Introduction
Colorectal cancer (CRC) is the fourth most common
cause of cancer-associated mortality worldwide (1). In the advanced stages of the disease,
the prognosis for patient with CRC remains poor due to the high
frequency of recurrence, high rate of distant metastasis and
resistance to chemotherapeutics. Therefore, novel treatments are
urgently required and a number of different intervention strategies
are being explored. The potential of immunotherapy is increasingly
gaining attention. One reason for the growing interest in
immunotherapy is that tumors that develop resistance to
chemotherapy or radiation may continue to be suitable immunotherapy
targets (2–4). CRCs are typically characterized by the
infiltration of multiple types of stromal cells, including the
tumor-infiltrating lymphocytes (TILs) that can serve as prognostic
and predictive factors (5–8).
TILs include natural killer (NK) cells, cluster of
differentiation (CD) 8+ T cells and CD4+ T
cells, which are further subdivided into T helper (Th) 1, Th2, Th17
and regulatory T (Treg) cells. These various TILs exhibit different
effects on tumorigenesis. Th1 cells produce the cytokines
interleukin (IL)-2 and interferon (IFN)-γ, which possess anti-tumor
activities. Additionally, IL-2 and IFN-γ perform a critical role in
cellular immunity by activating CD8+ T cells, which are
the main effector cells involved in cytotoxic T lymphocyte (CTL)
activity. Th2 cells produce IL-4, IL-5, IL-9 and IL-13 and protect
against gastrointestinal parasites (9). Polarization of Th1 can overcome the
ability of cancer cells to escape the immune response, thus killing
more residual tumor cells. By contrast, Th2 polarization leads to
an immunosuppressive state that can result in tumor cells escaping
immune detection. Th17 cells promote tumor growth by secreting
IL-17, which enhances net angiogenic activity and promotes the
growth of non-small cell lung cancer in vivo (10). IL-17 also supports the tumor promoting
microenvironment at tumor sites, and its effects on myeloid derived
suppressor cells represents an important tumor promoting mechanism
(11). The critical role performed by
TILs in cancer pathogenesis supports the hypothesis that
immunotherapy has the potential to be an effective option for the
treatment of CRC or for preventing relapse in patients with
CRC.
Carcinoembryonic antigen-related cell adhesion
molecule (CEACAM) 6 is an important cell adhesion associated
protein and a member of the CEACAM family. CEACAM family members
are useful clinical biomarkers with promising therapeutic targets
in melanoma and lung, colorectal and pancreatic cancer (12). CEACAM6 has been demonstrated to be an
independent prognostic factor associated with a higher risk of CRC
relapse (13). Therefore, CEACAM6 is
a promising target for CRC malignancy and metastatic control.
The 4-1BB ligand (4–1BBL) is a type II surface
glycoprotein member of the tumor necrosis factor (TNF) superfamily
that is generally expressed by antigen presenting cells (APC),
including dendritic cells, macrophages and activated B cells
(13). 4-1BBL binds to 4-1BB [also
termed cluster of differentiation (CD) 137], a member of the TNF
receptor superfamily, and enhances T cell activation (14). The interaction of 4-1BB and 4-1BBL
provides an important co-stimulatory T cell activation signal that
is independent of CD28, and has attracted a lot of attention in
previous immunology studies (15,16).
We previously demonstrated that recombinant CEACAM6
and 4-1BBL genes could be used to deliver CEACAM6 and 4-1BBL
antigens effectively via attenuated Salmonella. This
Salmonella-based vaccine efficiently inhibited the
development of 1,2-dimethylhydrazine (DMH) induced colorectal
tumors in rats (17). Tumor treatment
using the Salmonella-based vaccine was accompanied by an
increased number of CD3+ and CD8+ TILs and NK
cells, and decreased forkhead/winged-helix transcription factor box
P3 (FOXP3) positive T cells. The present study continues the
investigation of this vaccine, and explores whether the vaccine is
capable of inducing immune memory and also investigates the
mechanisms by which the vaccine influences Th cell polarization. To
investigate the mechanisms involved in the anti-tumor immunological
activity of the recombinant DNA vaccine the present study analyzed
CD45RO+, IL-4 and IL-17 expression in induced rat
tumors, and measured IFN-γ, CD3+, CD4+,
CD8+, CD56+, FOXP3+, IL-4 and
IL-17 levels in splenic tissues.
Materials and methods
Animals
A total of 24 male 6–8 week old Sprague Dawley rats
were obtained from the Experimental Animal Center of Soochow
University (Soochow, China). The rats were housed with free access
to water and food in a specific-pathogen-free facility under a 12 h
light/dark cycle at 50±10% humidity and 21±2°C. The body weight of
each rat was measured weekly. All experimental protocols in this
study were approved by the Ethics Committee of the First Affiliated
Hospital of Soochow University (Suzhou, China).
Bacteria
Salmonella enterica serovars Typhimurium
LB5000 and attenuated Typhimurium SL3261 were generously provided
by Professor Stocker from Stanford University (Stanford, CA, USA).
The plasmid pIRES2-EGFP was purchased from Clontech Laboratories,
Inc. (Mountainview, CA, USA). The pIRES, pIRES-CEACAM6,
pIRES-4-1BBL and pIRES-CEACAM6-4-1BBL plasmids were transformed
into the SL3261 Salmonella strain, following a previously
described method (17).
Rat colorectal tumor model
establishment
A total of 20 mg/kg of subcutaneous DMH
(Sigma-Aldrich; Merck Millipore, Billerica, MA, USA) was
administered to all animals each week for 18 consecutive weeks. A
total of 8 weeks subsequent to the first DMH administration, the
rats were randomly divided into treatment groups and administered 2
ml PBS containing 2×109 pIRES-transformed SL3261A,
pIRES-4-1BBL-transformed SL3261B, pIRES-CEACAM6-transformed SL3261C
or pIRES-CEACAM6-4-1BBL-transformed SL3261D via oral gavage every
other week (4 doses over 8 weeks). DMH injections continued for 18
weeks. Subsequent to 18 weeks, the rats from the pIRES/SL3261,
pIRES-4-1BBL/SL3261, pIRES-CEACAM6/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261 groups were anesthetized by intravenous
injections of pentobarbital (1.5 mg/100 g), blood samples were
collected and the rats were subsequently sacrificed by using carbon
dioxide. Following euthanasia, colons and spleens were dissected
for immunohistochemical (IHC) staining.
Gross and histological
examination
All colorectal tissue (from the cecum to the anus of
each rat) was removed, washed with ice-cold saline, cut
longitudinally and laid flat on a board. The total number of tumors
was counted by two independent blinded investigators. Since it is
difficult to distinguish lymph node metastasis in rats, Duke's
staging system (18) was used with
minor modifications to evaluate CRC disease stages as follows:
Stage A, tumors are only present in the mucosa; stage B-C, tumors
can be observed invading the muscularis propria, but no distant
metastasis can be identified; and stage D, tumors with distant
metastasis are present.
IHC analysis
All tumor specimens were analyzed with IHC for
CD45RO+ T cell infiltration, IL-4 and IL-17. Additionally, splenic
tissues were analyzed for IFN-γ, CD3+, CD4+, CD8+, CD56+, FOXP3+,
IL-4 and IL-17. Tissue sections (4 µm) were prepared from 10%
formalin-fixed (for 24 h at 25°C) and paraffin-embedded tissues.
Following deparaffinization and rehydration with ethanol (70–100%),
the slides were heated to 100°C in 10 mmol/l sodium citrate buffer
(pH, 6) for 15 min to for antigen retrieval. Endogenous peroxidase
activity was blocked by incubating at 25°C with 0.6%
H2O2 in methanol for 20 min. Sections were
subsequently blocked with 10% normal horse serum (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 5 min. Following
blocking, sections were incubated with the following antibodies:
Mouse monoclonal anti-rat CD3 (dilution, 1:100; catalog no.
sc-20047; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-IL-4 (dilution, 1:100; catalog no. sc-71020; Santa Cruz
Biotechnology, Inc.), anti-CD56 (dilution, 1:100; catalog no.
ab80025; Abcam, Cambridge, MA, USA), anti-CD45RO (dilution, 1:10;
catalog no. ab86080; Abcam), anti-FOXP3 (1:50; catalog no. ab450;
Abcam, Cambridge), rabbit polyclonal antibodies anti-rat CD4
(dilution, 1:100; catalog no. sc-7219; Santa Cruz Biotechnology,
Inc.), CD8 (dilution, 1:100; catalog no. sc-7188; Santa Cruz
Biotechnology), IL-17 (dilution, 1:100; catalog no. ab134074;
Abcam) and rabbit monoclonal antibody anti-rat INF-γ (dilution,
1:100; catalog no. ab134040; Abcam). Sections were incubated with
primary antibodies at room temperature for 2 h.
The slides were incubated with
streptavidin-horseradish peroxidase conjugated biotinylated
secondary antibodies horse-anti-mouse immunoglobulin G (IgG)
(dilution, 1:2,000; catalog no. K5006; Dako; Agilent Technologies,
Inc.) and horse-anti-rabbit IgG (dilution, 1:2,000; catalog no.
K5007; Dako; Agilent Technologies, Inc.) for 30 min at room
temperature. Following incubation, an avidin/strepavidin complex
(Dako; Agilent Technologies, Inc.) was added. A non-specific
staining blocker (GeneTex Corporation, Shanghai, China) and
enzyme-labeled sheep anti-rabbit IgG polymer reagent (GeneTex
Corporation) were added according to the manufacturer's protocol.
The antigen detection was conducted via a color reaction with
3,3′-diaminobenzidine (Dako, Agilent Technologies Denmark ApS,
Glostrup, Denmark). Sections were counterstained using hematoxylin
(AppliChem Inc., St Louis, MO, USA) and mounted with Aquatex (Merck
Millipore). A total of 5 randomly selected fields (magnification,
×400) were assessed using the Olympus BX53 microscope (Olympus
Corporation, Tokyo, Japan), and areas of necrosis were avoided. The
number of positive cells per field were estimated and assigned a
number as follows: 0) None; i) <1/100 cells; ii) ≥1/100 to 1/10
cells; iii) >1/10 to 1/3 cells; iv) >1/3 to 2/3 cells; and v)
>2/3 cells. Staining intensity was determined as follows: 0,
none; 1,weak; 2, intermediate; and 3, strong. The sum of the first
and second scores comprised the staining score, and the maximum
possible score was 8 for any given tissue (19). The IHC analysis was performed by 2
independent blinded investigators (author 1 and 2) and the results
were consistent between the two readings. Results were recorded as
the mean ± standard deviation for each group.
Statistical analysis
Data are represented as the mean ± standard
deviation. Differences among different groups were assessed using
one-way ANOVA, with the Tukey's honest significant difference post
hoc test. Differences between two groups were assessed using the
Student's t-test. Differences in the number of T-cells and
cytokines were investigated using the non-parametric Wilcoxon Rank
Sum test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using Predictive Analytics Software (PASW; version 18.0; IBM SPSS,
Armonk, NY, USA).
Results
SL3261D (pIRES-CEACAM6-4-1BBL/SL321)
vaccination reduces the number of colorectal tumors and restricted
the tumors to the mucosa
As demonstrated in a previous study (17), following colorectal tumor induction, 2
rats from the SL3261A (pIRES/SL3261) group exhibited bloody stools
18 weeks subsequent to the initial DMH treatment. Further analysis
of the animals with bloody stools revealed severe bloody ascites
and multiple tumor nodules of varying sizes in the mesentery and
posterior peritoneum. However, none of the other groups exhibited
an outcome this severe.
The number of tumors observed in the SL3261A
(pIRES/SL3261; 11.7±2.1) group following DMH treatment (duration,
18 weeks) was increased compared with the average number in the
SL3261B group (pIRES-4-1BBL/SL3261; 5.7±1.2) and the SL3261C group
(pIRES-CEACAM6/SL3261; 5.0±1.4). The SL3261D
(pIRES-CEACAM6-4-1BBL/SL3261) group had an average of 4.3±1.4
tumors, which was reduced compared to all other groups and
significantly reduced compared with the SL3261A group (P<0.05).
However, no significant differences were observed between the
pIRES-4-1BBL/SL3261, pIRES-CEACAM6/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261 vaccine groups (P>0.05).
Additionally, a number of the tumors observed in the SL3261A group
were advanced stage tumors (4 stage B-C tumors; 2 stage D tumors),
while the majority of the tumors observed in the SL3261C groups
were stage B-C (SL3261C, stage A, 1; stage B-C, 5). By contrast,
the majority of the tumors observed in the SL3261D group were stage
A tumors (stage A, 5; stage B-C, 1; P<0.05). This result
suggested that the SL3261D tumors were mainly restricted to the
mucosa with little or no invasion or metastasis.
Although no significant differences were identified
for body weight of rats between the 4 groups, the
SL3261D-vaccinated group had a marked but non-significant increase
in body weight 16 weeks following DMH treatment (17).
Population analysis of immune cells
and cytokines in the colon and spleen
In a previously published study, we observed that a
significant reduction in the number of tumors was accompanied by
higher densities of CD3+, CD8+ and CD56+ and a lower number of
FOXP3+ TIL cells in SL3261D rats (17). Therefore, to investigate the mechanism
underlying the tumor growth inhibition induced by this vaccine, the
present study analyzed CD45RO+ TIL infiltration and the expression
of IL-4 and IL-17 by IHC analysis. The present study also analyzed
splenic tissues for IFN-γ, CD3+, CD4+, CD8+, CD56+, P3+, IL-4 and
IL-17 expression. It was observed that colon tumor tissues from the
vaccinated groups had significantly higher CD45RO+ TIL antibody
staining when compared with the SL3261A group (P<0.05). However,
among the SL3261B, SL3261C and SL3261D groups, the SL3261D group
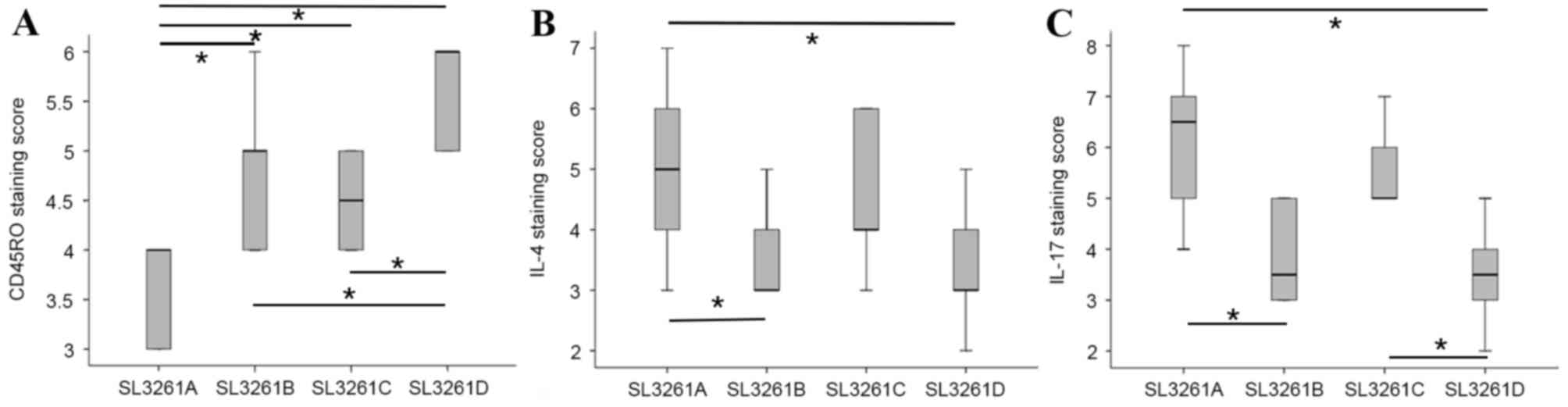
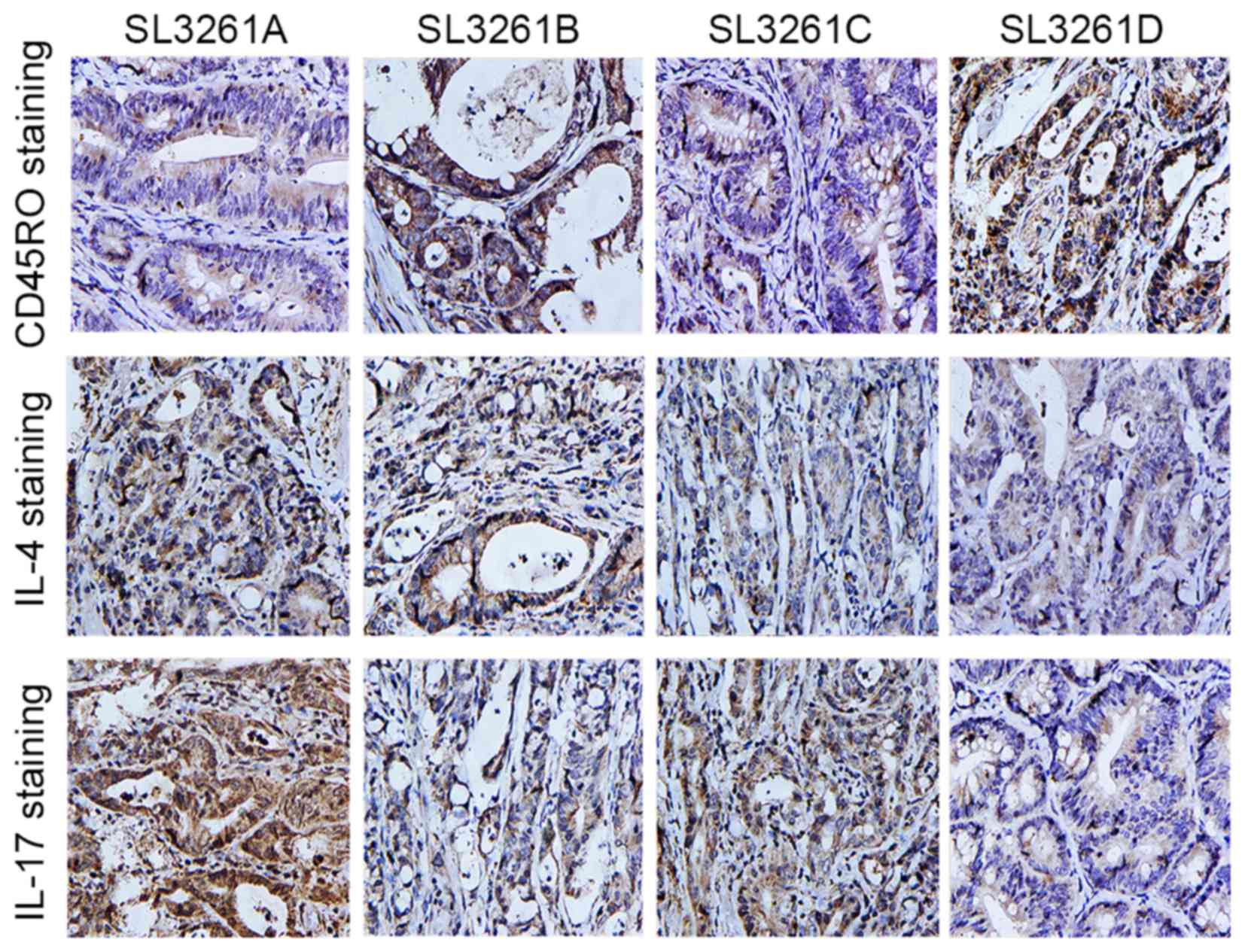
exhibited the highest rate of CD45RO+ cell infiltration (Fig. 1A). By contrast, the SL3261D group
exhibited significantly lower staining for IL-4 and IL-17 when
compared with the SL3261A group (P<0.05; Fig. 1B and C) (Fig. 2).
Splenic tissue analysis revealed that IFN-γ and
CD3+ (but not CD4+) were expressed at
significantly higher levels in the SL3261B and SL3261D groups when
compared with the SL3261C and SL3261A groups. CD56+ T
cell expression was higher in the SL3261B and SL3261D groups when
compared with the SL3261A group. Additionally, CD8+ T
cell expression was significantly higher in the SL3261D and SL3261B
groups when compared with the other groups. By contrast, the
SL3261B and SL3261D groups exhibited significantly lower expression
of FOXP3, IL-4 and IL-17 when compared with the SL3261A group
(P<0.05). Finally, the SL3261D group exhibited the lowest IL-17
expression levels in the spleen among all the groups examined
(Figs. 3 and 4).
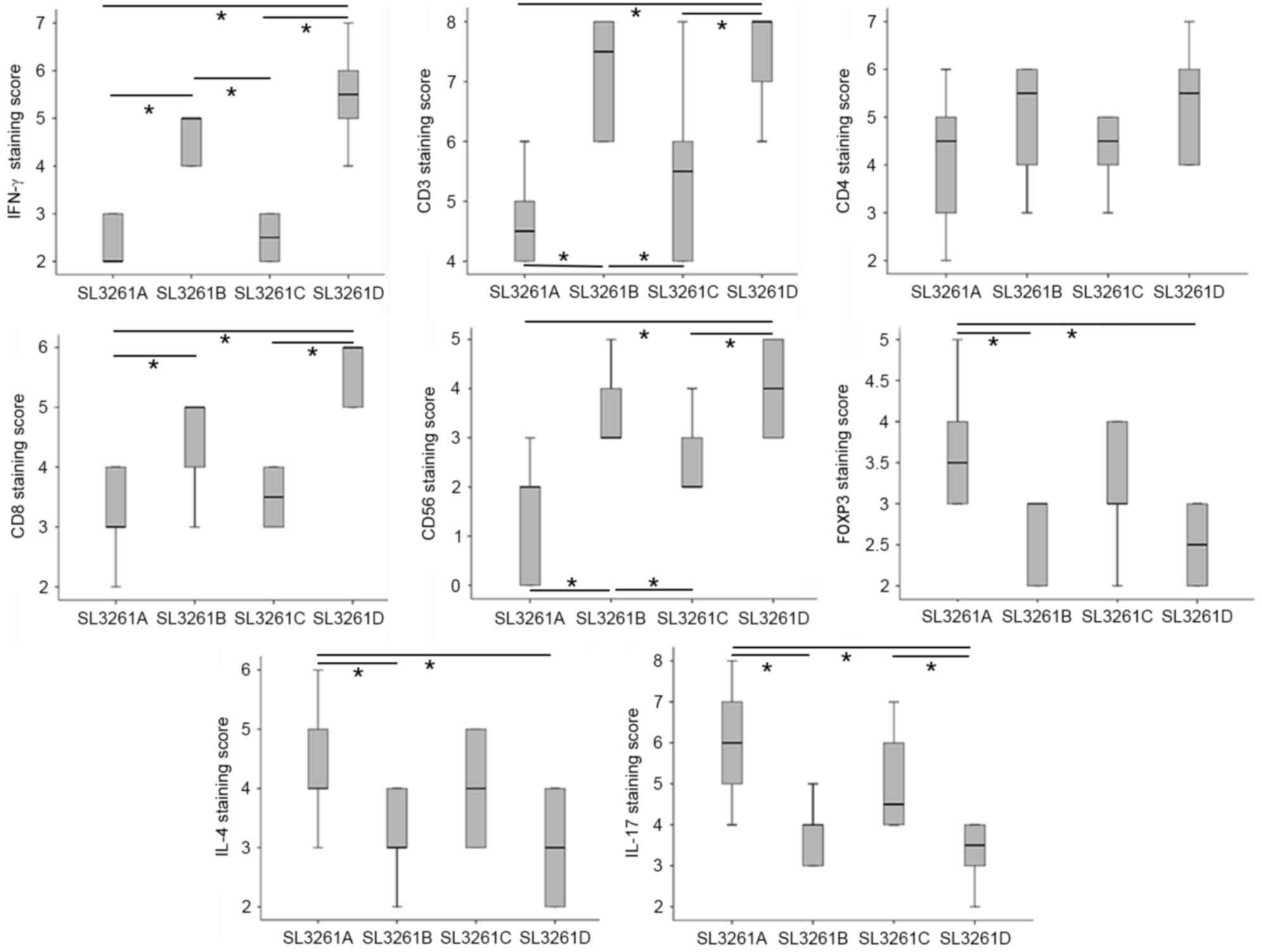 | Figure 3.Immunohistochemical staining of
splenic tissue. Immunostaining scores for IFN-γ, CD3+,
CD4+, CD8+, CD56+,
FOXP3+, IL-4 and IL-17 in splenic tissues from the 4
treatment groups. *P<0.05 IFN, interferon; CD, cluster of
differentiation; FOXP3, forkhead/winged-helix transcription factor
box P3; IL, interleukin. |
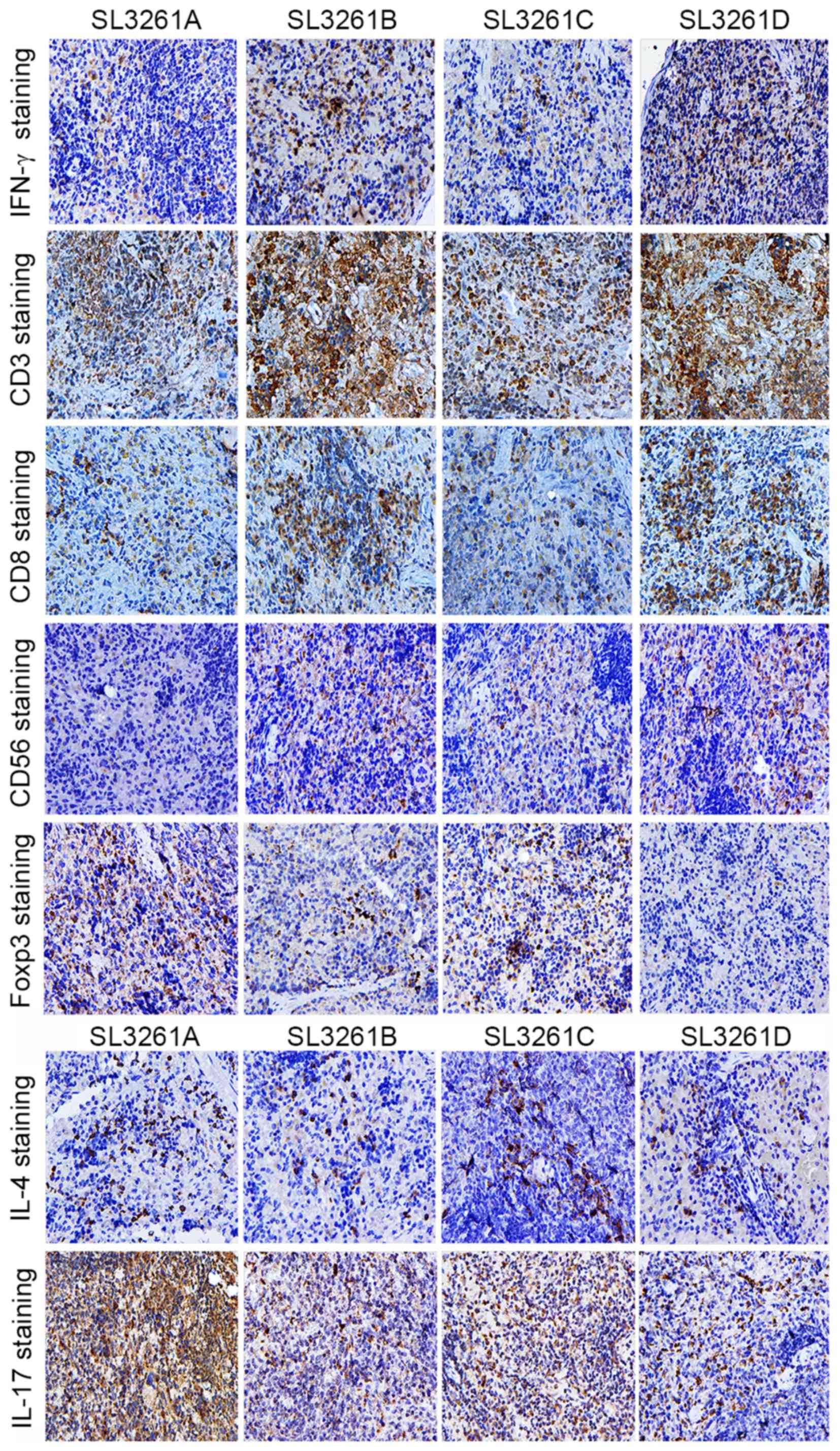 | Figure 4.Representative images of
immunohistochemical staining of spleen sections. IFN-γ,
CD3+, CD8+, CD56+,
FOXP3+, IL-4 and IL-17 staining in spleen sections
derived from the 4 treatment groups (SL3261A, SL3261B, SL3261C and
SL3261D; magnification, ×200). IFN, interferon; CD, cluster of
differentiation; FOXP3, forkhead/winged-helix transcription factor
box P3; IL, interleukin. |
Discussion
A previous study observed the appearance, subsequent
to 12 weeks of DMH administration, of a cell mass exhibiting
abnormal morphology and an accumulation of undifferentiated cells
with pleiomorphic and conspicuous nucleoli in a small cluster of
neighboring crypts of the colon (20). A total of 15 weeks subsequent to the
initiation of DMH treatment, microscopic carcinomatous foci were
observed (20). Therefore, in the
present study, based on this background information, the vaccine
was administered 8 weeks subsequent to the initiation of DMH
treatment.
pIRES-4-1BBL/SL3261, pIRES-CEACAM6/SL3261 and
pIRES-CEACAM6-4-1BBL/SL3261 vaccine treatment suppressed tumor
growth when compared with pIRES/SL3261 treatment. The present study
observed a small decrease in the number of colorectal tumors in the
pIRES-CEACAM6-4-1BBL/SL3261 group.
Staging of the observed tumors revealed that the
majority of the tumors in the pIRES/SL3261 groups were in a late
stage, while the majority of tumors in the pIRES-4-1BBL/SL3261 and
pIRES-CEACAM6/SL3261 groups were in an early or middle stage. By
contrast, the pIRES-CEACAM6-4-1BBL/SL3261 group primarily exhibited
early stage tumors. These data indicate that the individual
recombinant bacteria-based 4-1BBL and CEACAM6 vaccines effectively
inhibit CRC development, but the recombinant bacteria-based
combined CEACAM6+4-1BBL vaccine synergistically inhibits CRC
development and tumor growth.
To investigate the mechanism behind the tumor
suppressive activity of these vaccines, the present study studied
the subsets of immune cells that infiltrated colon tumor tissues
and the spleen in the DMH treated rats. It was observed that the
pIRES-CEACAM6-4-1BBL/SL3261 group exhibited significantly higher
CD3+, CD8+ and CD56+ and reduced
FOXP3+ TIL. It is important to note that this group had
the lowest number of tumors. Furthermore, analyzing cytokine
expression revealed that the pIRES-CEACAM6-4-1BBL/SL3261 and
pIRES-4-1BBL/SL3261 groups had reduced IL-4 and IL-17 staining when
compared with the pIRES/SL3261 and pIRES-CEACAM6/SL3261 groups. A
similar distribution of these molecules was observed in the splenic
tissue analysis. Among all groups, splenic IFN-γ expression was
highest in the pIRES-CEACAM6-4-1BBL/SL3261 group. These results
suggest that the recombinant Salmonella-based CEACAM6 and
4-1BBL vaccine inhibited the development of rat CRC by inducing
specific tumor suppressive immune responses and enhancing T cell
immunity.
The present study proposes that CEACAM6 presented on
the surface of APCs is the first T cell activation signal, and this
signal is aided by the co-stimulatory molecule 4-1BBL. Thus, the
combination of CEACAM6 and 4-1BBL leads to a strong induction of
specific anti-CEACAM6 CTLs. Additionally, the exogenous addition of
4-1BBL increases the functional activity of
CD3+CD56+ NK cells. Furthermore, these
vaccines may also promote Th1 polarization and inhibit Th2
polarization. This, in turn, promoted the formation of IFN-γ and
inhibited the formation of IL-4. The elevated IFN-γ then inhibited
the development of Th17 cells, which explains the reduction of
IL-17. Finally, this chain of molecular events led to the
inhibition of inflammation and tumor blood vessel formation, which
resulted in the observed reduction in tumor incidence.
Once memory T cells encounter an antigen, due to
prior infection, cancer or a vaccine, they can mount a fast and
strong immune response when the same antigen is encountered a
second time. CD45RA and CD45RO are specific markers that
distinguish T cells from memory T cells (21). CD45RO+ T cells are
considered memory T cells since they proliferate in response to
recalled antigens and following PHA activation by cord blood
mononuclear cells (21).
CD45RO+ T cells have also been identified as the main
anti-tumor effector cells in early CRCs and high CD45RO+
T cell infiltration levels are correlated with the absence of early
metastatic invasion, less advanced pathologic stages and increased
survival rate (22,23). The present study observed higher
CD45RO+ TIL cell staining in the
pIRES-CEACAM6-4-1BBL/SL3261 group, which also exhibited the lowest
number of tumors among the 4 groups. This indicates that the
vaccine activates tissue-resident memory T cells and induces an
effective secondary immune response.
Tregs are a small subset of
CD4+CD25+ T cells that are identified by
FOXP3 expression. FOXP3 is a transcription factor that is critical
for the differentiation and suppressive function of Tregs (24). FOXP3+ expression has been
associated with disease progression in several malignancies
(25,26). The present study observed lower
FOXP3+ expression in the pIRES-CEACAM6-4-1BBL/SL3261
group. This finding suggests that the recombinant
Salmonella-based CEACAM6 and 4-1BBL vaccine may contribute
to CRC inhibition in part by decreasing FOXP3 expression. However,
this mechanism of action needs to be confirmed by future
studies.
In summary, the present results indicate that the
Salmonella based vaccine efficiently inhibits DMH-induced
colorectal tumor development in rats. This inhibition was
accompanied by an increase in the number of CD45RO+ TIL
cells, a decrease in the number of FOXP3+ cells, which
promoted Th1 polarization and inhibited Th2 and Th17 polarization.
These findings have the potential to provide the basis for new
immunotherapies designed to treat or suppress CRC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81172166),
the Natural Science Foundation of the Jiangsu Province of China
(grant no. BK2008171) and the Natural Science Foundation of the
Jiangsu Higher Education Institutions of China (grant no.
11KJB320016).
Glossary
Abbreviations
Abbreviations:
|
CEACAM6
|
non-specific cross-reacting
antigen
|
|
4-1BBL
|
4-1BB ligand
|
|
DMH
|
1,2-dimethylhydrazine
|
|
FOXP3
|
forkhead/winged-helix transcription
factor box P3
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
Th cell
|
T helper cell
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weng D, Song B, Durfee J, Sugiyama V, Wu
Z, Koido S, Calderwood SK and Gong J: Induction of cytotoxic T
lymphocytes against ovarian cancer-initiating cells. Int J Cancer.
129:1990–2001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahara A, Koido S, Ito M, Nagasaki E,
Sagawa Y, Iwamoto T, Komita H, Ochi T, Fujiwara H, Yasukawa M, et
al: Gemcitabine enhances Wilms' tumor gene WT1 expression and
sensitizes human pancreatic cancer cells with
WT1-specificT-cell-mediated antitumor immune response. Cancer
Immunol Immunother. 60:1289–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weng D, Song B, Koido S, Calderwood SK and
Gong J: Immunotherapy of radioresistant mammary tumors with early
metastasis using molecular chaperone vaccines combined with
ionizing radiation. J Immunol. 191:755–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner P, Koch M, Nummer D, Palm S,
Galindo L, Autenrieth D, Rahbari N, Schmitz-Winnenthal FH,
Schirrmacher V, Büchler MW, et al: Detection and functional
analysis of tumor infiltrating T-lymphocytes (TIL) in liver
metastases from colorectal cancer. Ann Surg Oncol. 15:2310–2317.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pagès F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masopust D, Vezys V, Marzo AL and
Lefrançois L: Preferential localization of effector memory cells in
nonlymphoid tissue. Science. 291:2413–2417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vezys V, Yates A, Casey KA, Lanier G,
Ahmed R, Antia R and Masopust D: Memory CD8 T-cell compartment
grows in size with immunological experience. Nature. 457:196–199.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Numasaki M, Watanabe M, Suzuki T,
Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze
MT, Kolls JK and Sasaki H: IL-17 enhances the net angiogenic
activity and in vivo growth of human non-small cell lung cancer in
SCID mice through promoting CXCR-2-dependent angiogenesis. J
Immunol. 175:6177–6189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He D, Li H, Li H, Yusuf N, Elmets CA, Li
J, Mountz JD and Xu H: IL-17 promotes tumor development through the
induction of tumor promoting microenvironments at tumor sites and
myeloid-derived suppressor cells. J Immunol. 184:2281–2288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beauchemin N and Arabzadeh A:
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs)
in cancer progression and metastasis. Cancer Metastasis Rev.
32:643–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jantscheff P, Terracciano L, Lowy A,
Glatz-Krieger K, Grunert F, Micheel B, Brümmer J, Laffer U, Metzger
U, Herrmann R and Rochlitz C: Expression of CEACAM6 in resectable
colorectal cancer: A factor of independent prognostic significance.
J Clin Oncol. 21:3638–3646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheuk AT, Mufti GJ and Guinn BA: Role of
4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther.
11:215–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan JT, Whitmire JK, Ahmed R, Pearson TC
and Larsen CP: 4-1BB ligand, a member of the TNF family, is
important for the generation of antiviral CD8 T cell responses. J
Immunol. 163:4859–4868. 1999.PubMed/NCBI
|
|
16
|
Rabu C, Quéméner A, Jacques Y,
Echasserieau K, Vusio P and Lang F: roduction of recombinant human
trimetric CD137L (4-1BBL). Cross-linking is essential to its T cell
co-stimulation activity. J Biol Chem. 280:41472–41481. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin C, Liu Y, Zhu J, Xia T, Zhang B, Fei
Y, Ma B, Ye J and Chen W: Recombinant Salmonella-based CEACAM6 and
4-1BBL vaccine enhances T-cell immunity and inhibits the
development of colorectal cancer in rats: In vivo effects of
vaccine containing 4-1BBL and CEACAM6. Oncol Rep. 33:2837–2844.
2015.PubMed/NCBI
|
|
18
|
Kyriakos M: The President's cancer, the
Dukes classification, and confusion. Arch Pathol Lab Med.
109:1063–1066. 1985.PubMed/NCBI
|
|
19
|
Kawai H, Ishii A, Washiya K, Konno T, Kon
H, Yamaya C, Ono I, Minamiya Y and Ogawa J: Estrogen receptor alpha
and beta are prognostic factors in non-small cell lung cancer. Clin
Cancer Res. 11:5084–5089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maskens AP: Histogenesis and growth
pattern of 1, 2-dimethylhydrazine-induced rat colon adenocarcinoma.
Cancer Res. 36:1585–1592. 1976.PubMed/NCBI
|
|
21
|
Janeway CA Jr: The T cell receptor as a
multicomponent signaling machine: CD4/CD8 co-receptors and CD45 in
T cell activation. Ann Rev Immunol. 10:645–674. 1992. View Article : Google Scholar
|
|
22
|
Woodland DL and Kohlmeier JE: Migration,
maintenance and recall of memory T cells in peripheral tissues. Nat
Rev Immunol. 9:153–161. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wakatsuki K, Sho M, Yamato I, Takayama T,
Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K and Nakajima Y:
Clinical impact of tumor-infiltrating CD45RO+ memory T
cells on human gastric cancer. Oncol Rep. 29:1756–1762.
2013.PubMed/NCBI
|
|
24
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prevalence of FOXP3+ regulatory T cells increases
during the progression of pancreatic ductal adenocarcinoma and its
premalignant lesions. Clin Cancer Res. 12:5423–5434. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi N, Hiraoka N, Yamagami W, Ojima
H, Kanai Y, Kosuge T, Nakajima A and Hirohashi S: FOXP3+ regulatory
T cells affect the development and progression of
hepatocarcinogenesis. Clin Cancer Res. 13:902–911. 2007. View Article : Google Scholar : PubMed/NCBI
|