Introduction
Liver cancer, primarily hepatocellular carcinoma
(HCC), is the third most common cause of mortality from cancer
worldwide. Diagnostic and treatment strategies for liver cancer
have improved, with hepatic resection being the preferred
first-line treatment (1,2). Patients' safety is paramount when
performing hepatic surgery, although postoperative mortality
occasionally occurs. Therefore, accurate assessment of hepatic
functional reserve is important, particularly for hepatitis B virus
(HBV)-related HCC patients, since they often possess impaired
livers.
Hepatic functional reserve is determined by the
quality and quantity of liver cells. The Child-Pugh score (CPS) is
widely used to assess the quality of the liver, and hepatic
surgeons frequently use it to select HCC patients for resection.
The CPS is calculated by five parameters: Presence or absence of
encephalopathy, degree of ascites, total serum bilirubin, albumin
concentration and prothrombin time. According to the total point
score, CPS can be divided into grade A (5–6 points), B (7–9 points)
or C (10–15 points) (3). In clinical
practice, the majority of HCC patients who are eligible for
resection are Child-Pugh classification A patients (2,4). However,
postoperative liver dysfunction (PLD) and postoperative mortality
occasionally occur in Child-Pugh A patients. It appears that the
Child-Pugh classification alone may be unreliable for predicting
postoperative outcomes.
To overcome the possible limitations of Child-Pugh
classification for predicting postoperative outcomes, liver
computed tomography (CT) volumetry has been used to assist in the
assessment of hepatic functional reserve in recent years,
particularly when selecting HCC patients for major hepatic
resection (5). Future liver remnant
(FLR) volume measured preoperatively by three-dimensional CT
reconstruction can accurately reflect the size of the remnant liver
(6,7).
Patients with a smaller FLR are at a higher risk of developing PLD
or postoperative liver failure (8–10). The
main limitation of CT volumetry is the fact that volumetric
assessment of the remnant liver does not take into account the
quality of the liver parenchyma, and therefore is not reliable in
predicting PLD in patients with impaired livers. The safe limit of
FLR ranges from 35 to 50% in patients with chronic liver diseases
(2,9,11). To
improve the prediction of postoperative outcomes, CT volumetry
should be complemented with a method that assesses hepatic
function.
In the present study, the CPS was used to assess the
quality of the remnant liver, and the standardized FLR (sFLR)
measurement was used to evaluate the size of the remnant liver. In
addition, risk factors were investigated in relation to PLD in
patients with HBV-related HCC. The CPS was combined with the sFLR
measurement in an attempt to predict PLD more accurately.
Patients and methods
Patients
Between March 2014 and February 2015, 70 patients
with HCC underwent three-dimensional CT reconstruction for the
preoperative measurement of remnant liver volume prior to
hepatectomy at the Department of Hepatobiliary Surgery, Xiangya
Hospital, Central South University (Changsha, Hunan, China). Of
these 70 patients, 1 patient who had undergone hepatectomy for HCC
within 1 year of the present study and 8 patients who were negative
for hepatitis B surface antigen were excluded. Ultimately, 61
patients with chronic hepatitis B were included. None of these
patients had biliary obstruction prior to surgery or evidence of
hepatitis C virus (HCV)-specific antibodies or alcoholic cirrhosis.
All surgery was performed through open hepatectomy by a single team
of surgeons. Informed consent for the clinical study was obtained
from all patients, and the present study was approved by the
Institutional Review Board of Central South University.
Postoperative liver dysfunction was defined as a prothrombin time
of >18 sec (normal range, 10–14 sec) or a peak serum bilirubin
level of >51.3 µmol/l (normal range, 5.1–17.1 µmol/l) (12,13) for 7
days after surgery.
Liver volumetry and calculation of the
FLR
Prior to surgery, all patients underwent a
contrast-enhanced CT angiography scan with a slice thickness of 0.8
mm. Automated volumetry was performed for three-dimensional
reconstruction of the liver using medical image analysis software
(Myrian®; Intrasense S.A.S., Montpellier, France) and
the results were modified by manual contour tracing of the hepatic
contour following automated reconstruction. The gall bladder,
hepatic inferior vena cava and main branches of the intrahepatic
vascular structures were excluded from the reconstructional volume
calculation, but the biliary structures were included. Preoperative
virtual hepatic resection was performed according to the size and
location of the tumors, which were evaluated by two experienced
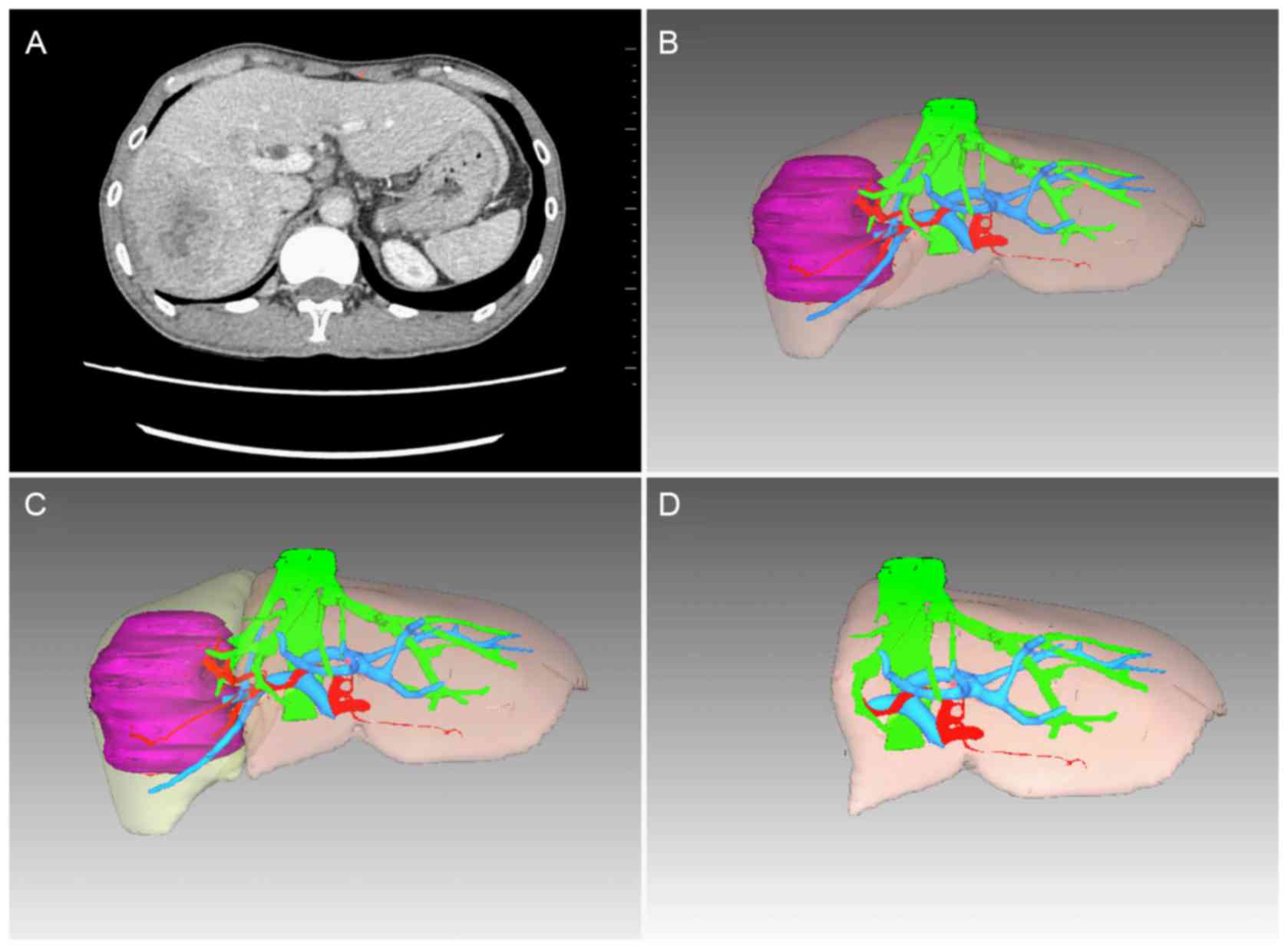
hepatic surgeons. The liver reconstruction and virtual hepatic
resection are shown in Fig. 1.
The total liver volume and FLR volume were
calculated using image analysis software. The percentage of FLR was
calculated as the sFLR using the following equation:
sFLR=FLR/standardized liver volume (SLV). The SLV was calculated
using the following equation: SLV (cm3)=706.2 × body
surface area (BSA; m2)+2.4 (14). The BSA was calculated using an
equation that includes bodyweight and height: BSA
(m2)=0.00607 × height (cm)+0.0127 × weight (kg)-0.0698
for men; and BSA (m2)=0.00586 × height (cm)+0.0126 ×
weight (kg)-0.0461 for women. The sFLR/CPS was calculated as a
combinatorial measure of sFLR and CPS.
Statistical analysis
Continuous variables are expressed as medians
(range) and were compared using the Mann-Whitney U test. Discrete
variables were compared using the χ2 test or Fisher's
exact test. Univariate analysis and multivariate logistic
regression analysis were performed to identify risk factors
associated with PLD. Correlation analyses were performed using
Pearson's correlation coefficient. The cut-off values for the
occurrence of PLD were determined by receiver operating
characteristic (ROC) curve analysis. Statistical analyses were
performed using SPSS 17.0 (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics
and modalities of hepatic resection
A total of 61 patients were enrolled in the present
study, comprising 52 men and 9 women, with a median age of 51 years
(range, 21–70 years). The surgical modalities were as follows: 18
patients underwent hemihepatectomy (right hemihepatectomy in 14 and
left hemihepatectomy in 4), 17 patients underwent bisectionectomy
(right anterior sectionectomy in 4, right posterior sectionectomy
in 6 and left lateral sectionectomy in 7) and 26 patients underwent
partial resection. None of the patients were allergic to anesthesia
or experienced cardiac arrest during surgery. Postoperative
pathological examinations revealed that 42 patients (68.9%) had
liver cirrhosis.
Postoperative liver dysfunction and
complications
In total, 18 of the 61 patients (29.5%) developed
PLD. Only 1 patient (1.6%) succumbed to intra-abdominal bleeding 15
days after surgery. Of the 61 patients, 19 (31.1%) developed one or
more complications after surgery and 11 (18.0%) developed
postoperative infection, which was the most common complication.
The following additional complications were observed: Pleural
effusions in 5 patients (8.2%), bile leakage in 3 patients (4.9%),
postoperative hemorrhage in 2 patients (3.3%) and temporary atrial
fibrillation in 1 patient (1.6%).
Child-Pugh classification and
postoperative liver dysfunction
According to the Child-Pugh classification criteria,
58 patients were of Child-Pugh grade A (with a CPS of 5 in 42
patients and a CPS of 6 in 16 patients) and 3 patients were of
Child-Pugh grade B (with a CPS of 7 in 2 patients and a CPS of 8 in
1 patient). A total of 16 of the 58 (27.6%) Child-Pugh A patients
developed PLD, and 2 of the 3 Child-Pugh B patients developed PLD.
Of the 58 Child-Pugh A patients, 8 of the 42 (19.0%) patients with
a CPS of 5 developed PLD and 8 of the 16 (50.0%) patients with a
CPS of 6 developed PLD. This difference was significant (Fisher's
exact test, P=0.026), indicating that the incidence of PLD for
patients with a CPS of 6 was higher than that for patients with a
CPS of 5.
Prothrombin time and sFLR are
independent risk factors for postoperative liver dysfunction
The univariate analysis revealed no differences
between the two groups in terms of age, body mass index, surgical
duration, albumin, total bilirubin, alanine aminotransferase,
aspartate aminotransferase, blood sugar, cholesterol, platelet
count, presence of inflow occlusion, presence of blood transfusion,
presence of hepatitis B e antigen, presence of HBV-DNA or presence
of liver cirrhosis (P>0.05; Table
I). However, tumor diameter (P=0.043), blood loss (P=0.015),
prothrombin time (PT) (P=0.007), CPS (P=0.007) and sFLR
(P<0.001) were significantly different between the two groups
(Table I).
 | Table I.Univariate analysis of risk factors of
postoperative liver dysfunction. |
Table I.
Univariate analysis of risk factors of
postoperative liver dysfunction.
|
| Postoperative liver
dysfunction |
|
|---|
|
|
|
|
|---|
| Variables | Yes (n=18) | No (n=43) | P-value |
|---|
| Age, years | 49.0 (26–65) | 51.0 (21–70) | 0.716 |
| BMI,
kg/m2a | 22.1 (17.9–30.4) | 21.1 (17.6–30.5) | 0.211 |
| Tumor diameter,
cm | 8.0 (2.3–14.7) | 4.4 (2.0–12.6) | 0.043 |
| Operating time,
min | 156.5 (90–280) | 135.0 (70–260) | 0.143 |
| Blood loss, ml | 600.0 (100–3000) | 400.0 (40–1200) | 0.015 |
| ALB, g/l | 40.8 (30.6–53.3) | 40.8 (34.7–48.5) | 0.482 |
| Total bilirubin,
µmol/l | 14.2 (6.5–27.8) | 11.8 (4.0–30.2) | 0.209 |
| ALT, U/l | 45.8
(16.5–140.9) | 36.7 (9.4–142.8) | 0.060 |
| AST, U/l | 46.9 (17.1–76.9) | 37.0
(13.4–117.2) | 0.056 |
| Blood sugar,
mmol/l | 5.2 (4.1–7.4) | 5.0 (3.7–8.4) | 0.496 |
| Cholesterol,
mmol/l | 4.2 (1.7–6.1) | 4.2 (3.0–5.8) | 0.289 |
| PT, sec | 13.9 (11.9–16.4) | 13.2 (11.5–15.0) | 0.007 |
| Platelet count,
×109/l | 149.5 (32–247) | 129.0 (48–437) | 0.664 |
| Child-Pugh score | 6.0 (5–8) | 5.0 (5–7) | 0.007 |
| sFLR | 0.501
(0.352–0.794) | 0.755
(0.361–1.101) | <0.001 |
| Inflow
occlusionb |
|
| 0.134c |
| Yes | 10 | 15 |
|
| No | 8 | 28 |
|
| Blood
transfusion |
|
| 0.429d |
| Yes | 4 | 5 |
|
| No | 14 | 38 |
|
| HBeAg |
|
| 0.411d |
|
Positive | 3 | 4 |
|
|
Negative | 15 | 39 |
|
| HBV-DNA |
|
| 0.301c |
| Positive
(>500/copies) | 13 | 25 |
|
|
Negative (<500/copies) | 5 | 18 |
|
| Liver
cirrhosis |
|
| 0.811c |
|
Yes | 12 | 30 |
|
| No | 6 | 13 |
|
A multivariate logistic regression analysis was
performed to identify risk factors for PLD. Age, body mass index,
operating time, albumin, total bilirubin, alanine aminotransferase,
blood sugar, cholesterol, platelet count, inflow occlusion,
positive HBeAg, liver cirrhosis, PT, blood loss, tumor diameter and
sFLR were entered into the multivariate logistic regression model
to avoid collinearity (Table II). A
prolonged PT and small sFLR were identified as significant
independent predictors of PLD (Table
II).
 | Table II.Multivariate logistic regression
analysis for risk factors of postoperative liver dysfunction. |
Table II.
Multivariate logistic regression
analysis for risk factors of postoperative liver dysfunction.
|
| 95% confidence |
|
|---|
|
|
|
|
|---|
| Variables | Odds ratio | P-value | Interval |
|---|
| Age >60
years | 9.643 | 0.533–174.458 | 0.125 |
| BMI >25
kg/m2a | 1.561 | 0.130–18.736 | 0.725 |
| Tumor diameter
>5 cm | 5.687 | 0.283–114.154 | 0.256 |
| Operating time
>150 min | 4.342 | 0.463–40.699 | 0.198 |
| Blood loss >400
ml | 2.190 | 0.238–20.122 | 0.489 |
| ALB <40 g/l | 2.409 | 0.224–25.881 | 0.468 |
| Total bilirubin
>17.1 µmol/l | 7.434 | 0.384–143.988 | 0.185 |
| ALT >40 U/l | 4.106 | 0.283–59.634 | 0.301 |
| Blood sugar >6.1
mmol/l | 1.487 | 0.052–42.484 | 0.817 |
| Cholesterol >4.2
mmol/l | 1.734 | 0.140–21.476 | 0.668 |
| PT >13.3
sec | 26.697 | 2.366–301.169 | 0.008 |
| Platelet count
<100×109/l | 0.259 | 0.012–5.805 | 0.394 |
| sFLR<0.55 | 27.014 | 1.356–538.118 | 0.031 |
| Inflow
occlusion | 17.864 | 0.865–368.948 | 0.062 |
| Positive HBeAg | 11.419 | 0.149–877.696 | 0.272 |
| Liver
cirrhosis | 10.073 | 0.511–198.729 | 0.129 |
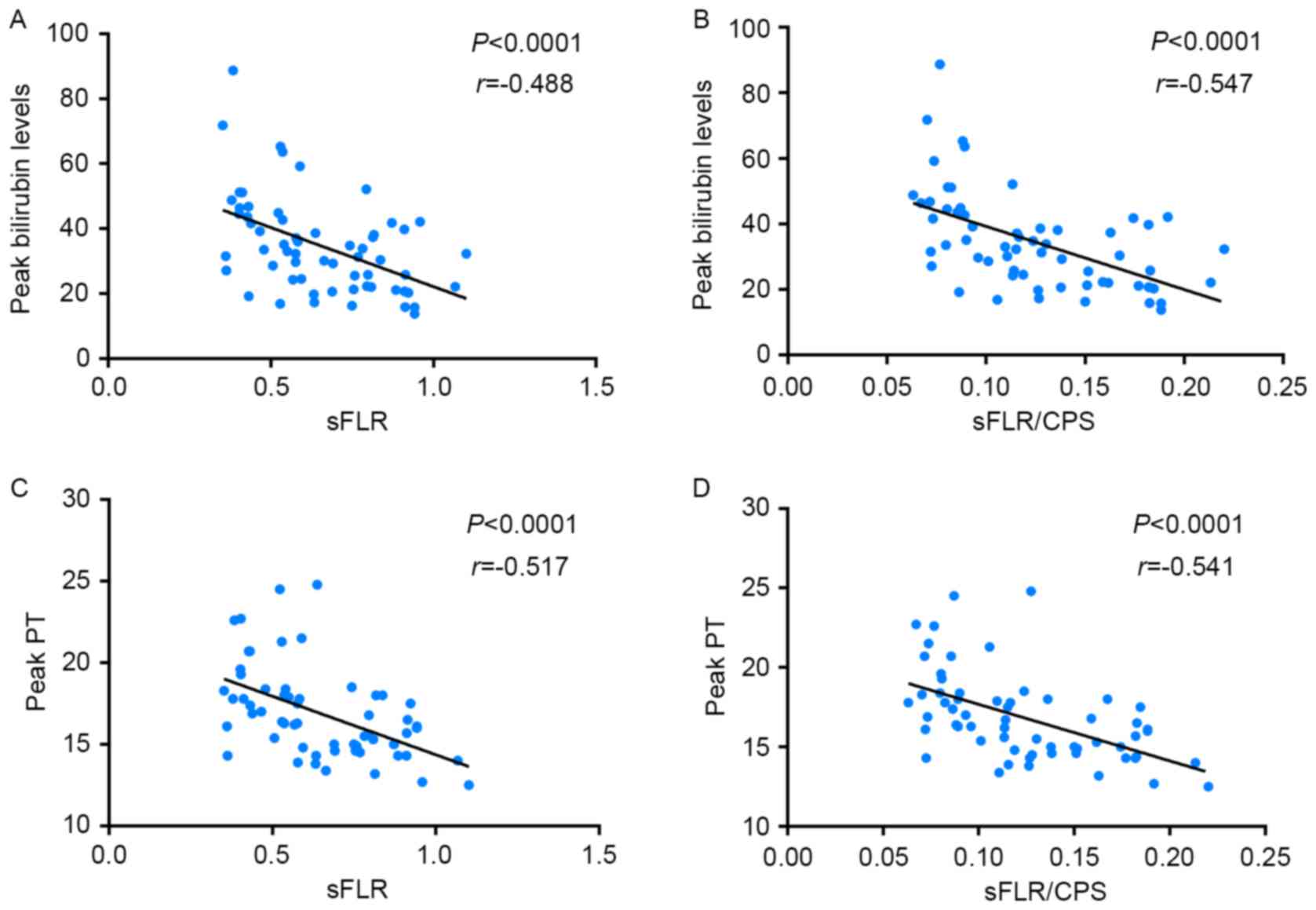
sFLR and sFLR/CPS correlate with
postoperative peak bilirubin levels and postoperative peak
prothrombin time
Pearson's correlation analysis revealed that the
postoperative peak bilirubin level had a stronger negative
correlation with sFLR/CPS (P<0.0001, r=−0.547) than with sFLR
(P<0.0001, r=−0.488) (Fig. 2). The
postoperative peak PT had a stronger negative correlation with
sFLR/CPS (P<0.0001, r=−0.541) than with sFLR (P<0.0001,
r=−0.517) (Fig. 2).
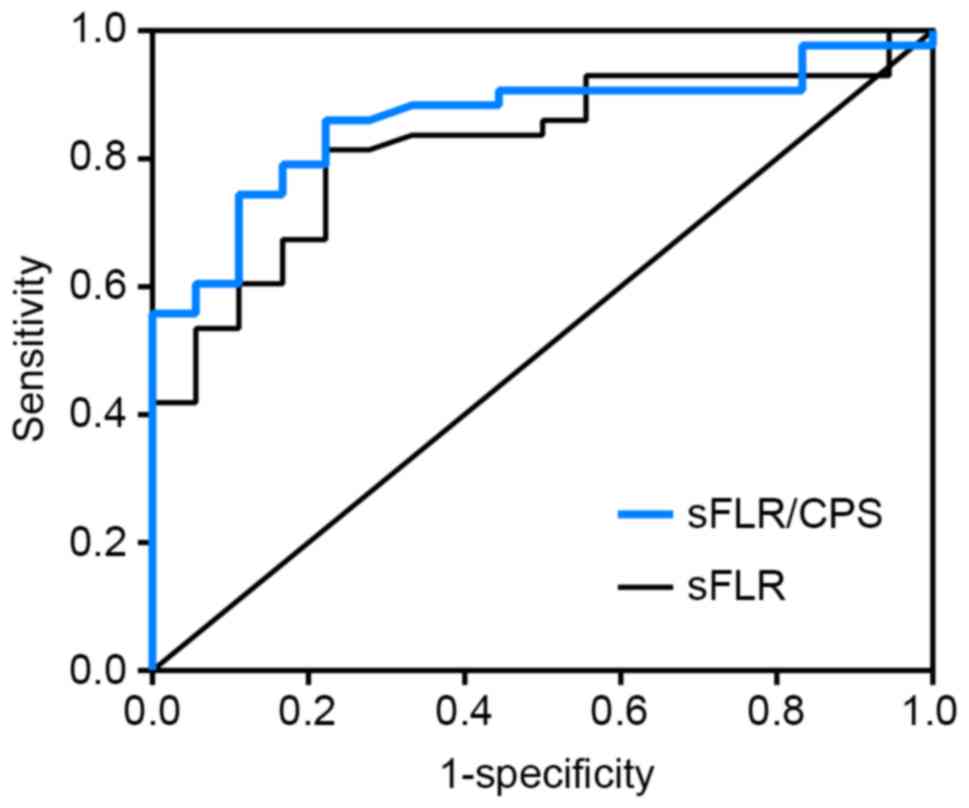
sFLR and sFLR/CPS are predictors of
postoperative liver dysfunction
ROC curve analysis revealed that the cut-off value
of sFLR for predicting PLD was 54.5%, with 81.4% sensitivity and
77.8% specificity (Fig. 3). In total,
14 of the 22 (63.6%) patients with an sFLR <54.5% developed PLD,
compared with 4 of the 39 (10.3%) patients with a larger sFLR. This
difference was statistically significant (χ2=19.268,
P<0.001). The cut-off value of sFLR/CPS for predicting PLD was
0.0916, with 86.0% sensitivity and 77.8% specificity (Fig. 3). Of the 20 patients with an sFLR/CPS
of <0.0916, 14 (70.0%) developed PLD compared with 4 of 41
(9.8%) patients with a higher sFLR/CPS, and this difference was
statistically significant (χ2=23.455, P<0.001). This
result indicates that sFLR/CPS was a more useful predictor of PLD
in HBV-related HCC patients following hepatic resection compared
with sFLR alone.
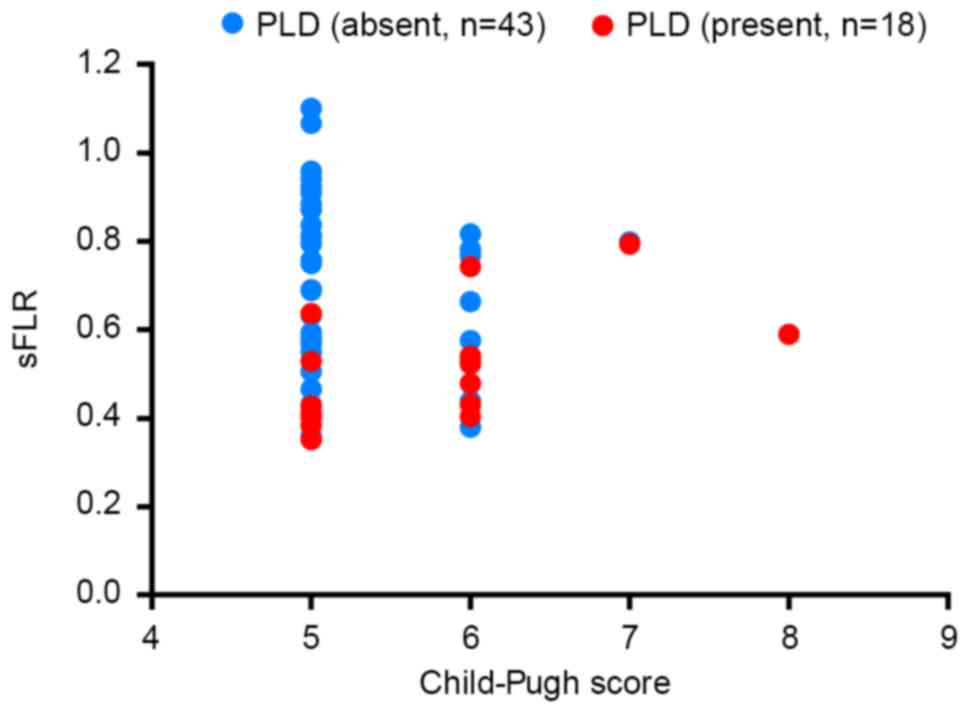
sFLR and CPS are linked to postoperative liver
dysfunction. The distribution of PLD in patients with different
CPSs and different sFLRs is demonstrated in Fig. 4. According to the distribution of PLD,
the majority of patients with a CPS of 5 who developed PLD had an
sFLR of <43%, and the majority of the patients with a CPS of 6
who developed PLD had an sFLR of <54%.
Discussion
In the present study, the incidence of PLD among
Child-Pugh A patients was revealed to be significantly higher in
patients with a CPS of 6 than in those patients with a CPS of 5
(P<0.05), indicating that hepatic function may not be the same
for all HCC patients with Child-Pugh A. For a number of years, the
selection of HCC patients for hepatic resection has been based on
the Child-Pugh classification (15);
however, flaws in this classification system have been described
recently (16–18). First, a number of the variables
included in the Child-Pugh classification are interrelated (e.g.,
ascites and serum albumin levels). Second, the grading of ascites
and encephalopathy is subjective. Third, the cut-off value for each
variable is selected empirically. Finally, the Child-Pugh
classification system does not offer a wide degree of
discrimination for HCC patients undergoing hepatic resection, the
majority of whom have Child-Pugh A disease. Certain studies have
reported the unreliability of the Child-Pugh classification system
for predicting postoperative outcomes (17,19–21). The
data from the present study revealed that heterogeneity may exist
in Child-Pugh A patients. There is a requirement to identify ‘good
risk’ and ‘poor risk’ Child-Pugh A patients. Furthermore, even for
multiple patients with a given CPS, different postoperative
outcomes were observed in the present study, possibly since the
remnant liver volume varied for these patients.
More recently, with the advancement of
three-dimensional imaging technologies, the importance of
preoperative volumetric analysis for major hepatic resection has
been increasingly emphasized (5,22).
Previous studies have demonstrated that a small FLR is associated
with worse postoperative hepatic function (23,24). As in
previous studies, the findings from the present study suggested
that sFLR is an independent risk factor for PLD. However, there
were patients in the present study who had a small FLR but did not
develop PLD, indicating that FLR may not be the only factor that
affects postoperative outcomes. There is little doubt that hepatic
function also plays an important role in predicting postoperative
outcomes (25,26). Theoretically, with a higher CPS, a
larger FLR is required to avoid PLD. Judging from the distribution
of PLD (Fig. 4), data from the
present study revealed that to avoid PLD in HBV-related HCC
patients following hepatic resection, an sFLR of 43% is relatively
safe when the CPS is 5, and an sFLR of 54% is relatively safe when
the CPS is 6. It is predicted that a higher sFLR is required when
the CPS is 7 or 8. These results require testing and verifying with
more cases in clinical practice, in particular with more patients
with a CPS of 7 or 8.
In addition, by using sFLR/CPS as a combinatorial
measure of sFLR and CPS, it was revealed that sFLR/CPS showed a
stronger negative correlation with postoperative peak bilirubin
levels and postoperative peak PT than sFLR, and ROC curve analysis
revealed that the cut-off value of sFLR/CPS could predict PLD more
accurately than sFLR. This indicates that sFLR/CPS is a more
accurate predictor of postoperative hepatic function than sFLR.
The present study has a number of limitations.
First, the time span of the study was short, and all the subjects
were enrolled from a single study center. In addition, the study
focused on HCC patients infected with HBV. It is possible that the
results do not apply to HCC patients in other countries where HCV
infection or alcoholic cirrhosis may be the most common cause of
HCC. Therefore, these study results require validation in Western
and Asia-Pacific patient populations. Finally, no evaluation was
performed of the correlations between the preoperative liver
volumetry and the weight of the resected specimen.
In conclusion, the combination of sFLR and CPS was
identified as aiding a more accurate assessment of hepatic
functional reserve and improving prevention of the occurrence of
postoperative liver dysfunction compared with either CPS or sFLR
alone.
Acknowledgements
The authors would like to thank Dr Saidan Zhang
(Institute of Medical Science, Xiangya Hospital, Central South
University, Changsha, China) for providing assistance with the
statistical analysis. The present study was supported by the
National Nature Science Foundation of China (grant nos. 81372630
and 81372631).
References
|
1
|
Cauchy F, Soubrane O and Belghiti J: Liver
resection for HCC: Patient's selection and controversial scenarios.
Best Pract Res Clin Gastroenterol. 28:881–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fonseca AL and Cha CH: Hepatocellular
carcinoma: A comprehensive overview of surgical therapy. J Surg
Oncol. 110:712–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcea G, Ong SL and Maddern GJ:
Predicting liver failure following major hepatectomy. Dig Liver
Dis. 41:798–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim MC, Tan CH, Cai J, Zheng J and Kow AW:
CT volumetry of the liver: Where does it stand in clinical
practice? Clin Radiol. 69:887–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wigmore SJ, Redhead DN, Yan XJ, Casey J,
Madhavan K, Dejong CH, Currie EJ and Garden OJ: Virtual hepatic
resection using three-dimensional reconstruction of helical
computed tomography angioportograms. Ann Surg. 233:221–226. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simpson AL, Geller DA, Hemming AW,
Jarnagin WR, Clements LW, D'Angelica MI, Dumpuri P, Gonen M,
Zendejas I, Miga MI and Stefansic JD: Liver planning software
accurately predicts postoperative liver volume and measures early
regeneration. J Am Coll Surg. 219:199–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schindl MJ, Redhead DN, Fearon KC, Garden
OJ and Wigmore SJ: The value of residual liver volume as a
predictor of hepatic dysfunction and infection after major liver
resection. Gut. 54:289–296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clavien PA, Petrowsky H, DeOliveira ML and
Graf R: Strategies for safer liver surgery and partial liver
transplantation. N Engl J Med. 356:1545–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribero D, Amisamo M, Bertuzzo F, Langella
S, Lo Tesoriere R, Ferrero A, Regge D and Capussotti L: Measured
versus estimated total liver volume to preoperatively assess the
adequacy of the future liver remnant: Which method should we use?
Ann Surg. 258:801–807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wagener G: Assessment of hepatic function,
operative candidacy, and medical management after liver resection
in the patient with underlying liver disease. Semin Liver Dis.
33:204–212. 2013.(In Danish, English). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ribero D, Abdalla EK, Madoff DC, Donadon
M, Loyer EM and Vauthey JN: Portal vein embolization before major
hepatectomy and its effects on regeneration, resectability and
outcome. Br J Surg. 94:1386–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Facciuto M, Contreras-Saldivar A, Singh
MK, Rocca JP, Taouli B, Oyfe I, LaPointe Rudow D, Gondolesi GE,
Schiano TD, Kim-Schluger L, et al: Right hepatectomy for living
donation: Role of remnant liver volume in predicting hepatic
dysfunction and complications. Surgery. 153:619–626. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urata K, Kawasaki S, Matsunami H,
Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A and
Makuuchi M: Calculation of child and adult standard liver volume
for liver transplantation. Hepatology. 21:1317–1321. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Durand F and Valla D: Assessment of
prognosis of cirrhosis. Semin Liver Dis. 28:110–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaplan DE, Dai F, Aytaman A, Baytarian M,
Fox R, Hunt K, Knott A, Pedrosa M, Pocha C, Mehta R, et al:
Development and performance of an algorithm to estimate the
Child-Turcotte-Pugh Score from a national electronic healthcare
database. Clin Gastroenterol Hepatol. 13:2333–2341, e1-e6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider PD: Preoperative assessment of
liver function. Surg Clin North Am. 84:355–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoekstra LT, de Graaf W, Nibourg GA, Heger
M, Bennink RJ, Stieger B and van Gulik TM: Physiological and
biochemical basis of clinical liver function tests: A review. Ann
Surg. 257:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cieslak KP, Runge JH, Heger M, Stoker J,
Bennink RJ and van Gulik TM: New perspectives in the assessment of
future remnant liver. Dig Surg. 31:255–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirashita T, Ohta M, Iwashita Y, Iwaki K,
Uchida H, Yada K, Matsumoto T and Kitano S: Risk factors of liver
failure after right-sided hepatectomy. Am J Surg. 206:374–379.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Truant S, Boleslawski E, Sergent G,
Leteurtre E, Duhamel A, Hebbar M and Pruvot FR: Liver function
following extended hepatectomy can be accurately predicted using
remnant liver volume to body weight ratio. World J Surg.
39:1193–1201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stockmann M, Lock JF, Riecke B, Heyne K,
Martus P, Fricke M, Lehmann S, Niehues SM, Schwabe M, Lemke AJ and
Neuhaus P: Prediction of postoperative outcome after hepatectomy
with a new beside test for maximal liver function capacity. Ann
Surg. 250:119–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okabe H, Beppu T, Chikamoto A, Hayashi H,
Yoshida M, Masuda T, Imai K, Mima K, Nakagawa S, Kuroki H, et al:
Remnant liver volume-based predictors of postoperative liver
dysfunction after hepatectomy: Analysis of 625 consecutive patients
from a single institution. Int J Clin Oncol. 19:614–621. 2014.
View Article : Google Scholar : PubMed/NCBI
|