Introduction
Extranodal marginal zone lymphoma (EMZL) of mucosa-
associated lymphoid tissue (MALT) is a subtype of indolent
non-Hodgkin lymphoma that develops in the extranodal organs, such
as the stomach, salivary glands, ocular adrexa and the thyroid
(1). Repeated cytogenetic alterations
include reciprocal chromosomal translocations such as
t(11;18)(q21;q21) in Helicobacter pylori
infection-unassociated gastric EMZL (2), t(14;18)(q32;q21) in ocular adnexa EMZL,
t(1;4)(p22;q32) in intestinal and pulmonary EMZL and numerical
abnormalities such as trisomy of chromosome 3 or chromosome 18
(3,4).
These alterations are valuable as diagnostic markers and for
understanding the molecular pathophysiology of the lymphomagenesis
of EMZL (5). The aforementioned
chromosomal translocations are usually mutually exclusive, and
their frequencies vary widely depending on the primary tumor site.
Furthermore, these chromosomal translocations and numerical
abnormalities frequently co-exist in tumor cells from individual
patients.
Primary lymphoid neoplasms of the uterus are rare,
accounting for ~2.0% of extranodal lymphomas and for <0.5% of
gynecologic cancer (6). In addition,
the majority of primary uterus lymphomas are high-grade subtypes,
such as diffuse large B-cell lymphoma (DLBCL) (7), whilst the occurrence of EMZL of the
uterus is rare, with only 17 previously reported cases in the
English literature (8–21), and their cytogenetic/genetic
characteristics remain unknown. The present study reports a patient
with primary uterine cervical EMZL with the concomitant copy number
gains of MALT1 and B-cell lymphoma 2 (BCL2) genes,
which are located at chromosome 18q21. As the tumor cells also
harbored triple centromeres of chromosome 18, the lymphoma cells in
the patient of the present study were suggestive of trisomy 18. In
addition, the clinical features of previously reported cases of
EMZL of the uterus were reviewed. In this examination of the
English literature, the present study is the first case of uterine
cervical EMZL in which cytogenetic abnormalities were at least
partly determined.
Case report
Patient
A 71-year-old female was referred to University
Hospital of Kyoto Prefectural University of Medicine (Kyoto,
Japan), complaining of abnormal vaginal bleeding. She exhibited no
B symptoms, such as pyrexia, night sweating or body weight loss at
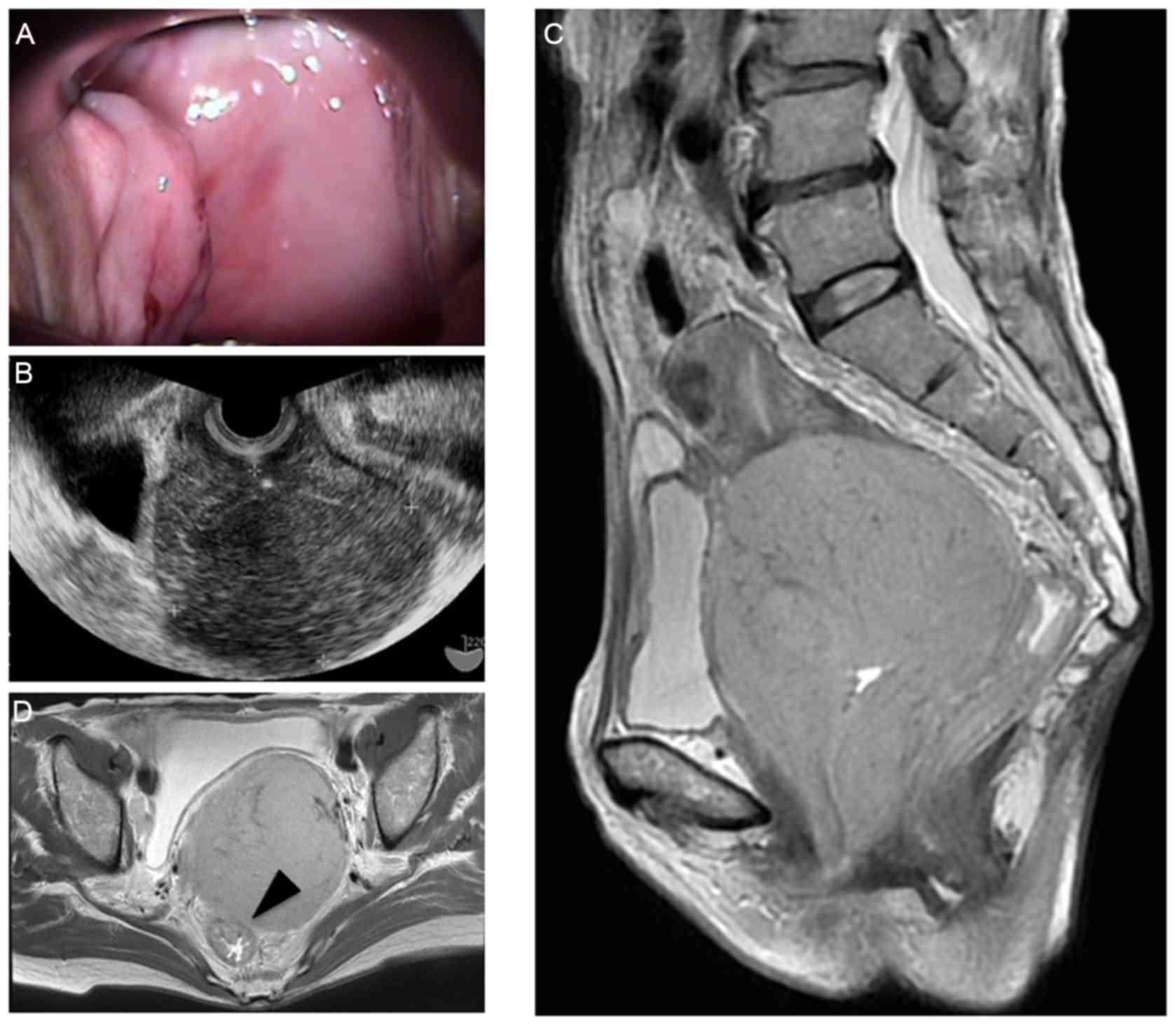
presentation. Vaginal examination identified abnormal thickening of
the vaginal wall (Fig. 1A), and
transvaginal ultrasound sonography detected a large mass, 80 mm in
diameter, at the uterine cervix (Fig.
1B). T2-weighted magnetic resonance imaging detected a slightly
high-intensity tumor at the uterus cervix that invaded directly to
the rectal serosa (Fig. 1C and D).
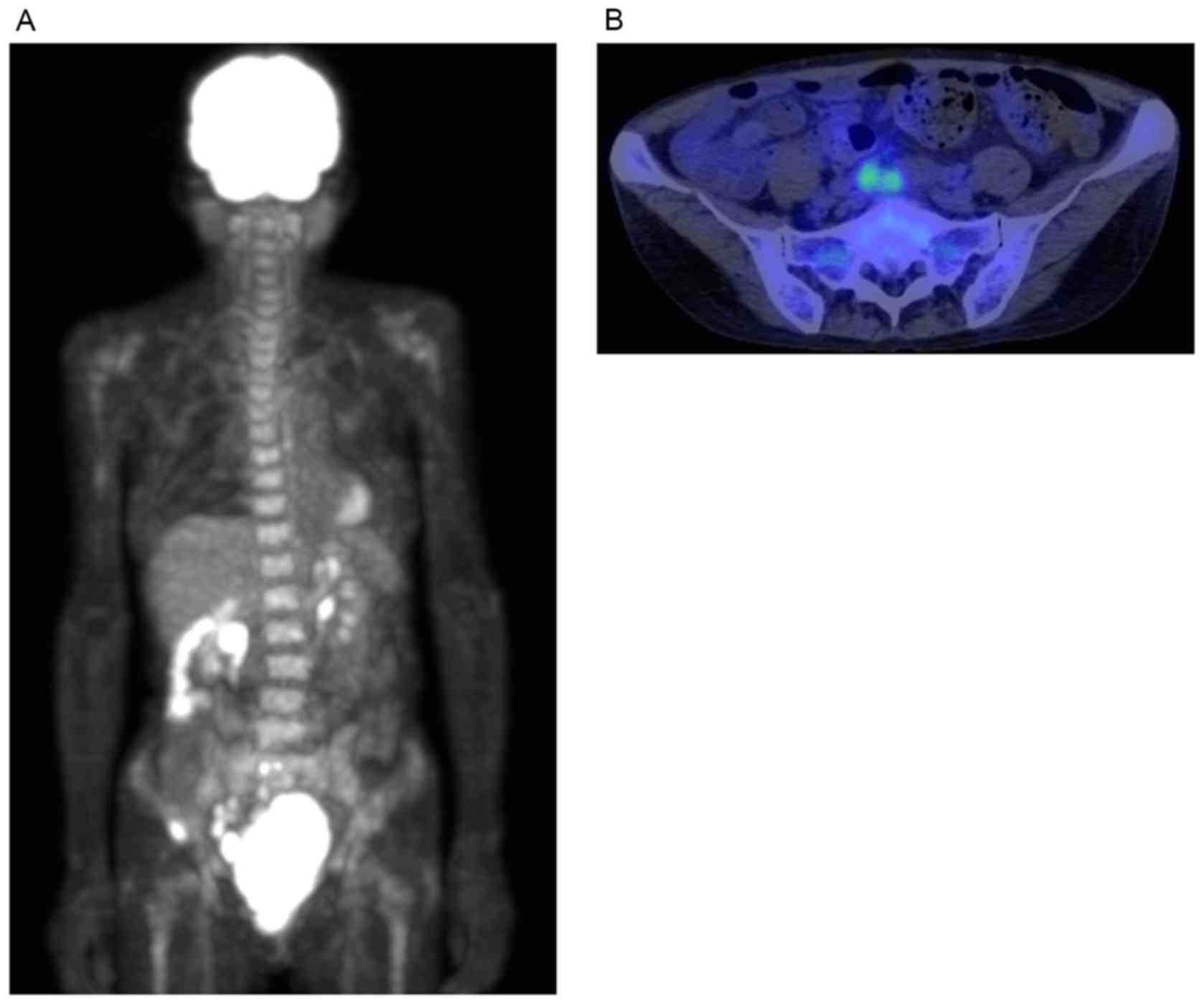
Positron emission tomography with 2-deoxy-2-(fluorine-18)
fluoro-D-glucose (FDG) integrated with computed tomography also
detected the presence of enlarged FDG-avid lymph nodes in the
pelvis, whilst other lesions were intact (Fig. 2). The serum soluble interleukin-2
receptor level was elevated to 1230 U/ml, normal range; 122–496
U/ml, whilst other laboratory tests were normal, including blood
cell counts, lactate dehydrogenase, C-reactive protein and albumin.
The serum antibody test for Chlamydia trachomatis was
negative. Histological examination of the biopsied specimen of the
tumor revealed infiltration of small and round-shaped abnormal
lymphoid cells with oval or convoluted nuclei that were positive
for cluster of differentiation (CD)20, CD79a and BCL2 markers
(Fig. 3A-D), but were negative for
CD5, CD10, cyclin D1, BCL6, or pan-cytokeratin markers (data not
shown). These results were consistent with the results obtained by
flow cytometric analysis, which detected the presence of
CD19-phycoerythrin (PE)-cyanin 5.1 (Beckman Coulter, Inc., Brea,
CA, USA) and CD20-fluorescein isothiocyanate (FITC), but the
absence of CD5-FITC or CD10-PE (BD Biosciences, San Jose, CA, USA).
Additionally, flow cytometry also revealed the expression of
surface immunoglobulin (Ig) λ chain of tumor cells (data not
shown). Collectively, the patient was diagnosed with EMZL, stage II
according to the Ann Arbor staging system (22). The patient was treated with 6 courses
of rituximab plus cyclophosphamide, doxorubicin, vincristine and
prednisolone (R-CHOP): Rituximab 375 mg/m2 on day 1,
cyclophosphamide 750 mg/m2 on day 2, doxorubicin 50
mg/m2 on day 2, vincristine 1.4 mg/m2 on day
2, prednisolone 100 mg/body on days 2–6, and attained complete
response (CR). She has maintained CR for 9 months at the time of
writing. Informed consent was obtained from the patient.
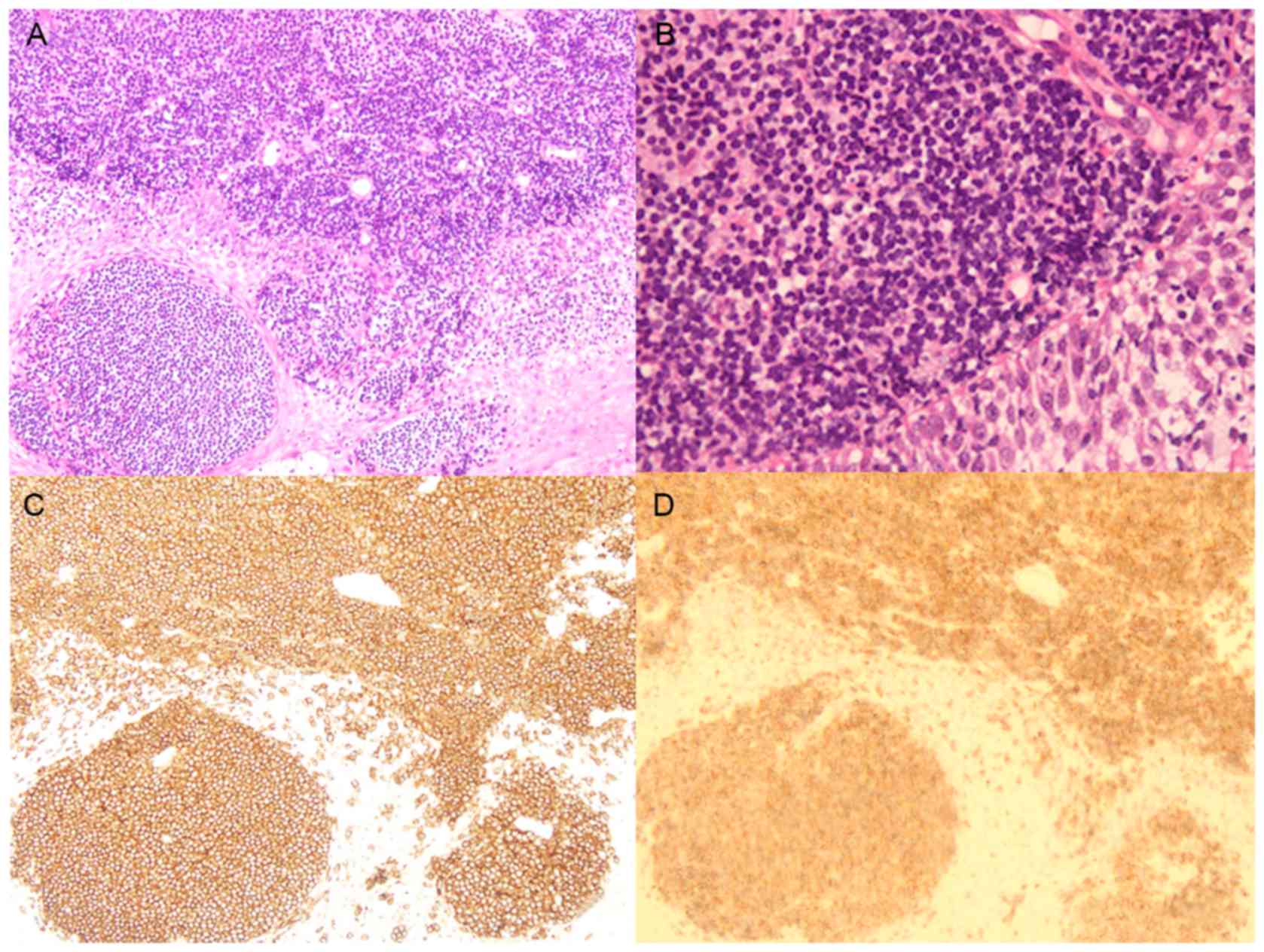 | Figure 3.Histological findings. Hematoxylin
& eosin staining of a biopsied specimen from the uterine
cervical tumor revealed infiltration of small and round-shaped
abnormal lymphoid cells with oval or convoluted nuclei in the
uterine cervix, leading to the diagnosis of extranodal marginal
zone lymphoma at (A) magnification, ×100 and (B) magnification,
×400 (light microscope). Immunohistochemical staining using the
Ventana iVIEW DAB Universal Kit (Ventana Medical Systems, Inc., Oro
Valley, AZ, USA) revealed that the abnormal lymphoid cells
expressed (C) cluster of differentiation 20 stained with anti-CD20
antibody (L26) (Roche Diagnostics, Branchburg, NJ, USA) at
magnification, ×100 and (D) B-cell lymphoma 2 stained with
anti-BCL2 antibody (clone 124) (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) at magnification, ×100. |
Procedure and result by the interphase
fluorescence in situ hybridization (FISH) analysis
Single- and double-color FISH in single cell
preparations of patient-derived lymphoma cells were performed as
previously described (23). In
addition, FISH was performed on paraffin-embedded tissue sections,
tissue-FISH, according to previously described methods (24). The LSI Dual Color sets for the IGH
Break Apart Rearrangement Probe (Abbott Molecular Inc., catalog no.
8L63-20), a mixture of 2 probes that hybridize to opposite sides of
the joining gene segment through constant regions of the
Immunoglobulin heavy chain (IgH) locus, were used to detect
chromosomal breakage at the IgH gene. The LSI Dual Color set for
the MALT1 Break Apart Rearrangement Probe was utilized to detect
the gene rearrangement of MALT1. The LSI Dual Color sets for
the fusion genes of MALT1/API2, MALT1/IgH,
BCL2/IgH, or MYC/IgH (Abbott Molecular Inc., Des
Plaines, Ill., USA) were also utilized in the present study. Vysis
Chromosomes Enumeration Probe 18 (CEP18) (catalog no. 05J08-028;
Abbott Molecular Inc.) was used to detect the centromeric region of
chromosome 18.
As the metaphase spreads were unavailable due to the
lack of dividing cells in the biopsied specimens, the cytogenetic
studies were performed using interphase FISH. Although the FISH
probes that specified chromosomal translocations at
t(11;18)(q21;q21) for the MALT1/API2 fusion gene,
t(14;18)(q32;q21) for the MALT1/IgH fusion gene and
t(14;18)(q32;q21) for the BCL2/IgH fusion genes did
not demonstrate the presence of the respective fusion genes, these
examinations identified that the tumor cells harbored triple copies
of the MALT1 and BCL2 genes (Fig. 4A-C). In addition, the tumor cells also
harbored three signals for chromosome enumeration probe 18,
indicating the existence of centromeres of chromosome 18 (Fig. 4D), and three copies of the
non-rearranged MALT1 gene (Fig.
4E). Collectively, these results suggested the existence of
trisomy 18 in the tumor cells. Whilst the FISH examination also
identified the IgH split signal, which indicated the
presence of IgH translocation, the translocation partner,
including c-MYC (MYC), was not identified (Fig. 4F and data not shown).
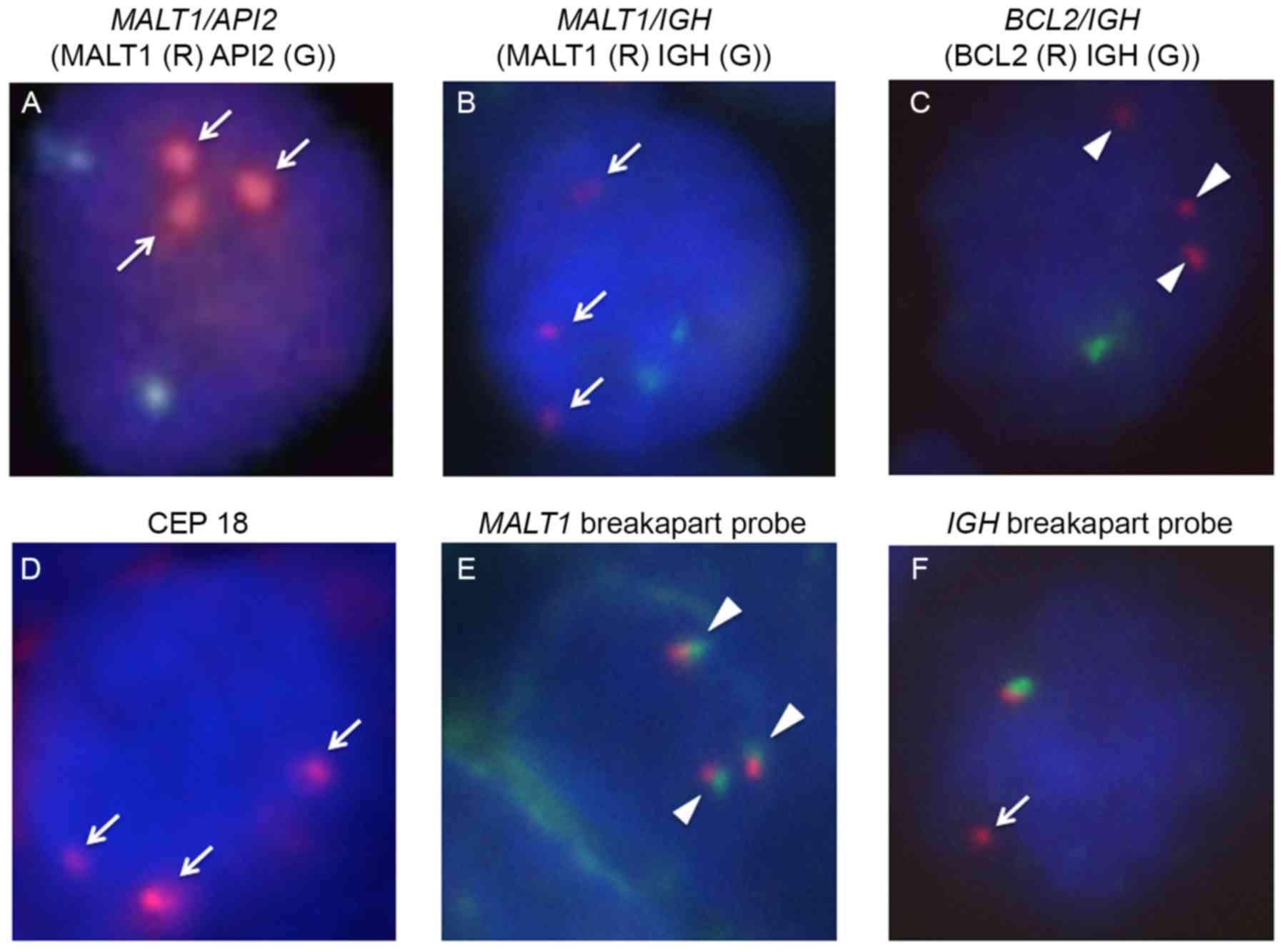 | Figure 4.FISH analyses. (A-C) Whilst
double-color FISH analyses for the API2/MALT1,
IgH/MALT1, and IgH/BCL2 fusion genes were negative,
these examinations revealed that the tumor cells harbored triple
copies of (A, B) MALT1 (arrows) and (C) BLC2
(arrowheads). The tumor cells harbored (D) three centromeres of
chromosome 18 (arrow), whilst (E) the MALT1 gene was not
rearranged (arrowheads). (F) FISH evaluation also identified the
IgH split signal, indicating the presence of IgH gene
rearrangement (arrow). FISH, fluorescence in situ
hybridization; CEP, chromosome enumeration probe; MALT1, mucosa
associated lymphoid tissue lymphoma translocation gene 1; IgH,
immunoglobulin heavy locus; BCL2, B-cell lymphoma 2. |
Discussion
Fox and More (25)
defined the criteria for the diagnosis of primary extranodal
lymphoma of the uterus as follows: Clinically confined to the
uterus, no evidence of leukemia and along interval between the
appearance of primary uterine lymphoma and the secondary tumor. The
patient of the present study fulfilled these criteria. Amongst the
previously reported cases of uterine EMZLs, including the patient
of the present study (Table I), the
development of EMZLs in the uterine cervix were even more rare,
with only 4 reported patients (19–21). With
respect to the gross appearance of the tumors of 18 cases, 15 were
small in size, located in grossly normal epithelium or in small
polyps, and were incidentally identified by the screening biopsy
(Cases 1–13, 15 and 17 in Table I).
Large tumors, as in the patient of the present study, were
extremely rare. Cytogenetic analyses using FISH were administered
to 6 previously reported cases, yet they failed to detect any
particular chromosomal abnormalities (Cases 1–6 in Table I) (8,9). In the
patient of the present study, trisomy 18 and IgH
translocation were suspected, although not determined definitively
due to the lack of sufficient material for metaphase spreads. Thus,
the present study is the first study to identify partial
chromosomal abnormalities in EMZL of the uterus.
 | Table I.List of cases of extranodal marginal
zone lymphoma of mucosa-associated lymphoid tissue of the
uterus. |
Table I.
List of cases of extranodal marginal
zone lymphoma of mucosa-associated lymphoid tissue of the
uterus.
|
|
|
|
| Gross appearance |
|
| FISH |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Age | Clinical
presentation | Site | Normal | Polyp | Tumor | Stage | Treatment | t(11;18) | t(14;18) | t(1;14) | Other | (Refs.) |
|---|
| 1 | 80 | Vaginal | Corpus | + | − | − | II | Hysterectomy | Neg. | Neg. | Neg. | − | (7) |
|
|
| prolapse |
| 2 | 58 | Incidental | Corpus | + | − | − | I | Hysterectomy | Neg. | Neg. | − | − | (8) |
| 3 | 46 | Bleeding | Corpus | + | − | − | I | Hysterectomy | Neg. | Neg. | − | − | (8) |
| 4 | 59 | Bleeding | Corpus | + | − | − | I | Hysterectomy | Neg. | Neg. | − | − | (8) |
| 5 | 72 | Bleeding | Corpus | + | − | − | I | None | Neg. | Neg. | − | − | (8) |
| 6 | 61 | Incidental | Corpus | + | − | − | I | Hysterectomy | − | Neg. | − | − | (9) |
| 7 | 43 | Bleeding | Corpus | + | − | − | II | TAH-BSO+LN | − | − | − | − | (10) |
|
|
|
|
|
|
|
|
| sampling |
| 8 | 47 | Dysmenorrhea | Corpus | + | − | − | I | TAH-BSO | − | − | − | − | (11) |
| 9 | 52 | Bleeding | Corpus | + | − | − | IV | TAH-BSO | − | − | − | − | (12) |
| 10 | 77 | Incidental | Corpus | + | − | − | I | Hysterectomy | − | − | − | − | (13) |
| 11 | 81 | Incidental | Corpus | − | + | − | I | − | − | − | − | − | (14) |
| 12 | 55 | Bleeding | Corpus | − | + | − | I | TAH-BSO | − | − | − | − | (15) |
| 13 | 65 | Bleeding | Corpus | − | + | − | I | TAH-BSO | − | − | − | − | (16) |
| 14 | 72 | Dysurea | Corpus | − | − | + | II | TAH+RT | − | − | − | − | (17) |
| 15 | 46 | Bleeding | Cervix | − | + | − | IV | ProMACE/ | − | − | − | − | (18) |
|
|
|
|
|
|
|
|
| CtyaBOM
hysterectomy |
| 16 | 56 | Vaginal | Cervix | − | − | + | I | Hysterectomy
with | − | − | − | − | (19) |
|
|
| spotting |
|
|
|
|
| bilateral
salpingo- |
|
|
|
|
|
|
|
|
| oophermectomy+ |
|
|
|
|
|
|
|
|
| RT+Rit |
| 17 | 53 | Cervical | Cervix | + | − | − | I | Conization | − | − | − | − | (20) |
|
|
| dysplasia |
| 18 | 71 | Bleeding | Cervix, | − | − | + | II | R-CHOP | Neg. | Neg. | Neg. | Trisomy | Present |
|
|
|
| Vagina |
|
|
|
|
|
|
|
| 18 | study |
Trisomy 18 has been associated with the levels of
clinical aggression in gastric EMZL (26). In addition, Sugimoto et al
(27) reported a case of DLBCL of the
uterus harboring trisomy 18 that was suspected to have been
transformed from EMZL. These suggest a potential association
between trisomy 18 and the large tumor at initial presentation in
the present study. Conversely, whilst rearrangement of the
IgH gene in the lymphoma cells was identified in the present
patient, the translocation partner was not. Furthermore,
investigation of the t(8;14)(q24;q32) for the IgH/MYC fusion
gene was negative (data not shown). In addition, as the
immunohistochemical analysis revealed that BCL6 was negative in the
tumor specimen, the t(3;14)(q27;q32) alteration was unlikely to
exist. Thus, the pathogenic involvement of the IgH
translocation remains unknown in the present study.
Whilst localized therapies, including surgical
resection and radiotherapy, have been generally adapted to EMZL in
the limited disease stage, systemic immunochemotherapy R-CHOP as
the initial treatment was administered to the patient in the
present study for several reasons. Firstly, the large tumor invaded
directly to the rectal serosa at presentation, and radiotherapy was
considered high risk for rectal penetration or rupture. It was also
anticipated that the wide field irradiation for the large tumor may
lead to unwanted adverse events in the pelvic viscera. Secondly,
surgical resection was also excluded, as complete resection of the
large tumor and the additionally involved lymph nodes required
pelvic evisceration. To avoid those invasive therapy-associated
complications, systemic immunochemotherapy was selected. However,
EMZL is generally a clinically indolent disease with a 5-year
overall survival ranging from 86 to 100% (28,29). The
optimal approach for the management of uterine EMZL has not yet
been established, and the treatment choice should be carried in
consideration of the tumor site, disease stage and clinical
manifestations on an individual basis. In conclusion, the present
study reports the first case of uterine cervical EMZL with trisomy
18 and IgH translocation with an unknown partner, as
detected by FISH analyses.
Acknowledgements
The authors are grateful to Ms. M Minatani and Ms. C
Ikawa for their excellent technical support. The present study was
supported in part by Grants-in-Aid for Scientific Research from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan (grant nos. J142004051, J152001060 and J162004043).
References
|
1
|
Isaacson PG: Update on MALT lymphomas.
Best Pract Res Clin Haematol. 18:57–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H, Ye H, Ruskone-Fourmestraux A, De
Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wündisch
T, et al: T(11;18) is a marker for all stage gastric MALT lymphomas
that will not respond to H. pylori eradication. Gastroenterology.
122:1286–1294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Penas Murga EM, Hinz K, Röser K,
Copie-Bergman C, Wlodarska I, Marynen P, Hagemeijer A, Gaulard P,
Löning T, Hossfeld DK and Dierlamm J: Translocations
t(11;18)(q21;q21) and t(14;18)(q32;q21) are the main chromosomal
abnormalities involving MLT/MALT1 in MALT lymphomas. Leukemia.
17:2225–2229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Streubel B, Simonitsch-Klupp I, Müllauer
L, Lamprecht A, Huber D, Siebert R, Stolte M, Trautinger F, Lukas
J, Püspök A, et al: Variable frequencies of MALT
lymphoma-associated genetic aberrations in MALT lymphomas of
different sites. Leukemia. 18:1722–1726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Streubel B, Lamprecht A, Dierlamm J,
Cerroni L, Stolte M, Ott G, Raderer M and Chott A:
T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal
aberration in MALT lymphoma. Blood. 101:2335–2339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kosari F, Daneshbod Y, Parwaresch R, Krams
M and Wacker HH: Lymphomas of the female genital tract: A study of
186 cases and review of the literature. Am J Surg Pathol.
29:1512–1520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris NL and Scully RE: Malignant
lymphoma and granulocytic sarcoma of the uterus and vagina. A
clinicopathologic analysis of 27 cases. Cancer. 53:2530–2545. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wright TM, Rule S, Liu H, Du MQ and Smith
ME: Extranodal marginal zone lymphoma of the uterine corpus. Leuk
Lymphoma. 53:1831–1834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahmasebi FC, Roy S, Kolitz JE, Sen F,
Laser J and Zhang X: Primary extranodal marginal zone lymphoma of
the endometrium: Report of four cases and review of literature. Int
J Clin Exp Pathol. 8:3036–3044. 2015.PubMed/NCBI
|
|
10
|
Heeren JH, Croonen AM and Pijnenborg JM:
Primary extranodal marginal zone B-cell lymphoma of the female
genital tract: A case report and literature review. Int J Gynecol
Pathol. 27:243–246. 2008.PubMed/NCBI
|
|
11
|
Frey NV, Svoboda J, Andreadis C, Tsai DE,
Schuster SJ, Elstrom R, Rubin SC and Nasta SD: Primary lymphomas of
the cervix and uterus: The university of Pennsylvania's experience
and a review of the literature. Leuk Lymphoma. 47:1894–1901. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nezhat CH, Dun EC, Wieser F and Mauricio
Z: A rare case of primary extranodal marginal zone B-cell lymphoma
of the ovary, fallopian tube, and appendix in the setting of
endometriosis. Am J Obstet Gynecol. 208:e12–e14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamadani M, Kharfan-Dabaja M, Kamble R,
Kern W and Ozer H: Marginal zone B-cell lymphoma of the uterus: A
case report and review of the literature. J Okla State Med Assoc.
99:154–156. 2006.PubMed/NCBI
|
|
14
|
Merritt AJ, Shenjere P, Menasce LP, Reid
F, Diss T, McVey RJ and Byers RJ: Primary extranodal marginal zone
B cell lymphoma of the uterus: A case study and review of the
literature. J Clin Pathol. 67:375–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Annibali O, Romeo AA, Agostinelli C,
Marchesi F, Natale A, De Muro M, Tirindelli MC, Pileri SA and
Avvisati G: A case of primary MALT lymphoma of the endometrium
presenting as an asymptomatic polyp. Ann Hematol. 88:491–493. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Tucci C, Pecorella I, Palaia I and
Panici Benedetti P: Endometrial marginal zone B-cell MALT-type
lymphoma: Case report and literature review. Crit Rev Oncol
Hematol. 88:246–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iyengar P and Deodhare S: Primary
extranodal marginal zone B-cell lymphoma of MALT type of the
endometrium. Gynecol Oncol. 93:238–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ballesteros E, Osborne BM and Matsushima
AY: CD5+ low-grade marginal zone B-cell lymphomas with localized
presentation. Am J Surg Pathol. 22:201–207. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossi G, Bonacorsi G, Longo L, Artusi T
and Rivasi F: Primary high-grade mucosa-associated lymphoid
tissue-type lymphoma of the cervix presenting as a common
endocervical polyp. Arch Pathol Lab Med. 125:537–540.
2001.PubMed/NCBI
|
|
20
|
Coon D, Beriwal S, Swerdlow SH and
Bhargava R: Mucosa-associated lymphoid tissue lymphoma of the
cervix. J Clin Oncol. 26:503–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vang R, Medeiros LJ, Ha CS and Deavers M:
Non-Hodgkin's lymphomas involving the uterus: A clinicopathologic
analysis of 26 cases. Mod Pathol. 13:19–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lister TA, Crowther D, Sutcliffe SB,
Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA and
Tubiana M: Report of a committee convened to discuss the evaluation
and staging of patients with Hodgkin's disease: Cotswolds meeting.
J Clin Oncol. 7:1630–1636. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuroda J, Matsumoto Y, Tanaka R, Kurita K,
Kobayashi T, Shimizu D, Kimura S, Ashihara E, Horiike S, Shimazaki
C and Taniwaki M: JAK2V617F-positive essential thrombocythemia and
multiple myeloma with IGH/CCND1 gene translocation coexist, but
originate from separate clones. Acta Haematol. 120:177–181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto Y, Nomura K, Matsumoto S, Ueda
K, Nakao M, Nishida K, Sakabe H, Yokota S, Horiike S, Nakamine H,
et al: Detection of t(14;18) in follicular lymphoma by dual-color
fluorescence in situ hybridization on paraffin-embedded
tissue sections. Cancer Genet Cytogenet. 150:22–26. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fox H and More JR: Primary malignant
lymphoma of the uterus. J Clin Pathol. 18:723–728. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura S, Ye H, Bacon CM, Goatly A, Liu
H, Banham AH, Ventura R, Matsumoto T, Iida M, Ohji Y, et al:
Clinical impact of genetic aberrations in gastric MALT lymphoma: A
comprehensive analysis using interphase fluorescence in situ
hybridization. Gut. 56:1358–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugimoto KJ, Imai H, Shimada A,
Wakabayashi M, Sekiguchi Y, Nakamura N, Sawada T, Izumi H, Ota Y,
Komatsu N and Noguchi M: Diffuse large B-cell lymphoma of the
uterus suspected of having transformed from a marginal zone B-cell
lymphoma harboring trisomy 18: A case report and review of the
literature. Int J Clin Exp Pathol. 6:2979–2988. 2013.PubMed/NCBI
|
|
28
|
Thieblemont C, Berger F, Dumontet C,
Moullet I, Bouafia F, Felman P, Salles G and Coiffier B:
Mucosa-associated lymphoid tissue lymphoma is a disseminated
disease in one third of 158 patients analyzed. Blood. 95:802–806.
2000.PubMed/NCBI
|
|
29
|
Zinzani PL, Magagnoli M, Galieni P,
Martelli M, Poletti V, Zaja F, Molica S, Zaccaria A, Cantonetti AM,
Gentilini P, et al: Nongastrointestinal low-grade mucosa-associated
lymphoid tissue lymphoma: Analysis of 75 patients. J Clin Oncol.
17:12541999. View Article : Google Scholar : PubMed/NCBI
|