Introduction
Cervical cancer is the leading cause of female
cancer worldwide (1,2). The incidence rate of cervical cancer is
high in developing countries. High-risk group (HR) papillomaviruses
(HPV) have been characterized as the etiological agents of cervical
cancer. HPV-infected cells may develop into non-invasive or
pre-malignant lesions referred to as cervical intraepithelial
neoplasia (CIN), also termed squamous intraepithelial lesions. The
classification of CIN depends on the morphological alteration in
pre-malignant lesion cells. CIN I and CIN II are mildly dysplastic
and rarely progress towards carcinoma, whereas CIN II and CIN III
lesions are precursors of cervical cancer due to the proliferation
of dysplastic cells. The development of cervical carcinoma is
established from high-grade squamous intraepithelial neoplasms,
which are frequently identified by exfoliative cytology screening
(conventional Pap smear) (3,4). If not treated, >25% of early lesions
progress to carcinoma in situ or invasive cancer (5).
The methylation of cytosine in CpG dinucleotides is
an epigenetic gene-regulation mechanism in mammalian genomes. The
methylation of gene sequences subsequently results in the
inactivation of gene expression (6,7). Aberrant
methylation of tumor suppressor genes has been detected in a high
proportion of human cancers (8). It
has been identified in almost all types of cancer and contributes
to malignant transformation by silencing multiple tumor suppressor
genes. Numerous previous studies have investigated DNA methylation
changes in cancer-associated gene sequences (8,9). The
aberrant methylation of tumor suppressor genes has been detected in
gynecological malignancies (10–12).
Wisman et al (13) proposed
that hypermethylation analysis may eventually become an additional
tool to identify patients with high-grade squamous intraepithelial
neoplasms or invasive cervical cancer, due to its increased
specificity compared with current cytomorphology-based screening
(13).
In particular, changes in hypermethylation of
promoter regions have been implicated in the onset and progression
of cervical cancer. However, the frequencies of hypermethylation of
tumor suppressor genes vary in the literature (14), and information concerning methylation
genes is not well documented in precancer, including CIN II and CIN
III. Therefore, the focus of the present study was on three tumor
suppressor genes and an inflammation-associated gene, and the
association between DNA methylation status and cytopathological
features of the genes was investigated.
Materials and methods
Clinical subjects
A community-based case-control study was conducted
in Kaohsiung City, and 529 patients (mean age, 46.2±13.2) were
enrolled in the present study between January 2008 and September
2010. The procedure of patient enrollment was as previously
described (15). Cervical scrapings
were obtained from female patients who attended the Department of
Obstetrics and Gynecology, Kaohsiung Medical University Hospital
(Kaohsiung, Taiwan) or from the community health clinics of Great
Kaohsiung City (southern part of Taiwan). Specimens were screened
for CIN by Pap smear, and the diagnosis was based on cervical
cytomorphological and histological characteristics. Normal
specimens were collected, as well as distinct stages of cervical
neoplasm specimens, including CIN I, CIN II, CIN III and squamous
cell carcinoma. The present study was approved by the Institutional
Review Board of Kaohsiung Medical University Hospital, and informed
consent was obtained from all patients.
Extraction of genomic DNA
Genomic DNA samples were collected from cervical
scrapings. Cells from cervical scrapings were concentrated by
centrifugation (425 × g for 10 min at room temperature) and
were solubilized in cell lysis solution (Cell lysis/binding buffer;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
cellular lysate was digested with 20 µg/ml proteinase K at 55°C
overnight. DNA extraction was performed with a Genomic DNA
Purification kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The cellular DNA was
finally dissolved in sterile water until subsequent reaction. The
genomic DNA was also determined for human papillomavirus infection
by screening HPV DNA using the Hybrid Capture II assay (Digene
Corporation, Gaithersburg, MD, USA).
Bisulfite modification
The methylation status of the promoter regions was
determined by sodium bisulfite reaction. The purified cellular DNA
was added to a cytosine conversion reagent in the MethylCode
Bisulfite Conversion kit (Invitrogen; Thermo Fisher Scientific,
Inc.). Bisulfite treatment of DNA converted unmethylated cytosine
into uracil, and methylated cytosine remained unchanged. DNA was
treated with sodium bisulfite using the MethylCode Bisulfite
Conversion kit, according to the manufacturer's protocol.
Methylation-specific polymerase chain
reaction (PCR)
The primers and reaction conditions for p16, COX-2
and CDH1 were as described previously (16–18). The
PCR thermocycling conditions for COX-2 was as follows: 94°C for 30
sec, 53°C for 30 sec and 72°C for 60 sec, for 40 cycles. The
methylated primers for RARb were 5′-GGGGGATTAAATTTTTTATGC−3′
(forward) and 5′-AAATCCTACCCCGACGATAC-3′ (reverse). The
unmethylated primers for RARb were 5′-GGGGGATTAGAATTTTTTATGTGA-3′
(forward) and 5′-AAATCCTACCCCAACAATACC-3′ (reverse).
Bisulfite-modified DNA (2 µl) was loaded in 25 µl PCR mixture
containing 1X PCR buffer, 0.2 µM of each primer, 0.2 mM dNTP, 1.5
mM MgCl2 and 0.7 unit HotStart Taq polymerase (Promega
Corporation, Madison, WI, USA). The annealing temperature for CDH1
and RARβ was initially 65°C, decreasing by 1°C for each cycle until
reaching 55°C, and amplification was at 94°C for 30 sec, 55°C for
30 sec and 72°C for 60 sec. The amplified PCR products were
visualized by 1.8% agarose gel electrophoresis. Either PCR products
were cloned into pGem-T vectors (Promega Corporation) for automated
sequencing, or direct sequencing was performed.
Statistical analysis
The statistical data were examined by χ2
test and Students t-test on Microsoft Excel software (2013;
Microsoft Corporation, Redmond, WA, USA). Each experiment
containing clinical samples was repeated three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
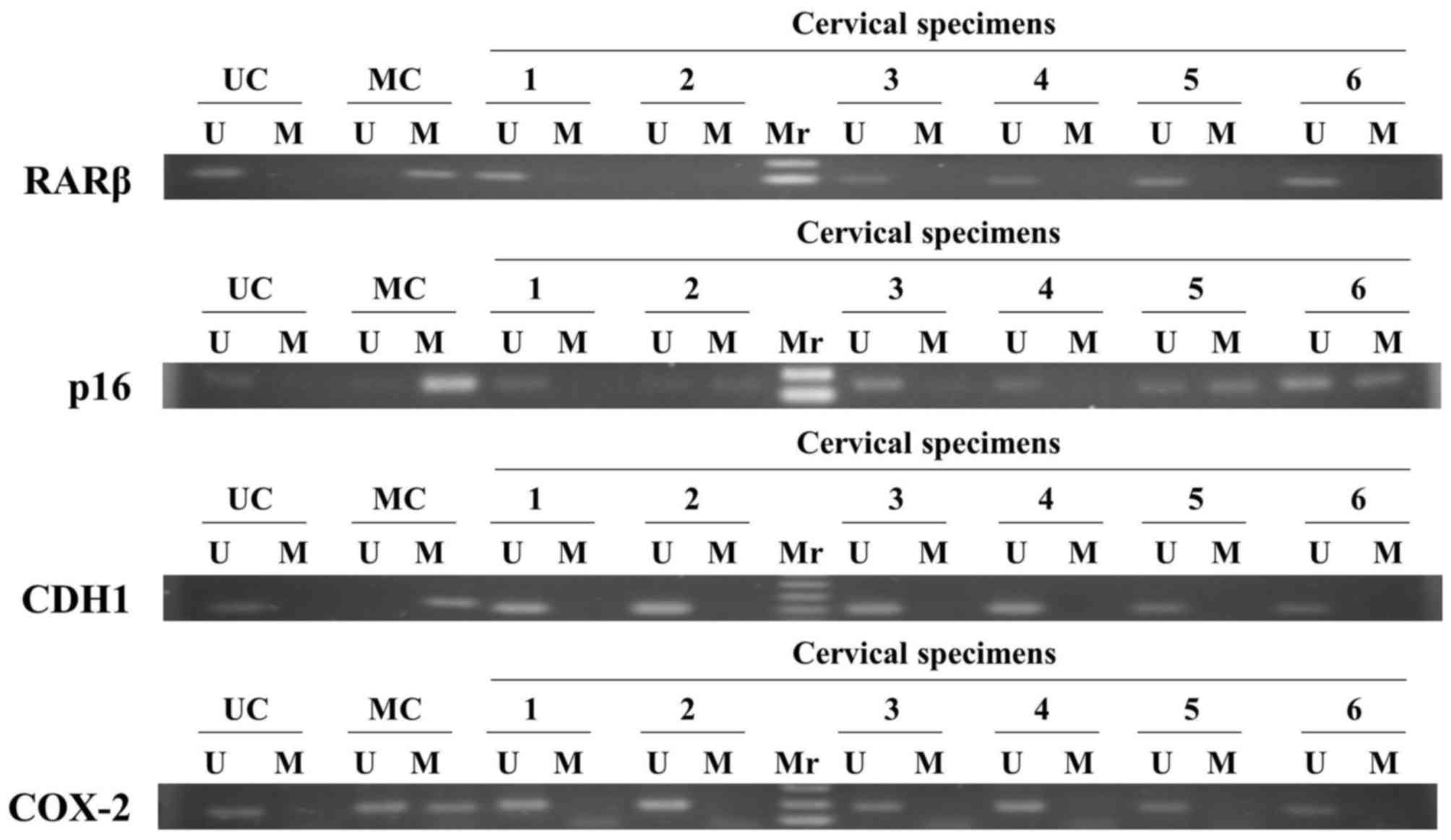
The methylation status of three tumor suppressor
genes, including RARβ, p16 and CDH1, and an inflammatory-associated
COX-2 gene, were examined in distinct stages of CIN by
methylation-specific PCR (Fig.
1).
The methylation rate of the COX-2 gene promoter was
detected in 1.2% of normal specimens, but in 0% of CIN I, CIN II,
CIN III and cervical squamous cell carcinoma. The results of the
present study indicated that the COX-2 gene was in a hypomethylated
or unmethylated form (Table I). It
was observed that the RARβ gene exhibited only a minimal change in
methylation frequency (Table I). The
methylation frequency of the CDH1 gene was 4.7 and 4.3% in normal
and precancerous stage CIN I, respectively. By contrast, the
methylation frequency was 7.1 and 7.9% in CIN II and CIN III,
respectively. The CDH1 gene methylation level was markedly
increased <2-fold between CIN I and carcinoma (P=0.249; Table I). This indicated that the increased
frequency of promoter methylation was associated with increasing
severity of cervical neoplasm changes. Notably, the methylation
frequency of p16 was 13.2% in normal specimens; 18.2% in CIN I;
35.7% in CIN II; 31.6% in CIN III; and 15.4% in squamous cell
carcinoma (Table I). The methylation
frequency of p16 progressively increased during the development of
malignant stages. Cervical specimens were screened for human
papillomavirus (HPV) by the method of Hybrid Capture II (Digene
Corporation). The results indicated that, particularly in the
absence of HR-HPV, the methylation frequency of p16 was 11.8% in
normal, 25% in CIN I and 58.3% in CIN II-carcinoma. In the presence
of HR-HPV, the methylation frequency of p16 was without any
apparent change; 21.3% in normal, 13.8% in CIN I and 23.8% for CIN
II, CIN III and carcinoma (Table
II).
 | Table I.Methylation status of 4 distinct genes
in distinct stages of cervical intraepithelial neoplasia. |
Table I.
Methylation status of 4 distinct genes
in distinct stages of cervical intraepithelial neoplasia.
| Gene | Normal, frequency
(%) | CIN I, frequency
(%) | CIN II, frequency
(%) | CIN III, frequency
(%) | Squamous cell
carcinoma, frequency (%) |
|---|
| RARβ | 12/311 (3.9) | 1/46 (2.2) | 0/23 (0.0) | 1/31 (3.2) | 0/7 (0.0) |
| p16 | 52/395 (13.2) | 10/55 (18.2) | 10/28 (35.7) | 12/38 (31.6) | 2/13 (15.4) |
| CDH1 | 18/384 (4.7) | 2/46 (4.3) | 2/28 (7.1) | 3/38 (7.9) | 0/12 (0.0) |
| COX-2 | 3/259 (1.2) | 0/35 (0.0) | 0/23 (0.0) | 0/31 (0.0) | 0/6 (0.0) |
 | Table II.Methylation frequency of p16 in
HPV-negative and HPV-positive specimens. |
Table II.
Methylation frequency of p16 in
HPV-negative and HPV-positive specimens.
| Grade | HPV-negative,
frequency (%) | HPV-positive,
frequency (%) |
|---|
| Normal | 32/271 (11.8) | 16/75 (21.3) |
| CIN I | 6/24
(25) | 4/29
(13.8) |
| CIN II-carcinoma | 7/12
(58.3) | 19/80 (23.8) |
| P-value | 0.0018 | 0.6510 |
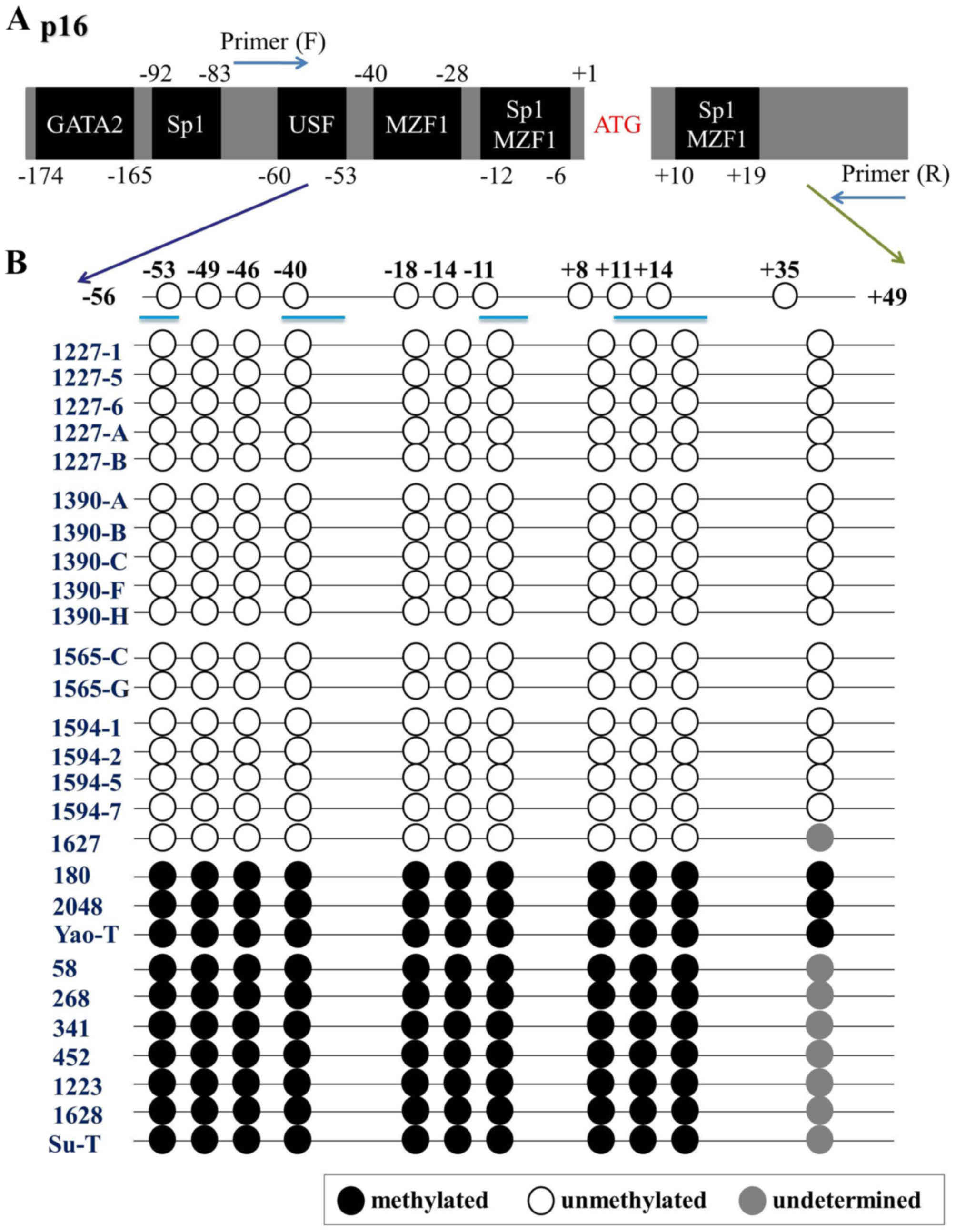
There are 11 CpG sites located within the
PCR-amplified 150 bp fragments of the p16 promoter, which encompass
a number of distinct transcription factor-binding sites (Fig. 2A). The PCR-amplified DNA fragments
were also cloned and sequenced following bisulfite treatment, and
the methylation status of all CG sites in the promoter region was
determined. The results from 10 individuals with CIN II and above
identified complete methylation at each CpG site (Fig. 2B). Bisulfite sequencing also revealed
that the CpG sites were all unmethylated from p16 unmethylated
individuals (Fig. 2B).
Discussion
DNA methylation is an important epigenetic mechanism
of transcriptional control. It performs a critical function in the
regulation of cellular gene expression. Aberrant DNA methylation
often occurs in numerous types of cancer; it contributes to
malignant transformation by silencing multiple tumor suppressor
genes. Therefore, DNA methylation has been increasingly recognized
as a promising epigenetic and diagnostic biomarker (8). In the present study, the methylation
status of tumor suppressor genes, including RARβ, p16 and CDH1, and
an inflammatory-associated COX-2 gene, was analyzed in distinct
stages of CIN using methylation-specific PCR. Retinoic acid is
required for the regulation of epithelial cell differentiation
(19). The RAR is an intracellular
molecule responsible for the binding to the RAR-responsive element
promoter (20). The rate of RARβ
methylation was progressively increased during oncogenesis of the
cervix (11). In the present study,
the RARβ promoter exhibited a minimal change in methylation rate,
and no significant difference was observed in the methylation
frequencies. Inflammation has numerous tumor-promoting effects in
the tumor microenvironment. COX-2 has been established as one of
the inflammatory factors involved in the process of angiogenesis
and cell adhesion (21). A previous
study reported that COX-2 expression was associated with higher
grades of oral epithelial dysplasia (22). Jo et al (16) also reported that COX-2 is
hypermethylated in cervical cancer, but their immunohistochemistry
results did not demonstrate an association between hypermethylation
and the expression pattern of COX-2. The present study revealed
that all COX-2 genes were in an unmethylated form throughout the
stages of CIN.
CDH1, also termed E-cadherin, is one of the
molecules involved in the Wnt signaling cascade pathway (23). CDH1 methylation was detected in 43%
(40/93) of serum samples of patients with cervical cancer (24). Chen et al (25) demonstrated that the inactivation of
CDH1 is due to hypermethylation at the promoter region. Narayan
et al (18) identified a high
degree of methylation of CDH1 in cervical cancers (51.1% of 90
cases); however, only 28.8 and 19.2% methylation was identified in
the studies of Dong et al (26) and Feng et al (11), respectively. By contrast, in the
present study, it was observed that the average methylation
frequency of CDH1 was 4.5% in normal and CIN I, 7.1% in CIN II and
7.9% in CIN III. The methylation rate was markedly increased
<2-fold between CIN I and carcinoma. It was observed that CDH1
exhibited a tendency to increased frequency of promoter methylation
with increasing severity of cervical neoplasm changes. The degree
of promoter methylation was proposed as a potential marker of the
early events of tumorigenesis.
Aberrant methylation of the p16 gene is widely
detected in the majority of types of tumor, and is the most studied
tumor suppressor gene in cervical cancers (27). p16 is a cell cycle regulatory gene,
and it performs an essential role in inhibiting cell abnormal
growth. Its function is involved in the inhibition of cell cycle
progression by encoding an inhibitor of cyclin-dependent kinase
(CDK) 4 and CDK6 (28). Wong et
al (29) and Dong et al
(26) detected 31.6% (31/98) and 30%
(16/53) of methylated p16 in squamous cervical carcinoma,
respectively. The results of the present study identified that the
methylation frequency of p16 was relatively low in precancerous
stages, and that the methylation frequency was increased in CIN II
and CIN III. The methylation frequency of p16 was progressively
increased during the development of malignant stages in CIN
(Table I).
In the presence of HR-HPV, p16 exhibited a
significant decrease in the methylation level between normal and
CIN I (P<0.001). However, between CIN I and CIN II-carcinoma,
the rate increased to 23.8%. Most notably, in the absence of
HR-HPV, the methylation frequency of p16 was 11.8% in normal, 25%
in CIN I and 58.3% in CIN II-carcinoma. The methylation frequency
of p16 was markedly increased between normal and CIN I, and between
CIN I and CIN II-carcinoma. It was concluded that p16 methylation
exhibited an apparent increase between normal and CIN I, and
between CIN I and CIN II-carcinoma, without the intervention of
virus infection. Furthermore, peripheral blood DNA obtained from
patients was analyzed for the levels of p16 methylation, however no
association was identified between these results and the DNA from
the cervical cells (data not shown). p16 exerted a critical role
across the cervical histological grades. However, in the presence
of HR-HPV, methylation of p16 served no role in the stages of
oncogenesis. It was hypothesized that virus infection or
integration of viral genome may activate the oncogenic expression
of p16 (30).
In conclusion, the results of the present study
revealed that the methylation frequencies of p16 and CDH1 were
progressively methylated in association with the development of
malignant stages in CIN. Promoter methylation analysis of cervical
cell specimens of p16 and CDH1 may be an additional diagnostic tool
for current cervical cytomorphology-based screening and prognosis
markers.
Acknowledgements
The present study was supported by the Taiwan
National Science Council (grant no. NSC102-2314-B037-070-MY3), the
National Health Research Institutes (grant no. NHRI-EX102-10226 PC)
and the Kaohsiung Medical University Aim for the Top Universities
Grant (grant no. KMU-TP105A00).
References
|
1
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dijkstra MG, Snijders PJ, Arbyn M,
Rijkaart DC, Berkhof J and Meijer CJ: Cervical cancer screening: On
the way to a shift from cytology to full molecular screening. Ann
Oncol. 25:927–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snijders PJ, Steenbergen RD, Heideman DA
and Meijer CJ: HPV-mediated cervical carcinogenesis: Concepts and
clinical implications. J Pathol. 208:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostör AG: Natural history of cervical
intraepithelial neoplasia: A critical review. Int J Gynecol Pathol.
12:186–192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daniel FI, Cherubini K, Yurgel LS, de
Figueiredo MA and Salum FG: The role of epigenetic transcription
repression and DNA methyltransferases in cancer. Cancer.
117:677–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bestor TH: The DNA methyltransferases of
mammals. Hum Mol Genet. 9:2395–2402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feinberg AP and Tycko B: The history of
cancer epigenetics. Nat Rev Cancer. 4:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen Y, Singer G, Lavie O, Dong SM,
Beller U and Sidransky D: The RASSF1A tumor suppressor gene is
commonly inactivated in adenocarcinoma of the uterine cervix. Clin
Cancer Res. 9:2981–2984. 2003.PubMed/NCBI
|
|
11
|
Feng Q, Balasubramanian A, Hawes SE, Toure
P, Sow PS, Dem A, Dembele B, Critchlow CW, Xi L, Lu H, et al:
Detection of hypermethylated genes in women with and without
cervical neoplasia. J Natl Cancer Inst. 97:273–282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang S, Kim HS, Seo SS, Park SY, Sidransky
D and Dong SM: Inverse correlation between RASSF1A
hypermethylation, KRAS and BRAF mutations in cervical
adenocarcinoma. Gynecol Oncol. 105:662–666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wisman GB, Nijhuis ER, Hoque MO,
Reesink-Peters N, Koning AJ, Volders HH, Buikema HJ, Boezen HM,
Hollema H, Schuuring E, et al: Assessment of gene promoter
hypermethylation for detection of cervical neoplasia. Int J Cancer.
119:1908–1914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wentzensen N, Sherman ME, Schiffman M and
Wang SS: Utility of methylation markers in cervical cancer early
detection: Appraisal of the state-of-the-science. Gynecol Oncol.
112:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai HT, Tsai YM, Yang SF, Wu KY, Chuang
HY, Wu TN, Ho CK, Lin CC, Kuo YS and Wu MT: Lifetime cigarette
smoke and second-hand smoke and cervical intraepithelial neoplasm-a
community-based case-control study. Gynecol Oncol. 105:181–188.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo H, Kang S, Kim JW, Kang GH, Park NH,
Song YS, Park SY, Kang SB and Lee HP: Hypermethylation of the COX-2
gene is a potential prognostic marker for cervical cancer. J Obstet
Gynaecol Res. 33:236–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang GH, Lee S, Lee HJ and Hwang KS:
Aberrant CpG island hypermethylation of multiple genes in prostate
cancer and prostatic intraepithelial neoplasia. J Pathol.
202:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Narayan G, Arias-Pulido H, Koul S, Vargas
H, Zhang FF, Villella J, Schneider A, Terry MB, Mansukhani M and
Murty VV: Frequent promoter methylation of CDH1, DAPK, RARB and
HIC1 genes in carcinoma of cervix uteri: Its relationship to
clinical outcome. Mol Cancer. 2:242003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Germain P, Chambon P, Eichele G, Evans RM,
Lazar MA, Leid M, de Lera AR, Lotan R, Mangelsdorf DJ and
Gronemeyer H: International union of pharmacology. LX. retinoic
acid receptors. Pharmacol Rev. 58:712–725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvarez S, Germain P, Alvarez R,
Rodriguez-Barrios F, Gronemeyer H and de Lera AR: Structure,
function and modulation of retinoic acid receptor beta, a tumor
suppressor. Int J Biochem Cell Biol. 39:1406–1415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z: The role of COX-2 in oral cancer
development and chemoprevention/treatment of oral cancer by
selective COX-2 inhibitors. Curr Pharm Des. 11:1771–1777. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warner DR, Smith SC, Smolenkova IA, Pisano
MM and Greene RM: Inhibition of p300 histone acetyltransferase
activity in palate mesenchyme cells attenuates Wnt signaling via
aberrant E-cadherin expression. Exp Cell Res. 342:32–38. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Widschwendter A, Ivarsson L, Blassnig A,
Müller HM, Fiegl H, Wiedemair A, Müller-Holzner E, Goebel G, Marth
C and Widschwendter M: CDH1 and CDH13 methylation in serum is an
independent prognostic marker in cervical cancer patients. Int J
Cancer. 109:163–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen CL, Liu SS, Ip SM, Wong LC, Ng TY and
Ngan HY: E-cadherin expression is silenced by DNA methylation in
cervical cancer cell lines and tumours. Eur J Cancer. 39:517–523.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong SM, Kim HS, Rha SH and Sidransky D:
Promoter hypermethylation of multiple genes in carcinoma of the
uterine cervix. Clin Cancer Res. 7:1982–1986. 2001.PubMed/NCBI
|
|
27
|
Herman JG, Merlo A, Mao L, Lapidus RG,
Issa JP, Davidson NE, Sidransky D and Baylin SB: Inactivation of
the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA
methylation in all common human cancers. Cancer Res. 55:4525–4530.
1995.PubMed/NCBI
|
|
28
|
Serrano M: The tumor suppressor protein
p16INK4a. Exp Cell Res. 237:7–13. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong YF, Chung TK, Cheung TH, Nobori T, Yu
AL, Yu J, Batova A, Lai KW and Chang AM: Methylation of p16INK4A in
primary gynecologic malignancy. Cancer Lett. 136:231–235. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto A, Kumakura S, Uchida M, Barrett
JC and Tsutsui T: Immortalization of normal human embryonic
fibroblasts by introduction of either the human papillomavirus type
16 E6 or E7 gene alone. Int J Cancer. 106:301–309. 2003. View Article : Google Scholar : PubMed/NCBI
|