, therefore contributing to a personalized
chemotherapy regimen. It is also possible that Musashi-1 may be
used as a molecular target for gastric cancer treatment.
Acknowledgements
This study was supported by the Research Foundation
of Science Technology Department of Zhejiang Province (grant no.
2008C33040) and the Medical Research Program of Zhejiang Province,
China (grant nos. 2007A013 and 2013KYA018). The authors thank Ms.
Wen-Juan Xu for assistance with specimen collection and
follow-up.
References
|
1
|
Tore LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WQ, Zheng RS, Zhang SW, Zeng HM and
Zou XN: The incidences and mortalities of major cancers in China,
2010. Chin J Cancer. 33:402–405. 2014.PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11 (Internet)International Agency
for Research on Cancer. Lyon, France: 2013 http://globocan.iarc.frAccessed. July
26–2015
|
|
5
|
Liu W, Yang Q, Liu B and Zhu Z: Serum
proteomics for gastric cancer. Clin Chim Acta. 431:179–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Röcken C: Ways to personalized medicine
for gastric cancer. Pathologe. 34:403–412. 2013.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Humphries JM, Penno MA, Weiland F,
Klingler-Hoffmann M, Zuber A, Boussioutas A, Ernst M and Hoffmann
P: Identification and validation of novel candidate protein
biomarkers for the detection of human gastric cancer. Biochim
Biophys Acta. 1844:1051–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okano H, Kawahara H, Toriya M, Nakao K,
Shibata S and Imai T: Function of RNA-binding protein Musashi-1 in
stem cells. Exp Cell Res. 306:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
MacNicol MC, Cragle CE and MacNicol AM:
Context-dependent regulation of Musashi-mediated mRNA translation
and cell cycle regulation. Cell Cycle. 10:39–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siddall NA, McLaughlin EA, Marriner NL and
Hime GR: The RNA-binding protein Musashi is required intrinsically
to maintain stem cell identity. Proc Natl Acad Sci USA.
103:8402–8407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song X, Zhou C, Zhou S, Zhang L, Feng G,
Zhao D and Huang F: The expression patterns of Msi1 related with
the glioma grade and the cytoplasmic Msi1 promotes angiogenesis.
Tissue Cell. 45:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bobryshev YV, Freeman AK, Botelho NK, Tran
D, Levert-Mignon AJ and Lord RV: Expression of the putative stem
cell marker Musashi-1 in Barrett's esophagus and esophageal
adenocarcinoma. Dis Esophagus. 23:580–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan LF, Dong WG, Jiang CQ, Xia D, Liao F
and Yu QF: Expression of putative stem cell genes Musashi-1 and
beta1-integrin in human colorectal adenomas and adenocarcinomas.
Int J Colorectal Dis. 25:17–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Peng X, Yan D, Tang H, Huang F, Yang
Y and Peng Z: Msi-1 is a predictor of survival and a novel
therapeutic target in colon cancer. Ann Surg Oncol. 18:2074–2083.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu DC, Yang ZL and Jiang S:
Identification of musashi-1 and ALDH1 as carcinogenesis,
progression, and poor-prognosis related biomarkers for gallbladder
adenocarcinoma. Cancer Biomark. 8:113–121. 2010.-2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kelsch R, Schüring AN and Kiesel L: Increased expression of the
adult stem cell marker Musashi-1 in endometriosis and endometrial
carcinoma. J Pathol. 215:317–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Jiang CQ and Fan LF: Correlation
of Musashi-1, Lgr5, and pEGFR expressions in human small intestinal
adenocarcinomas. Tumour Biol. 36:6075–6082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XY, Penalva LO, Yuan H, Linnoila RI,
Lu J, Okano H and Glazer RI: Musashi1 regulates breast tumor cell
proliferation and is a prognostic indicator of poor survival. Mol
Cancer. 9:2212010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen PX, Li QY and Yang Z: Musashi-1
expression is a prognostic factor in ovarian adenocarcinoma and
correlates with ALDH-1 expression. Pathol Oncol Res. 21:1133–1140.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ravindran G and Devaraj H: Prognostic
significance of neural stem cell markers, Nestin and Musashi-1, in
oral squamous cell carcinoma: Expression pattern of Nestin in the
precancerous stages of oral squamous epithelium. Clin Oral
Investig. 19:1251–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kayahara T, Sawada M, Takaishi S, Fukui H,
Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H and Chiba
T: Candidate markers for stem and early progenitor cells, Musashi-1
and Hes1, are expressed in crypt base columnar cells of mouse small
intestine. FEBS Lett. 535:131–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuang RG, Kuang Y, Luo QF, Zhou CJ, Ji R
and Wang JW: Expression and significance of Musashi-1 in gastric
cancer and precancerous lesions. World J Gastroenterol.
19:6637–6644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang T, Ong CW, Shi J, Srivastava S, Yan
B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B and
Salto-Tellez M: Sequential expression of putative stem cell markers
in gastric carcinogenesis. Br J Cancer. 105:658–665. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akasaka Y, Saikawa Y, Fujita K, Kubota T,
Ishikawa Y, Fujimoto A, Ishii T, Okano H and Kitajima M: Expression
of a candidate marker for progenitor cells, Musashi-1, in the
proliferative regions of human antrum and its decreased expression
in intestinal metaplasia. Histopathology. 47:348–356. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lauren P: The two histological main types
of gastric cancer: Diffuse and so-called intestinal type carcinoma.
An attempt at a histo-clinical classification. Acta Pathol
Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
28
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
3. 4th. IARC Press; Lyon: 2010
|
|
29
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. 7th. International Union
Against Cancer; New York, NY: 2009
|
|
30
|
Shou ZX, Jin X and Zhao ZS: Upregulated
expression of ADAM17 is a prognostic marker for patients with
gastric cancer. Ann Surg. 256:1014–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagata H, Akiba Y, Suzuki H, Okano H and
Hibi T: Expression of Musashi-1 in the rat stomach and changes
during mucosal injury and restitution. FEBS Lett. 580:27–33. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murata H, Tsuji S, Tsujii M, Nakamura T,
Fu HY, Eguchi H, Asahi K, Okano H, Kawano S and Hayashi N:
Helicobacter pylori infection induces candidate stem cell marker
Musashi-1 in the human gastric epithelium. Dig Dis Sci. 53:363–369.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi JE, Bae JS, Lee JH, Jang KY, Chung MJ
and Moon WS: Musashi-1 expression and clinicopathological
significance in young gastric cancer patients: A matched
case-control study. Int J Oncol. 44:1185–1192. 2014.PubMed/NCBI
|
|
34
|
Vo DT, Qiao M, Smith AD, Burns SC, Brenner
AJ and Penalva LO: The oncogenic RNA-binding protein Musashi1 is
regulated by tumor suppressor miRNAs. RNA Biol. 8:817–828. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okano H, Imai T and Okabe M: Musashi: A
translational regulator of cell fate. J Cell Sci. 115:1355–1359.
2002.PubMed/NCBI
|
|
36
|
Imai T, Tokunaga A, Yoshida T, Hashimoto
M, Mikoshiba K, Weinmaster G, Nakafuku M and Okano H: The neural
RNA-binding protein Musashi1 translationally regulates mammalian
numb gene expression by interacting with its mRNA. Mol Cell Biol.
21:3888–3900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fender AW, Nutter JM, Bertrand FE and
Sigounas G: Notch-1 promotes stemness and epithelial to mesenchymal
transition in colorectal cancer. J Cell Biochem. 116:2517–2527.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abravanel DL, Belka GK, Pan TC, Pant DK,
Collins MA, Sterner CJ and Chodosh LA: Notch promotes recurrence of
dormant tumor cells following HER2/neu-targeted therapy. J Clin
Invest. 125:2484–2496. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yen WC, Fischer MM, Axelrod F, Bond C,
Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, et al:
Targeting notch signaling with a notch2/notch3 antagonist
(tarextumab) inhibits tumor growth and decreases tumor-initiating
cell frequency. Clin Cancer Res. 21:2084–2095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5:103382015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Cao G, Yuan X, Zhang X, Zhang Q,
Zhu Y, Dong Z and Zhang S: Notch-1 knockdown suppresses
proliferation, migration and metastasis of salivary adenoid cystic
carcinoma cells. J Transl Med. 13:1672015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishimoto Y and Okano H: New insight into
cancer therapeutics: Induction of differentiation by regulating the
Musashi/Numb/Notch pathway. Cell Res. 20:1083–1085. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Battelli C, Nikopoulos GN, Mitchell JG and
Verdi JM: The RNA-binding protein Musashi-1 regulates neural
development through the translational repression of p21WAF-1. Mol
Cell Neurosci. 31:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Götte M, Greve B, Kelsch R, Müller-Uthoff
H, Weiss K, Masouleh Kharabi B, Sibrowski W, Kiesel L and Buchweitz
O: The adult stem cell marker Musashi-1 modulates endometrial
carcinoma cell cycle progression and apoptosis via Notch-1 and
p21WAF1/CIP1. Int J Cancer. 129:2042–2049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Muto J, Imai T, Ogawa D, Nishimoto Y,
Okada Y, Mabuchi Y, Kawase T, Iwanami A, Mischel PS, Saya H, et al:
RNA-binding protein Musashi1 modulates glioma cell growth through
the post-transcriptional regulation of Notch and PI3 kinase/Akt
signaling pathways. PLoS One. 7:e334312012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen K, Gao Q, Zhang W, Liu Z, Cai J, Liu
Y, Xu J, Li J, Yang Y and Xu X: Musashi1 regulates survival of
hepatoma cell lines by activation of Wnt signalling pathway. Liver
Int. 35:986–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Smith AR, Marquez RT, Tsao WC, Pathak S,
Roy A, Ping J, Wilkerson B, Lan L, Meng W, Neufeld KL, et al: Tumor
suppressive microRNA-137 negatively regulates Musashi-1 and
colorectal cancer progression. Oncotarget. 6:12558–12573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vo DT, Abdelmohsen K, Martindale JL, Qiao
M, Tominaga K, Burton TL, Gelfond JA, Brenner AJ, Patel V, Trageser
D, et al: The oncogenic RNA-binding protein Musashi1 is regulated
by HuR via mRNA translation and stability in glioblastoma cells.
Mol Cancer Res. 10:143–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang XY, Yu H, Linnoila RI, Li L, Li D, Mo
B, Okano H, Penalva LO and Glazer RI: Musashi1 as a potential
therapeutic target and diagnostic marker for lung cancer.
Oncotarget. 4:739–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rezza A, Skah S, Roche C, Nadjar J,
Samarut J and Plateroti M: The overexpression of the putative gut
stem cell marker Musashi-1 induces tumorigenesis through Wnt and
Notch activation. J Cell Sci. 123:3256–3265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sureban SM, May R, George RJ, Dieckgraefe
BK, McLeod HL, Ramalingam S, Bishnupuri KS, Natarajan G, Anant S
and Houchen CW: Knockdown of RNA binding protein musashi-1 leads to
tumor regression in vivo. Gastroenterology. 134:1448–1458. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lan L, Appelman C, Smith AR, Yu J, Larsen
S, Marquez RT, Liu H, Wu X, Gao P, Roy A, et al: Natural product
(−)-gossypol inhibits colon cancer cell growth by targeting
RNA-binding protein Musashi-1. Mol Oncol. 9:1406–1420. 2015.
View Article : Google Scholar : PubMed/NCBI
|