Introduction
Human cancer arises through the accumulation of
genetic alterations in multiple oncogenes and tumor suppressor
genes. However, the exact timing of the majority of molecular
genetic events during carcinogenesis and their correlation with
defined histopathological stages are largely unknown. (1–7). Invasive
ductal carcinoma (IDC) of the breast is the result of a multistep
process, beginning with ductal hyperplasia and followed by atypical
ductal hyperplasia, ductal carcinoma in situ (DCIS),
invasive ductal carcinoma and metastatic disease (1–3). Previous
studies in the literature (8–10) indicate that alterations in the p arm
of chromosome 9 may be a common denominator in human cancer, and
may have a role in the early stages of breast cancer, including
ductal hyperplasia and DCIS (11–14). Of
interest is the finding that loss of heterozygosity (LOH) in the p
arm of chromosome 9 may be involved in the pathogenesis of breast
cancer (15–19).
In the present study, laser capture microdissection
(LCM) was used to analyze paraffin-embedded tissues of the normal
breast, ductal hyperplasia, DCIS and IDC to obtain DNA from
selected populations of cells for molecular genetic analysis
(20–22). LCM was used in order to obtain cells
with a high degree of purity in their phenotypes, without
contamination of stromal, inflammatory or other cells that could
interfere with final conclusions of molecular analysis. The
isolated cells representing different stages of breast cancer
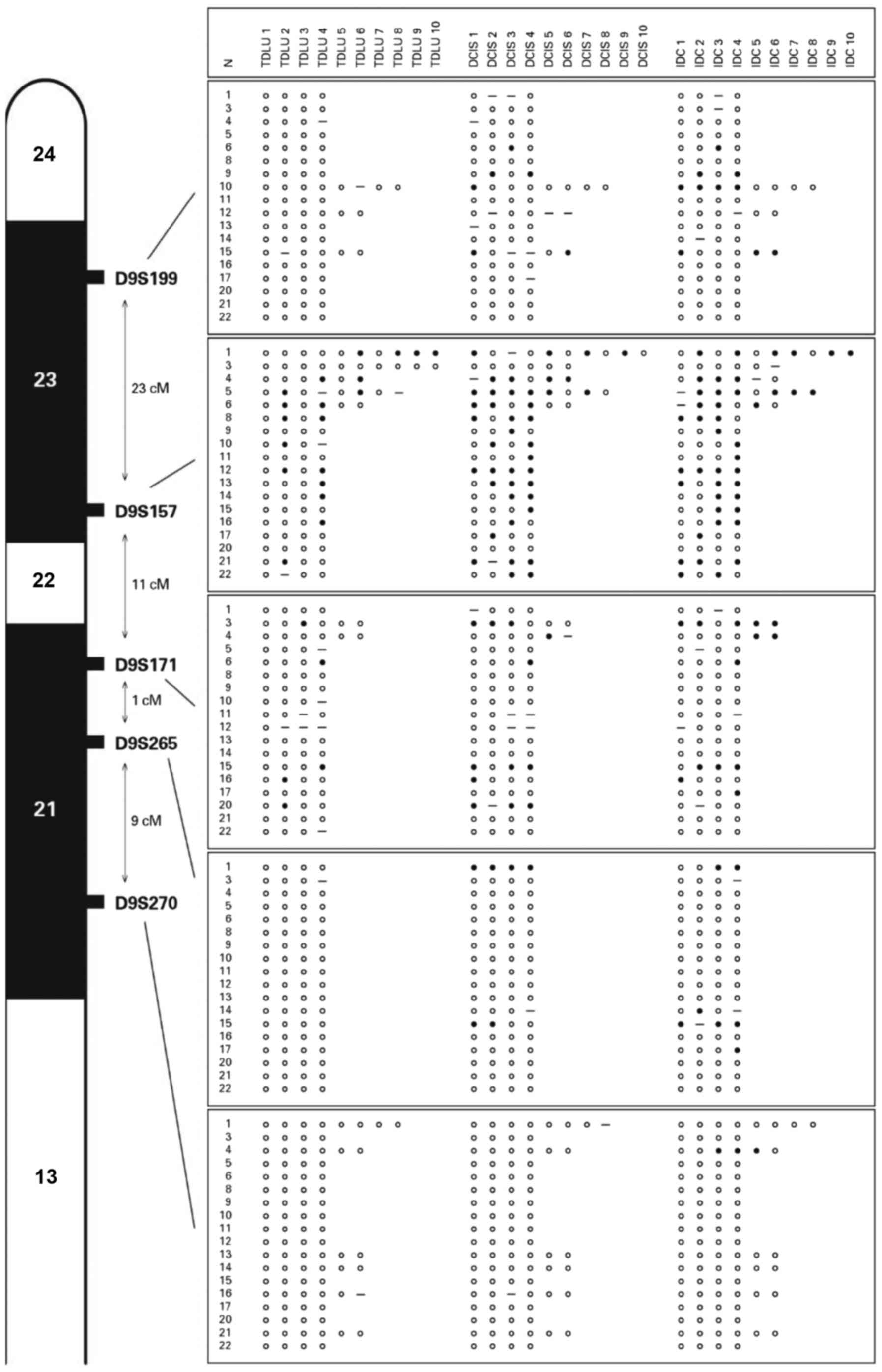
progression were used for detecting LOH using five microsatellite
markers: D9S199, D9S 157, D9S 171, D9S265 and D9S270. The present
study was conducted in an attempt to investigate the intratumoral
heterogeneity and to associate chromosomal alterations with
morphologic findings and proliferation state of the tumor.
Materials and methods
Tissue samples
Paraffin blocks from fourteen primary breast IDC
cases (mean age, 56; range, 27–86) that also contained areas of
carcinoma in situ were selected for the present study.
Paraffin blocks containing areas of normal tissue, including
breast, skin and lymph nodes, were available from the same
patients. Tissue blocks were obtained from the tumor bank of the
Breast Cancer Research Laboratory of the Fox Chase Cancer Center
(FCCC; Philadelphia, PA, USA). Six serial 5-µm sections were
obtained from each paraffin-embedded tissue block and stained with
hematoxylin and eosin (H&E). The first section was coverslipped
and the remaining five sections were dehydrated and air dried for
their use in LCM and DNA extraction. Tissue sections containing IDC
were selected on the basis that DCIS was also present in the same
section. The histopathological type of the carcinoma was classified
according to previously described criteria (23). Control tissues consisted of
phenotypically normal cells, which were obtained by LCM from: a)
Type 1 lobules or terminal duct lobular units (TDLUs) (24); b) normal skin obtained from the
mastectomy specimen; or c) lymph nodes free of metastases obtained
from axillary dissection from the same patient. This study was
approved by the Ethical Review Board (IRB 93–031) of the FCCC and
informed consent was obtained from patients for use of their
tissue.
LCM
Serial 5-µm thick sections containing IDC, DCIS and
normal tissue were utilized for microdissection. Areas containing
IDC, DCIS or normal tissue were identified in the slide that had
been stained with H&E and coverslipped. Preferentially, areas
containing microscopically homogeneous cells of each type of lesion
were selected. Tissues containing areas with dense stroma,
inflammatory cells, vascular or lymphatic vessels, muscle or
adipose tissue were avoided. Uncoverslipped serial 5-µm sections
slides were carefully matched with the respective area identified
in the coverslipped stained slide for verifying the accuracy of the
type of lesion selected for dissection. Tissue sections were
microdissected using a PixCell laser capture microdissection
apparatus (Arcturus Engineering, Mountain View, CA, USA) fitted
with cap in which a transparent thermoplastic film (ethylene vinyl
acetate polymer) was bonded to the underside. A cap was placed on
the specific lesion or normal tissue selected for dissection under
visual inspection by the operator. Then an infrared laser pulse was
activated and selected cells were transferred to the undersurface
of the cap, which was lifted off the tissue; the cells obtained at
each one of these laser shots were termed ‘a capture’. This process
was repeated successively in adjacent areas of the same lesion
twenty times using a 30-µm diameter laser beam. The caps containing
the captured tissues were placed into a 500-µl microcentrifuge tube
for molecular processing. Multiple foci from three to ten different
areas of in situ cancer, invasive carcinoma and ‘normal’
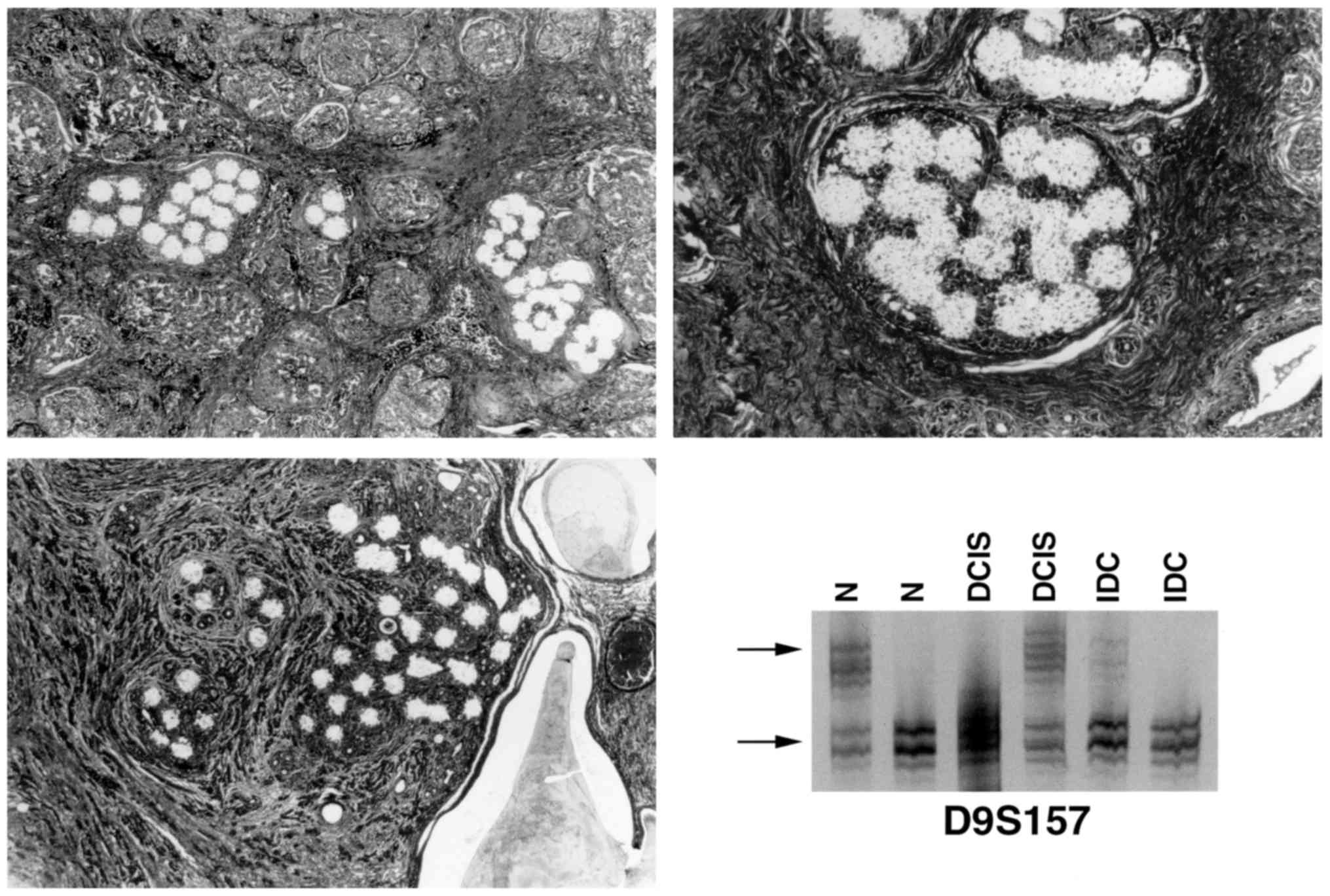
tissue were individually microdissected and separately analyzed
(Fig. 1). Finally, direct
visualization of the transferred tissue by light microscopy of the
capsule verified that the desired cells had been captured.
DNA extraction
DNA extraction from the selected tissues was
performed following the protocol provided by PixCell II™ (Arcturus
Engineering, Inc., Mountain View, CA, USA) Selected tissues were
digested for 16 h at 42°C in buffer containing: 10 mM Tris-HCL (pH
8.8), 1 mM EDTA, 1% Tween-20 and 0.05% Proteinase K. The lysate was
heated at 96°C for 8 min to inactivate Proteinase K and aliquots of
2 µl of this lysate were used directly as templates for PCR.
Polymerase chain reaction (PCR)
amplification and microsatellite analysis
Five microsatellite markers mapped to the short arm
of chromosome 9 (D9S199, D9S157, D9S171, D9S265 and D9S270) were
used for LOH analysis. Primers for PCR amplification were obtained
from Research Genetics Inc. (Huntsville, AL, USA) and all primer
sequence position of the markers, their levels of heterozygosity
and distances were obtained from Genome Database version February
2000 (Research Genetics, Inc.). PCRs were carried out according to
published study (25). The samples
were denatured for 5 min at 94°C and loaded onto a 6%
polyacrylamide gel. Electrophoresis was performed at room
temperature at 1,400 V for 2–3 h, depending on the length of the
marker. Following electrophoresis, gels were transferred to a 3 mm
Whatman paper, dried and autoradiographed using Kodak X-OMAT 35×43
film. Films were developed after a 48 to 72-h exposure.
Autoradiograms were analyzed following the guidelines of published
work (8).
Results
Invasive ductal carcinomas exhibited LOH for the
five markers tested, and the marker at 9p22-23 (D9S157) was the
most frequently identified, whereas the markers D9S171, D9S199,
D9S265 and D9S270 (Fig. 1) were less
frequently detected. LOH in the DCIS samples was found with 4/5 of
the markers tested. D9S157 locus was also present in the majority
of the samples, followed by 9p21 (D9S171), D9S199 and D9S265. There
are several reports in the literature indicating that other tumor
suppressor gene(s) may reside within different 9p loci, namely
9p22-23 (8,15,17,26–28).
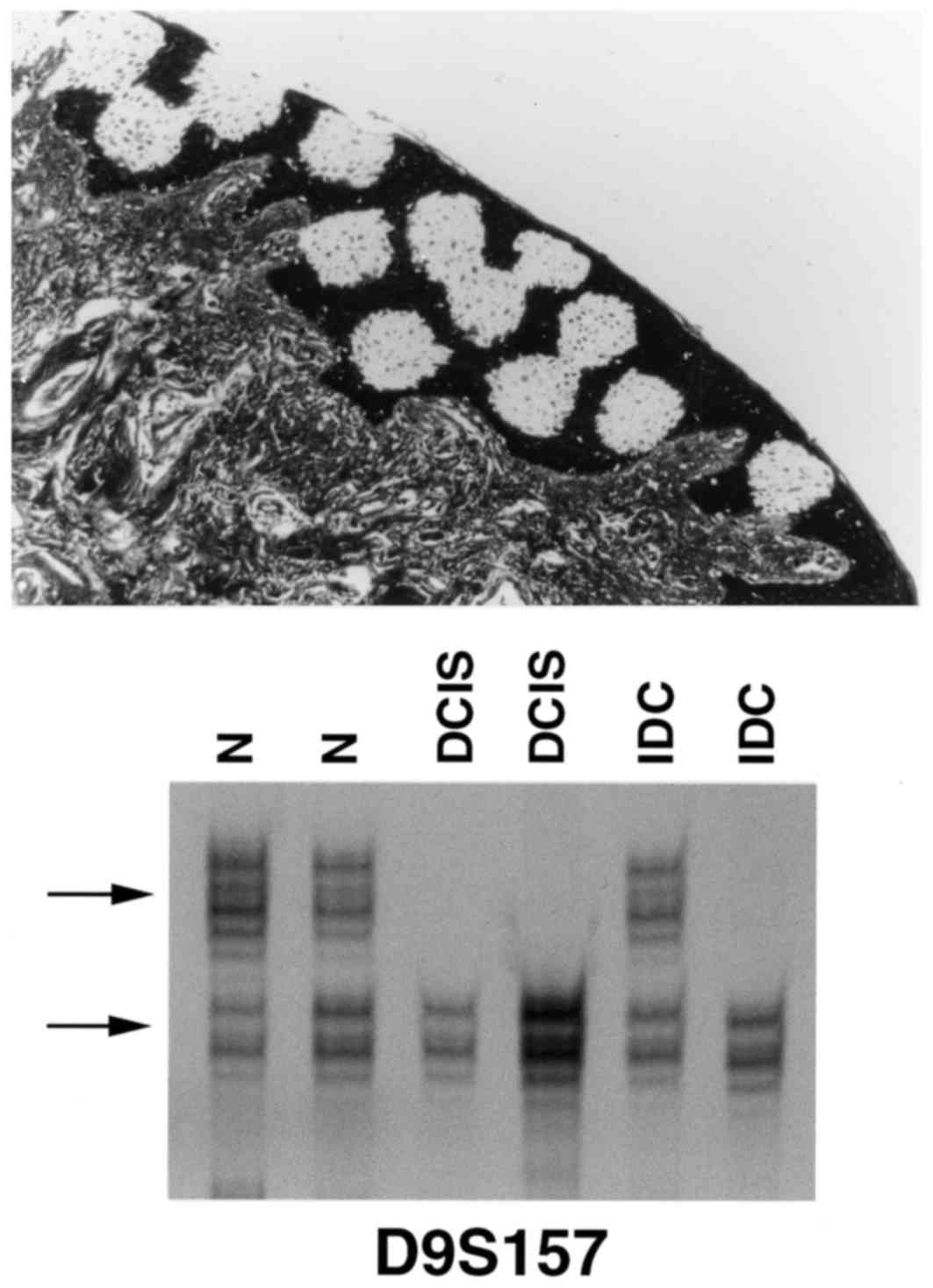
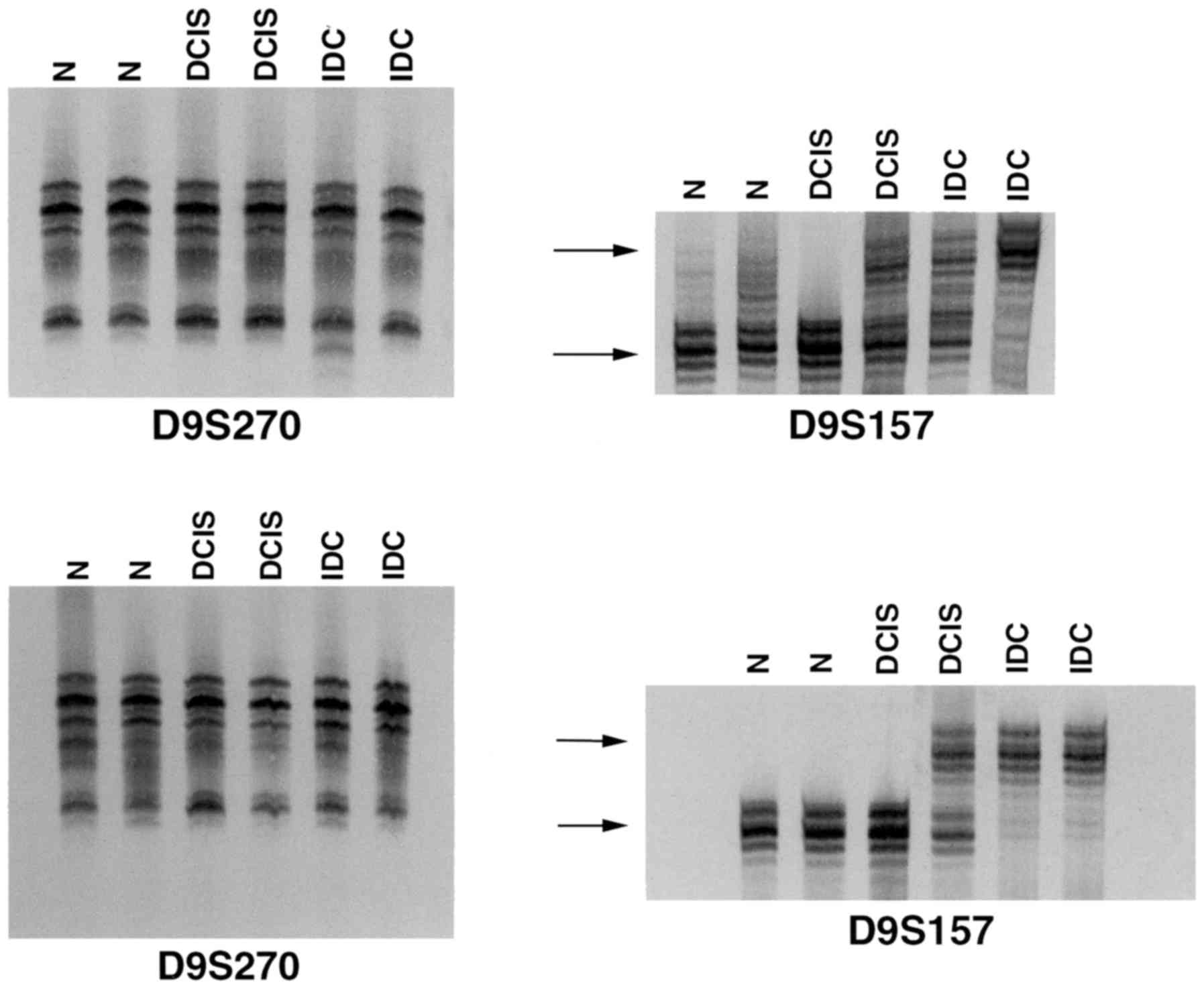
Notably, phenotypically normal breast tissues that were adjacent to
IDC and DCIS also exhibited LOH at D9S157 (Fig. 2) and/or D9S171. The finding that LOH
at these loci is also present in the normal tissue adjacent to
either DCIS or IDC is an indication that microsatellite instability
is an early event in the pathogenesis of breast cancer, and occurs
even earlier than any morphological changes are able to be
identified. The present study pursued further the validation of
these observations by performing LCM of normal skin and lymphocytes
from lymph nodes free of metastatic disease from 7 of the patients
and was unable to detect LOH in these other normal tissues.
(Fig. 3). This data supports previous
observations reported in the literature (29). It is notable that the practical
implications of these observations are of major importance in the
evaluation of the resected margins of conservative breast
surgery.
A novel finding was that LOH was heterogeneous in
its distribution, as it was exhibited in certain foci, but not in
all of the tumor foci studied (Fig.
4), suggesting that clones of cells with varied genetic
composition co-exist in the same lesion.
Discussion
The present data indicate that LOH at locus 9p22-23,
(D9S157) and to a lesser degree at 9p21 (D9S171), occurs during the
process of cancer initiation. More notably, clones of cells
co-exist within a single tumor, indicating that they do not share a
clonal origin and only those cells that have LOH at those loci may
progress. The monoclonal origin of cancer has been suggested in the
literature (30–32), and cytogenetic analyses have revealed
that breast cancers are polyclonal (33–35). The
use of LCM (36–38) has allowed the identification of more
chromosomal aberrations than is possible using DNA isolated from
tumor sections (39). By contrast to
previous reports (7,40–42) that
sustained clonal derivation from in situ cancer, the data
presented in the current study support the findings of Fujii et
al (8), who reported LOH
heterogeneity in multiple foci of individual DCIS lesions.
In conclusion, the present study demonstrated that
more than one clone of cells may exist in a simple lesion and that
genetic divergence occurs during cancer initiation and
progression.
Acknowledgements
This study was supported by National Cancer
Institute (grant nos. CA64896 and CA67238). Dr Margarida Dias has a
fellowship from The Luso-American Foundation for Development. The
authors would like to thank Dr Quivo Tahin for the critical
analysis of the microsatellite DNA polymorphism data, to Dr Xiang
Ao for the preparation of the slides used in laser capture
microdissection and to Dr Irma H. Russo (Breast Cancer Research
Laboratory, Fox Chase Cancer Center, Philadelphia PA, USA) for the
critical appraisal of the manuscript.
References
|
1
|
Russo J, Yang X, Hu YF, Bove BA, Huang Y,
Silva ID, Tahin Q, Wu Y, Higgy N, Zekri A and Russo IH: Biological
and molecular basis of human breast cancer. Front Biosci.
3:D944–D960. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Werner M, Mattis A, Aubele M, Cummings M,
Zitzelsberger H, Hutzler P and Höfler H: 20q13.2 amplification in
intraductal hyperplasia adjacent to in situ and invasive ductal
carcinoma of the breast. Virchows Arch. 435:469–472. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris JR and Hellman S: Natural history
of breast cancerDiseases of the Breast. Harris JR, Lippman ME,
Morrow M and Hellman S: Lippincott Raven; Philadelphia, PA: pp.
375–391. 1996
|
|
4
|
Lakhani SR: The transition from
hyperplasia to invasive carcinoma of the breast. J Pathol.
187:272–278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Werner M, Mattis A, Aubele M, Cummings M,
Zitzelsberger HH, Hhutzler P and Höfler H: 20q13.2 amplification in
intraductal hyperplasia adjacent to in situ and invasive ductal
carcinoma of the breast. Virchows Arch. 435:469–472. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eiriksdottir G, Sigurdsson A, Jonasson JG,
Agnarsson BA, Sigurdsson H, Gudmundsson J, Bergthorsson JT,
Barkardottir RB, Egilsson V and Ingvarsson S: Loss of
heterozygosity on chromosome 9 in human breast cancer: Association
with clinical variables and genetic changes at other chromosome
regions. Int J Cancer. 64:378–382. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuukasjärvi T, Karhu R, Tanner M, Kähkönen
M, Schäffer A, Nupponen N, Pennanen S, Kallioniemi A, Kallioniemi
OP and Isola J: Genetic heterogeneity and clonal evolution
underlying development of asynchronous metastasis in human breast
cancer. Cancer Res. 57:1597–1604. 1997.PubMed/NCBI
|
|
8
|
Fujii H, Marsh C, Cairns P, Sidransky D
and Gabrielson E: Genetic divergence in the clonal evolution of
breast cancer. Cancer Res. 56:1493–1497. 1996.PubMed/NCBI
|
|
9
|
Czerniak B, Chatuverdi V, Li L, Hodges S,
Johnston D, Ro JY, Luthra R, Logothetis C, Von Eschenbach AC,
Grossman HB, et al: Superimposed histologic and genetic mapping of
chromosome 9 in progression of human urinary bladder neoplasia:
Implications for a genetic model of multistep carcinogenesis and
early detection of urinary bladder cancer. Oncogene. 18:1185–1196.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campbell IG, Foulkes WD, Beynon G, Davis M
and Englefield P: LOH and mutation analysis of CDKN2 in primary
human ovarian cancers. Int J Cancer. 63:222–225. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakanishi H, Wang XL, Imai FL, Kato J,
Shiiba M, Myia T, Imai Y and Tanzawa H: Localization of a novel
tumor suppressor gene loci on chromosome 9p21-22 in oral cancer.
Anticancer Res. 19:29–34. 1999.PubMed/NCBI
|
|
12
|
Murphy DS, Hoare SF, Going JJ, Mallon EE,
George WD, Kaye SB, Brown R, Black DM and Keith WN:
Characterization of extensive genetic alterations in ductal
carcinoma in situ by fluorescence in situ hybridization and
molecular analysis. J Natl Cancer Inst. 87:1694–1704. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berns EM, Klijn JG, Smid M, Van Staveren
IL, Gruis NA and Foekens JA: Infrequent CDKN2 (MTS1/p16) gene
alterations in human primary breast cancer. Br J Cancer.
72:964–967. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quesnel B, Fenaux P, Philippe N, Fournier
J, Bonneterre J, Preudhomme C and Peyrat JP: Analysis of p16 gene
deletion and point mutation in breast carcinoma. Br J Cancer.
72:351–353. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu L, Sgroi D, Sterner CJ, Beauchamp RL,
Pinney DM, Keel S, Ueki K, Rutter JL, Buckler AJ and Louis DN:
Mutational analysis of CDKN2 (MTS1/p16INK4) in human breast
carcinomas. Cancer Res. 54:5262–5264. 1994.PubMed/NCBI
|
|
16
|
Brenner AJ and Aldaz M: Chromosome 9p
allelic loss and p16/CDK_N2 in breast cancer and evidence of p16
inactivation in immortal breast epithelial cells. Cancer Res.
55:2892–2895. 1995.PubMed/NCBI
|
|
17
|
An HX, Niederacher D, Picard F, Van Roeyen
C, Bender HG and Beckmann MW: Frequent allele loss on 9p21-22
defines a smallest common region in the vicinity of the CDKN2 gene
in sporadic breast cancer. Genes Chromosomes Cancer. 17:14–20.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minobe K, Onda M, Iida A, Kasumi F,
Sakamoto G, Nakamura Y and Emi M: Allelic loss on chromosome 9q is
associated with lymph node metastasis of primary breast cancer. Jpn
J Cancer Res. 89:916–922. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cairns P, Polascik TJ, Eby Y, Tokino K,
Califano J, Merlo A, Mao L, Heath J, Jenkins R, Westra W, et al:
Frequency of homozygous deletion at p16/CDKN2 in primary human
tumors. Nat Genet. 11:210–212. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dutrilaux B, Gerbault-Senreau M and
Zafrani B: Characterization of chromosomal abnormalities in human
breast cancer. A comparison of 30 paradiploid cases with few
chromosome changes. Cancer Genet Cytogenet. 49:203–217.
1990.PubMed/NCBI
|
|
21
|
Emmert-Buck MR, Bonner RF, Smith PD,
Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA and Liotta LA: Laser
capture microdissection. Science. 274:998–1001. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonner RF, Emmert-Buck M, Cole K, Pohida
T, Chuaqui R, Goldstein S and Liotta LA: Laser capture
microdissection: Molecular analysis of tissue. Science.
278:1481–1483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simone NL, Bonner RF, Gillespie JW,
Emmert-Buck MR and Liotta LA: Laser capture microdissection:
Opening the microscopic frontier to molecular analysis. Trends
Genet. 14:272–276. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russo J and Russo IH: The pathology of
breast cancer: Staging and prognostic indicators. J Am Med Womens
Assoc (1972). 47:181–187. 1992.PubMed/NCBI
|
|
25
|
Russo J, Gusterson BA, Rogers AE, Russo
IH, Wellings SR and van Zwieten MJ: Comparative study of human and
rat mammary tumorigenesis. Lab Invest. 62:244–278. 1990.PubMed/NCBI
|
|
26
|
Kellogg DE, Rybalkin I, Chen S,
Mukhamedova N, Vlasik T, Siebert PD and Chenchik A: TaqStart
Antibody: ‘hot start’ PCR facilitated by a neutralizing monoclonal
antibody directed against Taq DNA polymerase. Biotechniques.
16:1134–1137. 1994.PubMed/NCBI
|
|
27
|
Wu Y, Barnabas N, Russo IH, Yang X and
Russo J: Microsatellite instability and loss of heterozygosity in
chromosomes 9 and 16 in human breast epithelial cells transformed
by chemical carcinogens. Carcinogenesis. 18:1069–1074. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muzeau F, Flejou JF, Thomas G and Hamelin
R: Loss of heterozygosity on chromosome 9 and p16 (MTS1, CDKN2)
gene mutations in esophageal cancers. Int J Cancer. 72:27–30. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morita R, Fujimoto A, Hatta N, Takehara K
and Takata M: Comparison of genetic profiles between primary
melanomas and their metastases reveals genetic alterations and
clonal evolution during progression. J Invest Dermatol.
111:919–924. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng G, Lu Y, Zlotikov G, Thor AD and
Smith HS: Loss of heterozygosity in normal tissue adjacent to
breast carcinomas. Science. 274:2057–2059. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nowell PC: The clonal evolution of tumor
cell populations. Science. 194:23–28. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fialkow PJ: Clonal origin of human tumors.
Biochem Biophys Acta. 458:283–321. 1976.PubMed/NCBI
|
|
33
|
Noguchi S, Motomura K, Inaji H, Imaoka S
and Koyamma H: Clonal analysis of human breast cancer by means of
the polymerase chain reaction. Cancer Res. 52:6594–6597.
1992.PubMed/NCBI
|
|
34
|
Teixeira MR, Pandis N, Bardi G, Andersen
JA, Mitelman F and Heim S: Clonal heterogeneity in breast cancer:
karyotypic comparisons of multiple intra- and extra-tumorous
samples from 3 patients. Int J Cancer. 63:63–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teixeira MR, Pandis N, Bardi G, Andersen
JA and Heim S: Karyotypic comparisons of multiple tumors and
macroscopically normal surrounding tissue samples from patients
with breast cancer. Cancer Res. 56:855–859. 1996.PubMed/NCBI
|
|
36
|
Pandis N, Jin Y, Gorunova L, Petersson C,
Bardi G, Idvall I, Johansson B, Ingvar C, Mandahl N and Mitelman F:
Chromosome analysis of 97 primary breast carcinomas: Identification
of eight karyotypic subgroups. Genes Chromosomes Cancer.
12:173–185. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Böni R, Matt D, Voetmeyer A, Burg G and
Zhuang Z: Chromosomal allele loss in primary melanoma is
heterogeneous and correlates with proliferation. J Invest Dermat.
110:215–217. 1998. View Article : Google Scholar
|
|
38
|
Ornstein DK, Englert C, Gillespie JW,
Paweletz CP, Linehan WM and Emmert-Buck MR and Petricoin EF III:
Characterization of intracellular prostate-specific antigen from
laser capture microdissected benign and malignant prostatic
epithelium. Clin Cancer Res. 6:353–356. 2000.PubMed/NCBI
|
|
39
|
Milchgrub S, Wistuba II, Kim BK,
Rutherford C, Urban J, Cruz PD Jr..Gazdar AF: Molecular
identification of metastatic cancer to the skin using laser capture
microdissection: A case report. Cancer. 88:749–754. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aubele M, Mattis A, Zitzelsberger H, Walch
A, Kremer M, Hutzler P, Höfler H and Werner M: Intratumoral
heterogeneity in breast carcinoma revealed by laser-microdissection
and comparative genomic hybridization. Cancer Genet Cytogenet.
110:94–102. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhuang Z, Merino MJ, Chuaqui R, Liotta LA
and Emmert-Buck MR: Identical allelic loss on chromosome 11q13 in
microdissected in situ and invasive human breast cancer. Cancer
Res. 55:467–471. 1995.PubMed/NCBI
|
|
42
|
Radford DM, Phillips NJ, Fair KL, Ritter
JH, Holt M and Donis-Keller H: Allelic loss and the progression of
breast cancer. Cancer Res. 55:5180–5183. 1995.PubMed/NCBI
|