Introduction
Donor lymphocyte infusion (DLI) has been
demonstrated to promote disease regression in selected patients
with refractory and relapsed hematological malignancies, either by
inducing a direct graft-versus-tumor (GVT) effect or by indirectly
destroying the tumor cells (1–4). Several
studies have demonstrated that DLI can promote the formation of
donor-type chimerism, in addition to preventing or treating the
relapse following bone marrow stem cell transplantation (5,6),
particularly for the treatment of chronic myeloid leukemia. Thus,
this technique has gradually gained a wide application in the
management of other malignancies (7,8).
Unfortunately, the severe graft vs. host disease (GVHD) that may be
induced by DLI limits its application as a routine treatment for
the prevention of tumor relapse. It has been demonstrated that
irradiation of peripheral blood mononuclear cells (PBMCs) with at
≥25 Gy prior to infusion decreases the probability of GVHD
(9); however, the beneficial effects
of DLI are impaired. The irradiation of PBMCs has been performed in
certain clinical applications for over a decade (10–13).
Previous study from our group determined the optimum
dose of X-rays required to inhibit PBMC proliferation using an MTT
assay (7.5 Gy; Yu-Hua Sun, unpublished data). In the present study,
an additional proliferation assay using a WST-8 kit was performed
to confirm the optimum dose of X-rays, and then the cytotoxic
activity of irradiated and non-irradiated PBMCs was measured.
Additionally, the safety and efficacy of irradiated PBMCs was
assessed in patients following autologous hematopoietic stem cell
transplantation (HSCT) to avoid confounding factors from GVHD owing
to HLA-haploidentical bone marrow transplantation.
Materials and methods
Preparation of PBMCs
PBMCs were isolated using Ficoll-Hypaque density
gradient centrifugation of blood samples from three healthy donors
at the Peking University First Hospital (Beijing, China) between
January 2015 and March 2015, and separation of the samples using a
continuous automated flow cell separator (14). Granulocyte colony-stimulating factor
was not administered to the donors prior to PBMC collection. The
cells were stained with a MultiTEST™ IMK kit (BD Biosciences,
Franklin Lakes, NJ, USA) (cluster of differentiation
(CD)3/CD16CD56/CD45/CD19 and CD3/CD8/CD45/CD4) and analyzed by
using FACSCalibur flow cytometer (BD Biosciences) and CellQuest Pro
software v 6.0 (BD Biosciences). PBMCs were irradiated with 0, 2.5,
5, 7.5, 10, 15, 25, or 50 Gy using an X-ray source (Varian 21EX
Linear Accelerator; Varian Medical Systems, Inc., Palo Alto, CA,
USA) prior to the in vitro experiments. The non-irradiated
group (0 Gy) served as the control.
Cell proliferation assay
Irradiated or non-irradiated PBMCs were seeded at a
concentration of 1×106 cells/ml in Roswell Park Memorial
Institute-1640 medium containing penicillin and streptomycin,
supplemented with 10% fetal bovine serum (all Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), at 37°C with 5% CO2
in a humidified incubator, and all cells were cultured in
interleukin (IL)-2 (Peprotech, Inc., Rocky Hill, NJ, USA) at a
concentration of 200 IU/ml for 2 weeks. The IL-2 medium was
replenished each alternate day. Cell proliferation was analyzed
using a WST-8 assay kit [Cell Counting Kit-8 (CCK-8); Dojindo
Molecular Technologies, Inc., Kumamoto, Japan]. The cells (100 µl
cells/well), at a concentration of 1×106 cells/ml, were
seeded onto 96-well plates and incubated at 37°C with 5%
CO2. At the indicated time points (days 1 and 15), 10 µl
CCK-8 solution was added to each well and the plates were incubated
for 2 h. The absorbance was measured at 450 nm using a MultiSkan™
microplate reader (Thermo Fisher Scientific, Inc.). The results
were obtained from three independent experiments performed in
triplicate. The proliferation inhibition ratio was calculated using
the equation illustrated below. The control group consisted of
non-irradiated cells (0 Gy) while the blank control was fresh
medium alone.
(%)Proliferationinhibitionratio=ControlgroupOD–Experimental group
ODControlgroupOD–BlankcontrolgroupOD×100%
Cytotoxicity assay
Cytotoxicity was assessed using a lactate
dehydrogenase (LDH) assay following irradiation. Effector PBMC
cells were isolated from the peripheral blood of healthy donors
(4×106 cells/ml). Target (K562) and Adriamycin-resistant
(K562A) cells (100 µl/well at a concentration of 2×105
cells/ml), in addition to the serial dilutions of effector cells,
were incubated in a 96-well U-bottomed plate at 37°C for 4 h. Three
independent assays were performed in triplicate at each
effector:target (EF:TA) ratio used. The target or effector cells
alone were used as spontaneous LDH release controls. To measure the
maximum target cell LDH release, lysis solution was added to the
target cells and the supernatants were assayed for their LDH
concentration with the CytoTox 96® Non-Radioactive
Cytotoxicity Assay kit (Promega Corporation, Madison, WI, USA),
with measured at 492 nm. The percentage of cytotoxicity was
determined using the equation described below.
(%)Cytotoxicity=Experimental–EffectorSpontaneous–TargetSpontaneousTargetMaximum–TargetSpontaneous×100%
Patients
The study was approved before 2005 by the Ethics
Committee of Peking University First Hospital (Beijing, China;
approval no. 808), and the clinical data were assessed
retrospectively. Written informed consent was obtained from all
participants. The methodology used for risk stratification was
according to the World Health Organization classification (15). A total of 7 patients with
hematological malignancies who received X-ray-irradiated PMBCs from
day 9–330 following autologous HSCT at Peking University First
Hospital between January 2005 and January 2013 were included in the
present study. The PBMCs used were from HLA-haploidentical sibling
or offspring. Confounding factors from GVHD owing to allogeneic
stem cell transplantation could be avoided in these patients, in
order to accurately evaluate the effects of DLI. A total of 21
infusions were administered (range, 1–5 infusions/patient). The
median interval and average time were 34 and 80.6 days after HSCT,
respectively (range, 9–330 days). The dose and the time to HCST of
each infusion are shown in Table IV.
Prior to all the infusions, cells were irradiated with a 7.5 Gy
X-ray dose, as this was determined to be the optimum dose to
inhibit PBMC proliferation (Yu-Hua Sun, unpublished data). All
patients were followed up to assess the outcome of their treatment
and GVHD occurrence. A total of 5 patients received 7.5
Gy-irradiated infusions upon complete remission (CR) in an effort
to prevent relapse. Another patient was treated for partial
remission (PR), while the other patient relapsed with acute myeloid
leukemia (AML) following HSCT and received infusions to reinforce
the GVT effect. Patient clinicopathological characteristics and
outcomes are provided in Table I.
 | Table IV.Comparison of cytotoxic activity
between irradiated and non-irradiated peripheral blood mononuclear
cells against K562A cells. |
Table IV.
Comparison of cytotoxic activity
between irradiated and non-irradiated peripheral blood mononuclear
cells against K562A cells.
|
| Cytotoxicity
(%) |
|
|---|
|
|
|
|
|---|
| EF:TA radio | Median | 95% CI | SD | P-value (irradiated
vs. non-irradiated) |
|---|
| 1:1
(non-irradiated) | 9.46 | 6.91–11.91 | 2.39 |
|
| 1:1
(irradiated) | 8.10 | 5.92–9.92 | 2.05 | P>0.05 |
| 5:1
(non-irradiated) | 25.32 | 23.52–27.52 | 2.12 |
|
| 5:1
(irradiated) | 22.22 | 21.28–23.28 | 1.14 | P>0.05 |
| 10:1
(non-irradiated) | 44.85 | 44.60–45.60 | 0.26 |
|
| 10:1
(irradiated) | 47.98 | 46.33–50.33 | 1.90 | P>0.05 |
| 20:1
(non-irradiated) | 62.99 | 59.50–65.50 | 3.23 |
|
| 20:1
(irradiated) | 61.20 | 54.02–64.02 | 6.22 | P>0.05 |
 | Table I.Patient characteristics and treatment
outcome. |
Table I.
Patient characteristics and treatment
outcome.
| Patient no. | Disease | Risk
stratification | Age (years)/sex | Cell donor | Disease status
pre-HSCT | Disease status
pre-infusion | No. of infusions/cell
dose, ×107/kg (days after HSCT) | aGVHD status | Outcome |
|---|
| 1 | NHL (ENK/T) | Lugano II2 | 57/male | Haploidentical
(son) | CR1 | CR | 4.32 (+9), 7.57 (+12)
and 4.55 (+16) | No | CR for 9 years to
present |
| 2 | NHL (DLBCL) | Ann arbor IVB | 48/male | Haploidentical
(son) | PD | PR | 19.4 (+13), 14.1
(+20), 7.1 (+29) and 8.4 (+36) | No | PR for 7 months and
died with PD |
| 3 | NHL (AITL) | Ann arbor IVB | 45/male | Haploidentical
(daughter) | PR | Relapse | 8.7 (+120), 6.7
(+150), 7.3 (+180), 7.1 (+240) and 8.1 (+330) | No | CR at 2 years
followed by secondary malignancy |
| 4 | MM | DS-IIIB/ISS III IgA
κ | 58/male | Haploidentical
(son) | PR | CR | 3.23 (+106) | No | CR for 4 years to
present |
| 5 | AML M2 | Medium | 34/male | Haploidentical
(sister) | CR1 | CR | 8.72 (+14), 10.59
(+24) and 11.76 (+34) | No | CR for 7 years to
present |
| 6 | AML M4EO | Favorable
prognosis | 46/male | Haploidentical
(son) | CR1 | CR | 7.6 (+26), 6.1
(+37) and 4.5 (+243) | No | Reinforced with
CIK, and CR for 2 years to present |
| 7 | AML M2a | Medium | 38/male | Haploidentical
(brother) | CR1 | CR | 7.7 (+20) and 5.4
(+34) | No | CR at 5 months
followed by relapse |
Statistical analysis
SPSS software (version 19.0; IBM SPSS, Armonk, NY,
USA) was used to compare the cytotoxicity of fresh and irradiated
PBMCs. A paired t-test was used to compare cytotoxicity between
dependent samples (fresh PBMCs to K562 vs. K562A cells; irradiated
PBMCs to K562 vs. K562A cells). The Wilcoxon signed-rank test was
used to compare cytotoxicity between paired samples (non-irradiated
vs. irradiated PBMCs). P<0.05 was considered to indicate a
statistically significant difference.
Results
PBMC subpopulation analysis
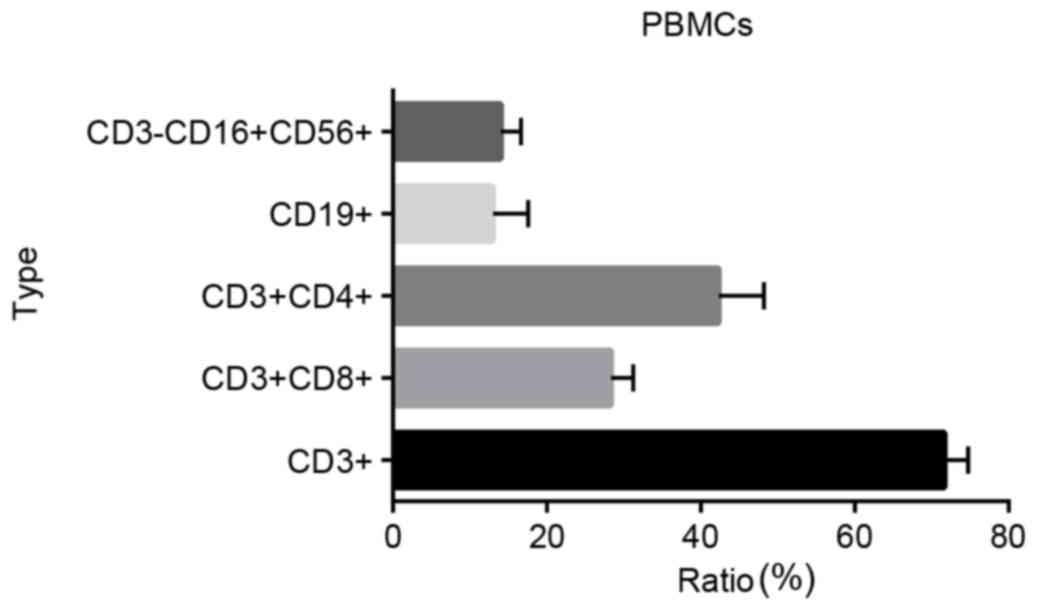
The phenotypes and ratios of PBMCs were analyzed
using flow cytometry. The proportion of CD3+ cells was
71.67±3.06%, the proportion of CD3+CD8+ cells
28.33±2.89%, the proportion of CD3+CD4+ cells
42.33±5.86%, the proportion of CD19+ cells 13.00±4.58%,
and the proportion of CD3-CD16+CD56+ cells
14.00±2.65% (Fig. 1). The majority of
the PBMCs were lymphocytes [T cell-B cell-natural killer (NK) cell
ratio, ~98.67%].
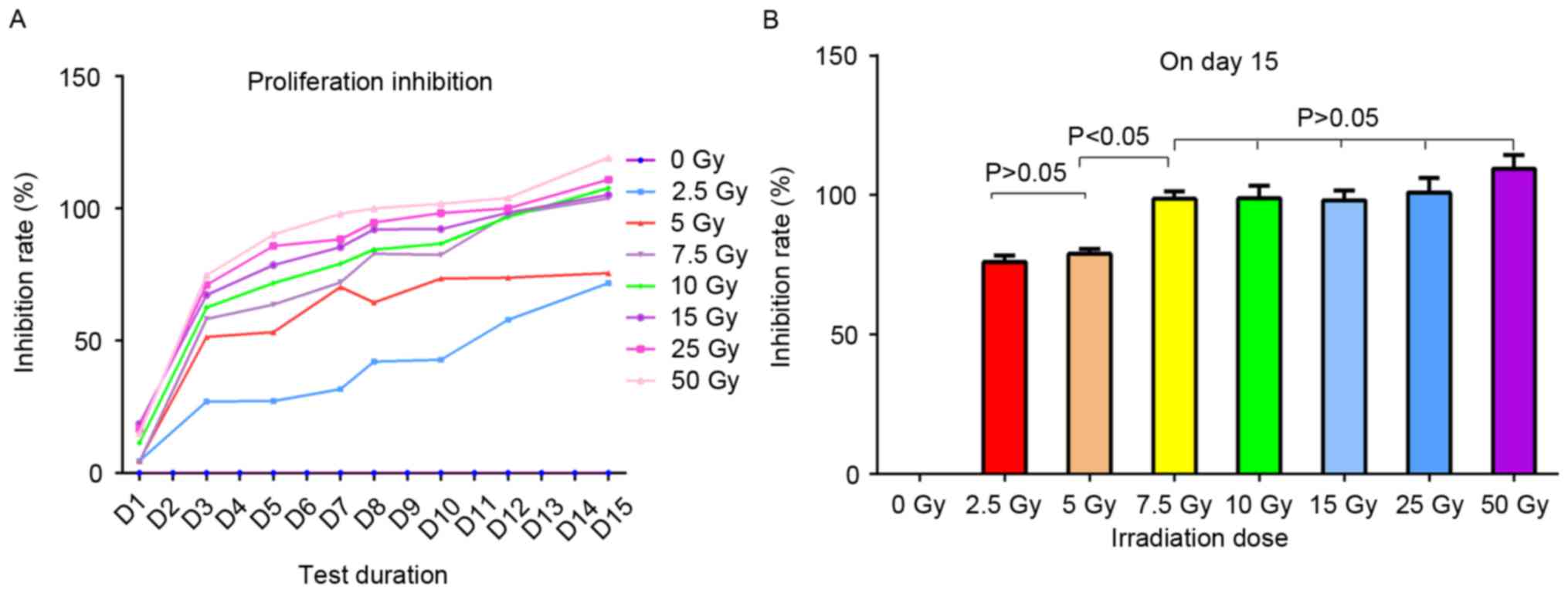
Determination of the optimum X-ray
dose for the inhibition of PBMC proliferation
To evaluate the effect of irradiation on the
proliferation of PBMCs, the cells were irradiated with eight
different doses of X-rays and cultured with IL-2 for 2 weeks. The
proliferation activity of the PBMCs was determined using a CCK-8
kit each alternate day and the proliferation inhibition rate was
calculated (Fig. 2). The
proliferation rate of non-irradiated cells was presumed to be 100%,
hence their inhibition rate at 0 Gy was 0%. Following irradiation,
the lymphocytes exhibited significant proliferation inhibition of
varying degrees compared with the non-irradiated cells. The
majority of the inhibition ratios of the seven irradiated groups
stabilized over time (Fig. 2A). The
proliferation inhibition ratios of two groups, 2.5 and 5.0 Gy, were
lower compared with that of the other five groups, 7.5, 10, 15, 25
and 50 Gy (Fig. 2B and Table II).
 | Table II.Proliferation inhibition rates of
PBMCs irradiated with different doses of X-rays. |
Table II.
Proliferation inhibition rates of
PBMCs irradiated with different doses of X-rays.
|
| Inhibition rate
(%) |
|---|
|
|
|
|---|
| Dose (Gy) | Median | 95% CI | SD |
|---|
| 0 | 0.0000 | 0.00 | 0.00000 |
|
2.5 | 76.0667 | 71.80–79.80 | 4.02658 |
|
5.0 | 78.8667 | 75.60–82.60 | 3.20208 |
|
7.5 | 98.6667 | 95.60–103–80 | 4.47363 |
| 10.0 | 98.9000 | 93.20–107.20 | 7.73111 |
| 15.0 | 98.1667 | 93.60–105.60 | 6.10437 |
| 25.0 | 103.1000 | 92.50–110.50 | 9.51420 |
| 50 | 111.1500 | 103.10–119.10 | 11.38442 |
On day 12, the five groups displayed ~100%
inhibition, and on day 15 the inhibition ratio was >100% as a
result of apoptosis. The proliferation inhibition ratios for all
seven irradiated groups were significantly different compared with
the non-irradiated PBMCs (set as a reference) (P<0.05; Fig. 2B). However, there was no statistically
significant difference between the proliferation inhibition rates
of irradiated PBMCs in the 2.5 and 5.0 Gy groups (P>0.05), or
between the other five groups (7.5, 10, 15, 25, and 50 Gy;
P>0.05; Fig. 2B). Additionally,
there was a statistically significant difference in inhibition rate
between the 2.5 and 5 Gy groups and the other five groups (7.5, 10,
15, 25 and 50 Gy; P<0.05; Fig.
2B). Therefore, 7.5 Gy was chosen as the putative X-ray dose to
irradiate the PBMCs prior to the application of DLI, in order to
rescue its GVT effect while avoiding GVHD.
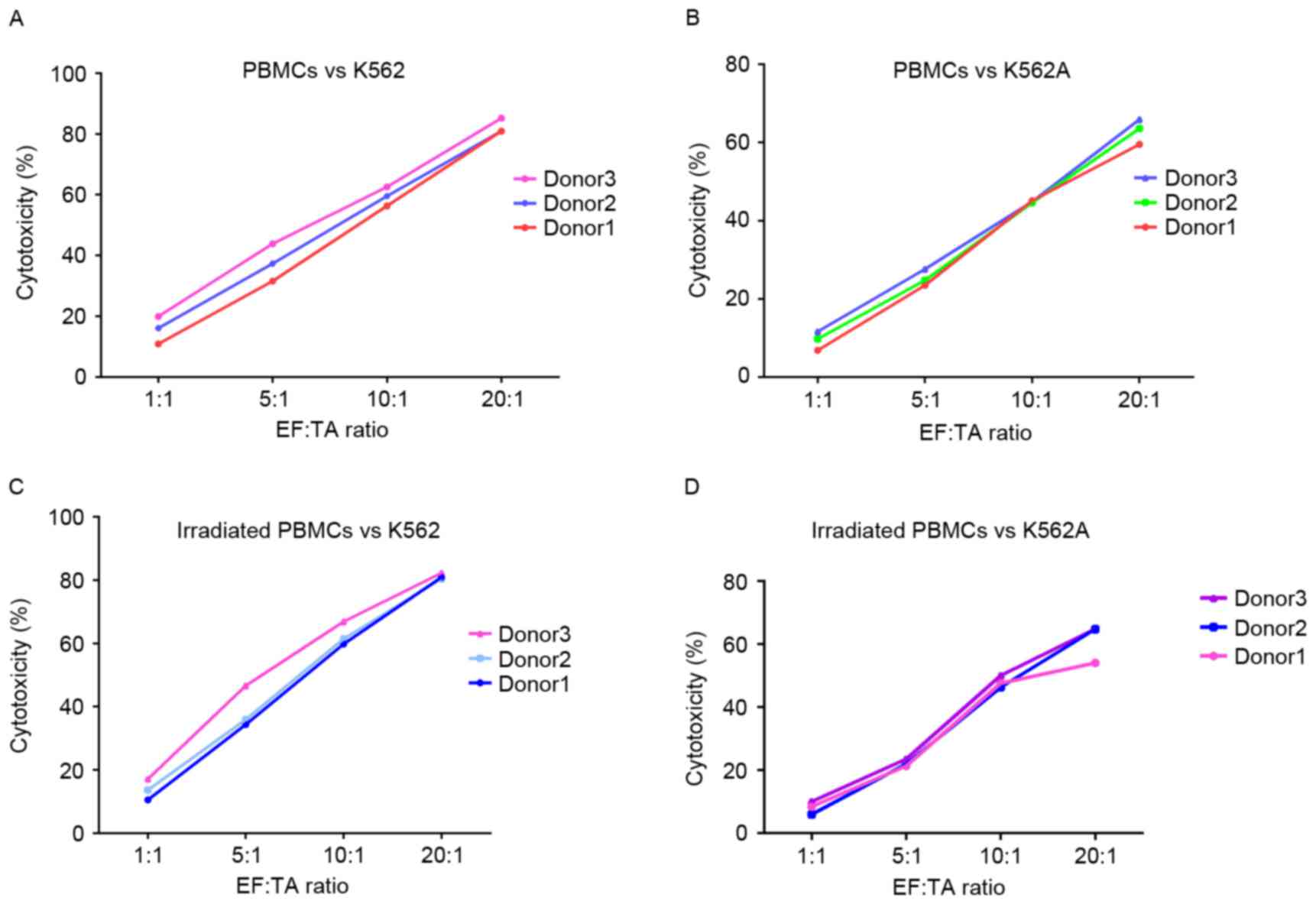
Cytotoxicity of PBMCs
The effect of the 7.5 Gy X-ray dose on the
cytotoxicity of PBMCs was analyzed. PBMCs isolated from the
peripheral blood of 3 healthy donors were tested as effector cells
in the cytotoxicity assay. Each PBMC group, irradiated and
non-irradiated, was from the same donor. The K562 cell line and the
corresponding Adriamycin-resistant strain, K562A, were the target
cells. Irradiated or non-irradiated PBMCs did not show any
significant difference in cytotoxic activity against either of the
leukemic cell lines, K562 and K562A, when mixed in a 1:1 EF:TA
ratio (Fig. 3). Increasing the EF:TA
ratio from 1:1 to 20:1 was resulted in a 2–5-fold increase in
cytotoxic activity (Fig. 3).
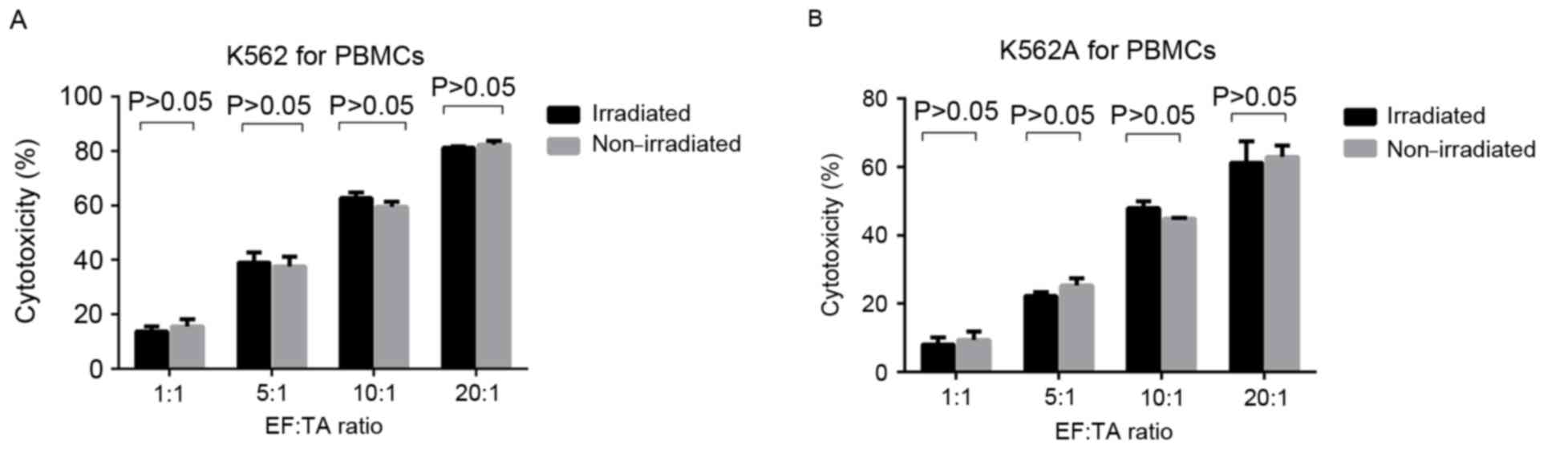
For each EF:TA ratio, there was no significant
difference in the cytotoxic activity against the K562 or K562A cell
lines compared between irradiated and non-irradiated PBMC groups
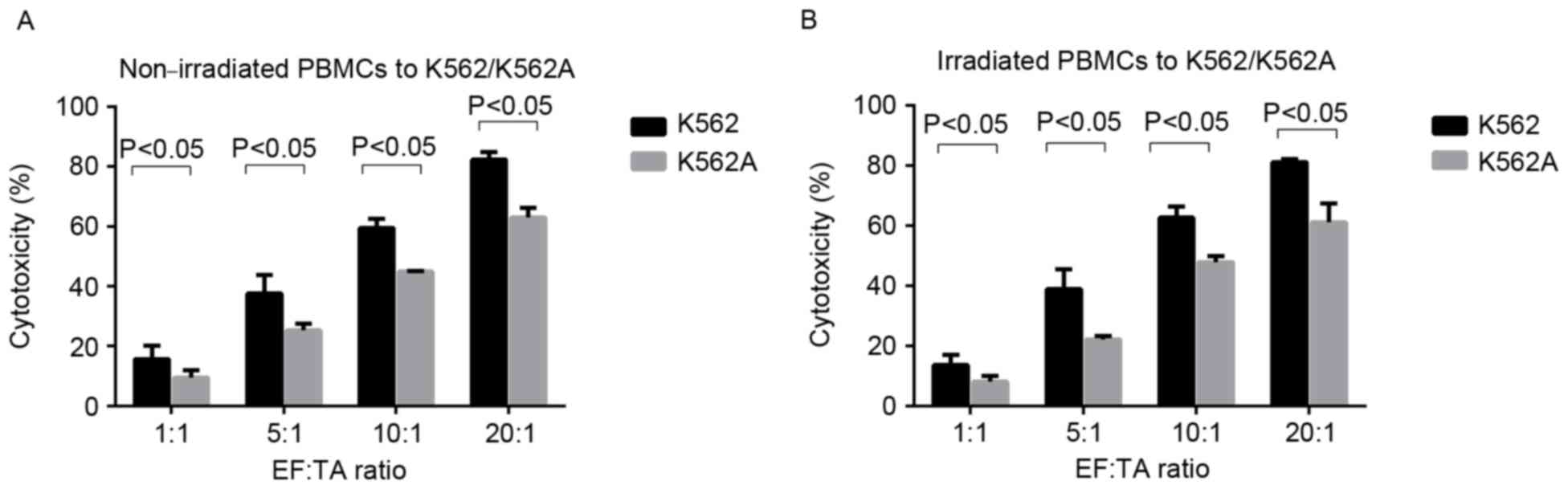
(P>0.05) (Fig. 4; Tables III and IV). However, the cytotoxicity of
non-irradiated PBMCs to K562 cells was significantly higher
compared with the cytotoxicity to K562A cells at all EF:TA ratios
tested (P<0.05; Fig. 5A), and the
same was observed for the irradiated PBMCs (P<0.05; Fig. 5B). However, for K562A cells, the
cytotoxic activity was still increasing with the EF:TA ratio and
scored ~60% at 20:1 (P<0.05; Fig.
5B).
 | Table III.Comparison of cytotoxic activity
between irradiated and non-irradiated peripheral blood mononuclear
cells against K562 cells. |
Table III.
Comparison of cytotoxic activity
between irradiated and non-irradiated peripheral blood mononuclear
cells against K562 cells.
|
| Cytotoxicity
(%) |
|
|---|
|
|
|
|
|---|
| EF:TA ratio | Median | 95% CI | SD | P-value (irradiated
vs. non-irradiated) |
|---|
| 1:1
(non-irradiated) | 15.60 | 10.85–19.85 | 4.55 |
|
| 1:1
(irradiated) | 13.73 | 10.51–17.51 | 3.30 | P>0.05 |
| 5:1
(non-irradiated) | 37.59 | 31.56–43.56 | 6.15 |
|
| 5:1
(irradiated) | 38.94 | 34.32–46.32 | 6.68 | P>0.05 |
| 10:1
(non-irradiated) | 59.48 | 56.34–62.34 | 3.12 |
|
| 10:1
(irradiated) | 62.69 | 59.78–66.78 | 3.71 | P>0.05 |
| 20:1
(non-irradiated) | 82.33 | 80.92–85.92 | 2.43 |
|
| 20:1
(irradiated) | 81.90 | 80.41–82.41 | 0.96 | P>0.05 |
Infusion of irradiated cells
As 7.5 Gy was determined as the optimum X-ray dose
in vitro, 7 patients who received 7.5 Gy X-ray-irradiated
PBMCs following HSCT were followed up to assess the outcome of
their treatment and GVHD occurrence. Differences in survival rates
were not significant because of the small cohort size (data not
shown). Therefore, each patient's condition is described
individually below.
Patient 1 suffered from extranodal NK/T-cell
lymphoma that originated from the small intestine, with lymph node
metastases in the popliteal and groin (Lugano II2). The patient did
not progress into a CR state following 7 courses of chemotherapy;
however, the patient achieved CR following a course of regular
chemotherapy and HSCT for a long duration. Following PBMC infusion,
the patient remained in continuous CR without GVHD for 9 years up
to the present.
Patient 2 suffered from diffuse large B-cell
lymphoma that originated from the small intestine, and was
diagnosed with multiple tumor metastases in the ileum, urinary
bladder and blood vessels during their initial consultation, where
the patient presented with emaciation and night sweats (Ann Arbor
IVB). Following 8 courses of chemotherapy, the disease continued to
progress. The patient received salvage HSCT and irradiated PBMCs
from their haploidentical son. The patient remained at PR for 7
months without GVHD prior to succumbing to the progressive
disease.
Patient 3 suffered from angioimmunoblastic T-cell
lymphoma and was diagnosed with multiple tumor invasions spread
throughout the body on their first visit to the clinic. Following 7
courses of chemotherapy accompanied with low sugar consumption, the
patient achieved PR, which was determined by positron emission
tomography-computed tomography; however, their lymph nodes remained
enlarged. The patient relapsed 7 weeks subsequent to HSCT.
Following the infusion of irradiated PBMCs, the patient remained in
CR without GVHD for 2 years, and then suffered from a secondary
malignancy.
Patient 4 suffered from multiple myeloma IIIB IgA κ
(Durie and Salmon), with acute renal insufficiency as the first
clinical manifestation. The patient achieved PR following 6 courses
of chemotherapy, then received 1 infusion with haploidentical
lymphocytes following HSCT, and has remained in continuous CR
without GVHD for >4 years.
A total of 3 patients (5, 6 and 7) suffered from AML
without a suitable matched donor for allogeneic hematopoietic stem
cell transplantation prior to achieving CR at the first time (CR1),
and received ASCT following CR1, with irradiated PBMCs preventing
relapse. Only 1 patient remained in continuous CR without GVHD for
7 years. Another sustained CR without GVHD for >2 years, while
the other relapsed at 5 months following 2 PBMC infusions without
GVHD, but succumbed to the complications of transplantation from a
related donor.
Patients were closely monitored for signs and
symptoms of GVHD development following PBMC infusion, and no acute
or chronic GVHD occurred in any of the 7 patients.
Discussion
DLI is one of the most effective treatment
strategies for patients with hematological malignancies, and
markedly improves survival rates. Allogeneic lymphocytes can induce
a beneficial GVT effect or detrimental GVHD (16–19).
Previous studies have primarily focused on minimizing the incidence
of GVHD while preserving the GVT effect, such as the infusion of
allodepleted donor T cells, delayed DLI time, gradually increasing
the infusion dose, gene modification, the infusion of specific
Epstein-Barr virus cytotoxic lymphocytes, and the infusion of
granulocyte colony-stimulating factor-mobilized peripheral blood
progenitor cells; however, these could not effectively prevent GVHD
(10,20–24).
Previous studies have demonstrated that
transfusion-associated GVHD can be entirely prevented with the
infusion of lymphocytes irradiated with X-rays or γ-rays at a dose
of ≥25 Gy. Lymphocytes do not perish immediately following
irradiation; hence, there is no sustained GVHD with a rising
proliferation inhibition rate (9,11,12). As a result, the transfusion of
irradiated PBMCs has been performed in certain clinical
applications for over a decade (9,11,12,25,26). The
aim of the present study was to confirm the optimum dose of X-rays,
under appropriate conditions, that can reduce the proliferation of
lymphocytes while preserving or enhancing their cytotoxic activity,
therefore improving the effectiveness of irradiation in the
clinic.
The in vitro data from the present study
demonstrated that PBMCs irradiated by different doses of X-ray
exhibit various levels of proliferation inhibition, and PBMCs
irradiated with ≥7.5 Gy exhibited a complete inhibition of
proliferation. No significant difference was observed in the
cytotoxicity between the irradiated or non-irradiated groups,
irrespective of the target cell, K562 or K562A. For PBMCs
irradiated with 7.5 Gy X-rays, the cytotoxicity activity towards
K562 cells was markedly stronger compared with that for K562A cells
(P<0.05). However, PBMCs still exhibited some cytotoxicity
towards K562A cells, indicating that they may have a potential
application for the treatment of drug-resistant tumors.
Additionally, the in vitro data from the
present study demonstrated that the proliferation of lymphocytes
irradiated with 7.5 Gy was inhibited effectively without any
significant decrease in cytotoxicity. Thus, 7.5 Gy may be the
appropriate dose to irradiate lymphocytes with in order to rescue
their GVT effect while avoiding GVHD.
Based on the in vitro data of the current
study, the safety and efficacy of 7.5 Gy-irradiated haploidentical
lymphocytes were reviewed in 7 patients. To test for the occurrence
of GVHD caused by DLI, 7 patients that had received an autologous
HSCT were chosen to avoid GVHD owing to HLA-haploidentical bone
marrow transplantation. Notably, none of the patients in the
present study developed GVHD following infusion with PBMCs
irradiated with a 7.5 Gy dose. The patients benefitted from the 7.5
Gy-irradiated PBMC infusion regardless of whether their tumors were
previously resistant to chemotherapy or not. Therefore, despite the
small cohort size, the data suggest that infusion with PBMCs
pre-irradiated with a 7.5 Gy dose of X-rays could be widely used as
a treatment for a variety of patients. However, further studies
with larger cohorts are required to verify the data from the
present study.
In conclusion, the proliferation capacity of PBMCs
was demonstrated to be inhibited by an X-ray dose of ≥7.5 Gy. There
was no significant difference between the cytotoxicity activities
for PBMCs in the 7.5 Gy-irradiated and non-irradiated groups;
however, there was a significant difference between the
cytotoxicity activities of the K562 and K562A cells, no matter the
irradiation status of PBMCs. Patients that received infusions of
PBMCs irradiated with a 7.5 Gy dose of X-rays achieved CR or had an
extended overall survival time, with a low incidence of GVHD in the
autologous HSCT/haploidentical DLI setting. Further studies with
larger cohorts are required to assess the potential for 7.5 Gy
X-irradiated PBMCs to rescue the GVT effect of HSCT while avoiding
GVHD.
Acknowledgements
The authors thank the expert clinical care provided
by the Department of Hematology transplant team at the Peking
University First Hospital, and assistance from the Hematology
Laboratory, Peking University First Hospital. The present study was
supported by a grant of the key topics fund of Beijing Municipal
Science and Technology Commission (grant no. Z141107002514017) and
the National Natural Science Fund of China (grant no.
81041002).
References
|
1
|
Wang Y, Liu DH, Xu LP, Liu KY, Chen H,
Zhang XH, Chen YH, Han W, Wang FR, Wang JZ, et al: Prevention of
relapse using granulocyte CSF-primed PBPCs following
HLA-mismatched/haploidentical, T-cell-replete hematopoietic SCT in
patients with advanced-stage acute leukemia: A retrospective
risk-factor analysis. Bone Marrow Transplant. 47:1099–1104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hasskarl J, Zerweck A, Wäsch R, Ihorst G,
Bertz H and Finke J: Induction of graft versus malignancy effect
after unrelated allogeneic PBSCT using donor lymphocyte infusions
derived from frozen aliquots of the original graft. Bone Marrow
Transplant. 47:277–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Cheikh J, Crocchiolo R, Furst S,
Ladaique P, Castagna L, Faucher C, Granata A, Oudin C, Lemarie C,
Calmels B, et al: Lenalidomide plus donor-lymphocytes infusion
after allogeneic stem-cell transplantation with reduced-intensity
conditioning in patients with high-risk multiple myeloma. Exp
Hematol. 40:521–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deol A and Lum LG: Role of donor
lymphocyte infusions in relapsed hematological malignancies after
stem cell transplantation revisited. Cancer Treat Rev. 36:528–538.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLarnon A, Piper KP, Goodyear OC, Arrazi
JM, Mahendra P, Cook M, Clark F, Pratt G, Craddock C and Moss PA:
CD8(+) T-cell immunity against cancer-testis antigens develops
following allogeneic stem cell transplantation and reveals a
potential mechanism for the graft-versus-leukemia effect.
Haematologica. 95:1572–1578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oettel KR, Wesly OH, Albertini MR, Hank
JA, Iliopolis O, Sosman JA, Voelkerding K, Wu SQ, Clark SS and
Sondel PM: Allogeneic T-cell clones able to selectively destroy
Philadelphia chromosome-bearing (Ph1+) human leukemia lines can
also recognize Ph1- cells from the same patient. Blood.
83:3390–3402. 1994.PubMed/NCBI
|
|
7
|
Mackinnon S, Papadopoulos EB, Carabasi MH,
Reich L, Collins NH, Boulad F, Castro-Malaspina H, Childs BH,
Gillio AP and Kernan NA: Adoptive immunotherapy evaluating
escalating doses of donor leukocytes for relapse of chronic myeloid
leukemia after bone marrow transplantation: Separation of
graft-versus-leukemia responses from graft-versus-host disease.
Blood. 86:1261–1268. 1995.PubMed/NCBI
|
|
8
|
Dazzi F, Szydlo RM, Cross NC, Craddock C,
Kaeda J, Kanfer E, Cwynarski K, Olavarria E, Yong A, Apperley JF
and Goldman JM: Durability of responses following donor lymphocyte
infusions for patients who relapse after allogeneic stem cell
transplantation for chronic myeloid leukemia. Blood. 96:2712–2716.
2000.PubMed/NCBI
|
|
9
|
British Committee for Standards in
Haematology, ; Milkins C, Berryman J, Cantwell C, Elliott C, Haggas
R, Jones J, Rowley M, Williams M and Win N: Guidelines for
pre-transfusion compatibility procedures in blood transfusion
laboratories. British Committee for Standards in Haematology.
Transfus Med. 23:3–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGuirk JP, Seropian S, Howe G, Smith B,
Stoddart L and Cooper DL: Use of rituximab and irradiated
donor-derived lymphocytes to control Epstein-Barr virus-associated
lymphoproliferation in patients undergoing related haplo-identical
stem cell transplantation. Bone Marrow Transplant. 24:1253–1258.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsirigotis P, Resnick IB, Kapsimalli V,
Dray L, Psarra E, Samuel S, Spyridonidis A, Konsta E, Vikentiou M,
Or R, et al: Irradiated mononuclear cells express significant in
vitro cytotoxic activity: Promise for in vivo clinical efficacy of
irradiated mismatched donor lymphocytes infusion. Immunotherapy.
6:409–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reynolds JT, Watkins JM, Dufan TA and
Kubsad SS: Irradiation of donor mononuclear cells for treatment of
chemorefractory metastatic solid cancers: A community-based immune
transplant pilot study. Cancer Res Treat. 44:133–141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu BY, Song CY, Guo KY, Yan DA, Yang YL,
Xiao LL and Wu GX: Treatment of malignant hematologic diseases by
peripheral blood stem cell transplantation combined with halotype
lymphocyte infusion. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
11:287–291. 2003.(In Chinese). PubMed/NCBI
|
|
14
|
Pierelli L, Menichella G, Paoloni A,
Teofili L, Sica S, Foddai ML, Rossi PL, Leone G, Mango G and Bizzi
B: Collection of peripheral blood stem cells using an automated
discontinuous flow blood cell separator. Haematologica. 75 Suppl
1:S29–S32. 1990.
|
|
15
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumors of the Haematopoietic and Lymphoid Tissues. 4th edition.
IARC Press; Lyon: 2008
|
|
16
|
Kolb HJ, Schmid C, Barrett AJ and Schendel
DJ: Graft-versus-leukemia reactions in allogeneic chimeras. Blood.
103:767–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia G, Truitt RL and Johnson BD:
Graft-versus-leukemia and graft-versus-host reactions after donor
lymphocyte infusion are initiated by host-type antigen-presenting
cells and regulated by regulatory T cells in early and long-term
chimeras. Biol Blood Marrow Transplant. 12:397–407. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kolb HJ: Graft-versus-leukemia effects of
transplantation and donor lymphocytes. Blood. 112:4371–4383. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stern M, Passweg JR, Meyer-Monard S, Esser
R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T,
Bader P, et al: Pre-emptive immunotherapy with purified natural
killer cells after haploidentical SCT: A prospective phase II study
in two centers. Bone Marrow Transplant. 48:433–438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun HD and Waller EK: Finding the sweet
spot for donor lymphocyte infusions. Biol Blood Marrow Transplant.
19:507–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porter DL, Collins RH Jr, Shpilberg O,
Drobyski WR, Connors JM, Sproles A and Antin JH: Long-term
follow-up of patients who achieved complete remission after donor
leukocyte infusions. Biol Blood Marrow Transplant. 5:253–261. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liga M, Triantafyllou E, Tiniakou M,
Lambropoulou P, Karakantza M, Zoumbos NC and Spyridonidis A: High
alloreactivity of low-dose prophylactic donor lymphocyte infusion
in patients with acute leukemia undergoing allogeneic hematopoietic
cell transplantation with an alemtuzumab-containing conditioning
regimen. Biol Blood Marrow Transplant. 19:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bar M, Sandmaier BM, Inamoto Y, Bruno B,
Hari P, Chauncey T, Martin PJ, Storb R, Maloney DG, Storer B and
Flowers ME: Donor lymphocyte infusion for relapsed hematological
malignancies after allogeneic hematopoietic cell transplantation:
Prognostic relevance of the initial CD3+ T cell dose. Biol Blood
Marrow Transplant. 19:949–957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levine JE, Braun T, Penza SL, Beatty P,
Cornetta K, Martino R, Drobyski WR, Barrett AJ, Porter DL, Giralt
S, et al: Prospective trial of chemotherapy and donor leukocyte
infusions for relapse of advanced myeloid malignancies after
allogeneic stem-cell transplantation. J Clin Oncol. 20:405–412.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waller EK, Ship AM, Mittelstaedt S, Murray
TW, Carter R, Kakhniashvili I, Lonial S, Holden JT and Boyer MW:
Irradiated donor leukocytes promote engraftment of allogeneic bone
marrow in major histocompatibility complex mismatched recipients
without causing graft-versus-host disease. Blood. 94:3222–3233.
1999.PubMed/NCBI
|
|
26
|
Nikiforow S and Alyea EP: Maximizing GVL
in allogeneic transplantation: Role of donor lymphocyte infusions.
Hematology Am Soc Hematol Educ Program. 2014:570–575.
2014.PubMed/NCBI
|