Introduction
Chronic myeloid leukemia (CML) is a
myeloproliferative disorder that arises in hematopoietic stem cells
(1). It is characterized by a
reciprocal t(9;22) translocation that leads to the formation of the
Philadelphia chromosome, which in turn produces the BCR-ABL1 fusion
protein. BCR-ABL1 is a constitutively active tyrosine kinase, which
transmits proliferation and survival signals through the Src family
kinases, Lyn and Hck, to the downstream targets, STAT5 and Ras/ERK
(2,3).
CML has a triphasic clinical course (1). In the chronic phase (CP), excessive
proliferation of mature myeloid cells occurs (1). In the accelerated phase (AP), CML cells
accumulate chromosomal and genetic abnormalities, and blasts in the
peripheral blood (PB) or bone marrow (BM) increase to 10–20%
(1). Eventually, the disease
progresses to the blastic phase (BP), where blasts account for
>20% of the cells in the PB or BM (1). In ~70% of BP cases the blast lineage is
myeloid, whereas in the remaining 20–30% of BP cases the lineage is
lymphoid (1).
Tyrosine kinase inhibitors (TKIs) have markedly
improved the prognosis of patients with CML. Currently, five
different TKIs are available for the treatment of CML: Imatinib,
nilotinib, dasatinib, bosutinib and ponatinib (4). Imatinib, nilotinib and dasatinib are
approved for first-line treatment of CML, and bosutinib and
ponatinib are available for resistant and intolerant CML patients.
As of July 2015, ponatinib is not yet available in Japan. Secondary
resistance to TKIs occurs in 20–30% of patients with CML, and is
mainly due to mutations in the ABL1 kinase domain (5,6). The most
intractable mutation is the T315I gatekeeper mutation, which
potently interferes with the binding of TKIs to BCR-ABL1 (7). Of the five TKIs, only ponatinib is
active against the T315I mutant (4,8–11). Stem cell transplantation is currently
reserved for patients with CML-AP/BP and selected cases of CML-CP
(4,12,13).
Bosutinib is a dual Src and ABL1 tyrosine kinase
inhibitor (14–21). It is active against various BCR-ABL1
mutations, including those associated with imatinib, dasatinib and
nilotinib resistance. However, bosutinib is not active against the
T315I and V299L BCR-ABL1 mutations (4,10,11). Bosutinib inhibits Src family kinases,
including Src, Lyn, Fgr and Hck (14). However, unlike other TKIs, bosutinib
has exhibited minimal inhibitory activity against platelet-derived
growth factor receptor and c-KIT (14). Therefore, bosutinib has a distinct
toxicity profile compared to other TKIs (15–21). The
present case study provides a report of a patient with
T315I-positive CML-BP who was successfully treated with bosutinib
as a fourth-line therapy prior to cord blood transplantation.
Materials and methods
Sequencing of the ABL1 kinase domain
of BCR-ABL1
Total RNA was extracted from total BM cells using
ISOGEN (Nippon Gene, Tokyo, Japan) and 1 µg from each sample was
reverse transcribed using a Transcriptor 1st Strand cDNA Synthesis
kit (Roche Diagnostics, Tokyo, Japan). The ABL1 tyrosine kinase
domain of BCR-ABL1 was amplified with nested RT-PCR with the
following primers: 1st forward, (designed on BCR gene),
5′-TTCAGAAGCTTCTCCCTGCAT-3′ and reverse (located in ABL gene),
5′-CTTCGTCTGAGATACTGGATTCCT-3′; 2nd forward (located in ABL gene),
5′-AAGCGCAACAAGCCCACTGTCTAT-3′ and reverse (located in ABL gene),
5′-CTTCGTCTGAGATACTGGATTCCT-3′. Amplified fragments were directly
sequenced using an ABI PRISM 310 Genetic Analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Written informed consent was obtained from the patient in the
present study, according to the Declaration of Helsinki.
Staining
BM aspirate smears were stained with May-Grünwald's
and Giemsa's staining solution. Images were acquired at room
temperature using a BX50 light microscope (Olympus Corporation,
Tokyo, Japan) equipped with a DS-Fi2 camera, Digital Sight DS-U3
controller and NIS-Elements D software version 3.20 (all Nikon
Corporation, Tokyo, Japan). BM biopsy samples were fixed in 10%
neutral buffered formalin solution, decalcified in Osteosoft (Merck
KGaA, Darmstadt, Germany), sectioned (3-µm thick), and stained with
hematoxylin and eosin. Images were acquired at room temperature
using a BX53 light microscope equipped with DP21 camera/controller
(both Olympus Corporation).
Case report
A previously healthy 35-year-old Japanese male
visited the Department of Surgery for a left inguinal hernia in
March 2014. The patient had mild hepatomegaly and marked
splenomegaly. His blood cell counts were: White blood cells (WBC)
345,820/µl (blasts 4.0%, promyelocytes 0.0%, myelocytes 24.5%,
metamyelocytes 3.5%, band 16.5%, segmented 44.5%, monocytes 1.0%,
lymphocytes 1.0%, eosinophils 2.0%, basophils 3.0%), hemoglobin 7.3
g/dl and platelets 113×103/µl. The following day, the
patient was referred to the Department of Hematology. BM aspiration
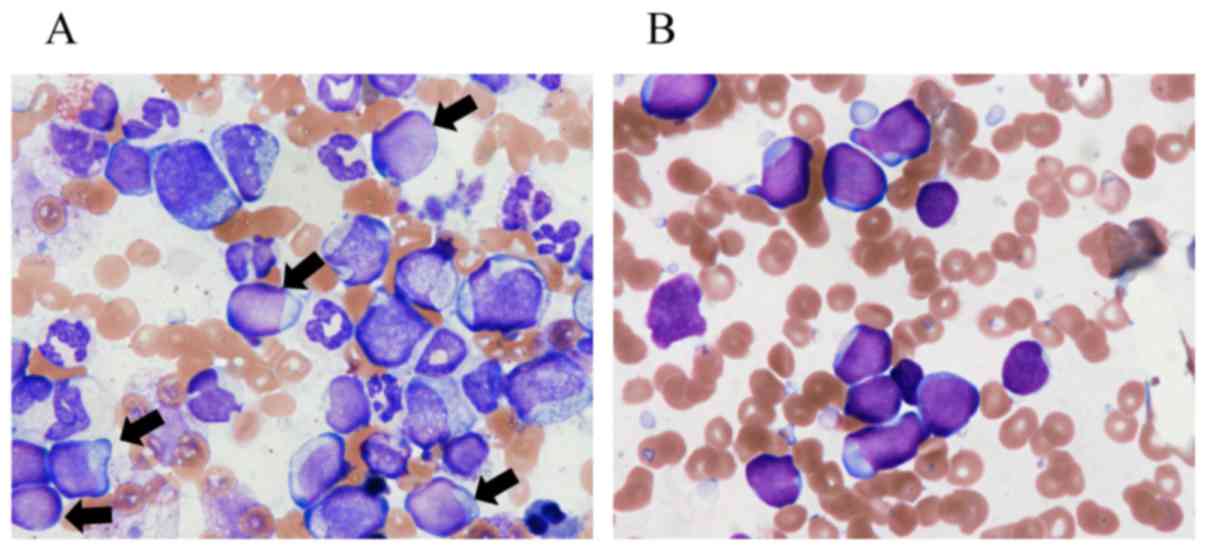
and biopsy revealed hypercellular marrow with 9.4% blasts (Fig. 1A) and the karyotype was 46,XY,
t(9;22)(q34;q11.2)[20/20]. The patient was diagnosed with CML-CP.
However, the disease rapidly progressed to AP (platelets
65×103/µl). The patient was hospitalized in April 2014,
and 100 mg dasatinib was prescribed. WBC and platelet counts
normalized, while hemoglobin levels increased. The splenomegaly was
also reduced from 8 to 3.5 cm below the umbilicus. On the 9th day
of hospitalization, the patient was discharged. The patient
continued to take dasatinib until his platelet count lowered to
49×103/µl, when dasatinib was discontinued one month
subsequent to the initial prescription. One month later,
administration of dasatinib was resumed. Subsequent to this visit,
no contact could be made with the patient.
In September 2014, the patient was hospitalized due
to active nasal bleeding. Blood test results revealed levels of:
WBC 3,790/µl, hemoglobin 7.6 g/dl and platelets
8×103/µl. BM aspiration and biopsy revealed
hypercellular marrow with peroxidase-negative blasts (85.5%,
Fig. 1B) characterized as
CD10+/CD19+/CD20−/CD34+/CD7dim.
Cytogenetic analysis revealed a Philadelphia chromosome with
additional aberrations: 46, XY, t(9;22)(q34;q11.2), del(15)(q?)[6/20], 47,idem, +der(22)t(9;22)[2/20], 50,idem,+X,del(13)(q?),+14,+21, +der(22)t(9;22)[3/20],46,XY[8/20]. A diagnosis of
CML B-lymphoid BP was made. Hyper-CVAD (cyclophosphamide,
vincristine sulfate, adriamycin, dexamethasone) chemotherapy was
started in combination with 140 mg dasatinib (22). The patient's platelet counts remained
as low as 10×103/µl, despite frequent transfusions. When
dasatinib induced thrombocytopenia in the patient by day 7, it was
discontinued and the patient was switched to 600 mg imatinib.
Active nasal bleeding persisted even following cauterization.
Therefore, dexamethasone was reduced from 8 doses to 5 doses to
reduce mucosal toxicity. When the bilirubin and alkaline
phosphatase levels reached 3.7 mg/dl and 1,012 IU/l, respectively,
by day 16, imatinib was discontinued. However, pancytopenia
persisted, and when BM aspiration and biopsy was performed on day
37, the blast level was 81.4%. Considering the high leukemia burden
of the patient, allogeneic stem cell transplantation was considered
to be a high risk treatment (4,12,13). Therefore, the patient underwent
follow-up care in an outpatient setting, with transfusions
performed 2–3 times per week.
In December 2014, the patient was re-admitted for
leukemia debulking with bosutinib. BM aspiration and biopsy
revealed hypercellular BM filled with blasts (96.1%). Cytogenetic
analysis revealed 46,XY,t(9;22)(q34;q11.2),del(15)(q?)[18/19] and 50,idem,+X,add(1)(p11), add(13)(q12),+14,+21,+der(22)t(9;22)[1/19]. Quantitative PCR detected
major BCR-ABL1 at 3.1×105 copy/µg RNA. The T315I
mutation was detected in the major BCR-ABL1 sequencing. In
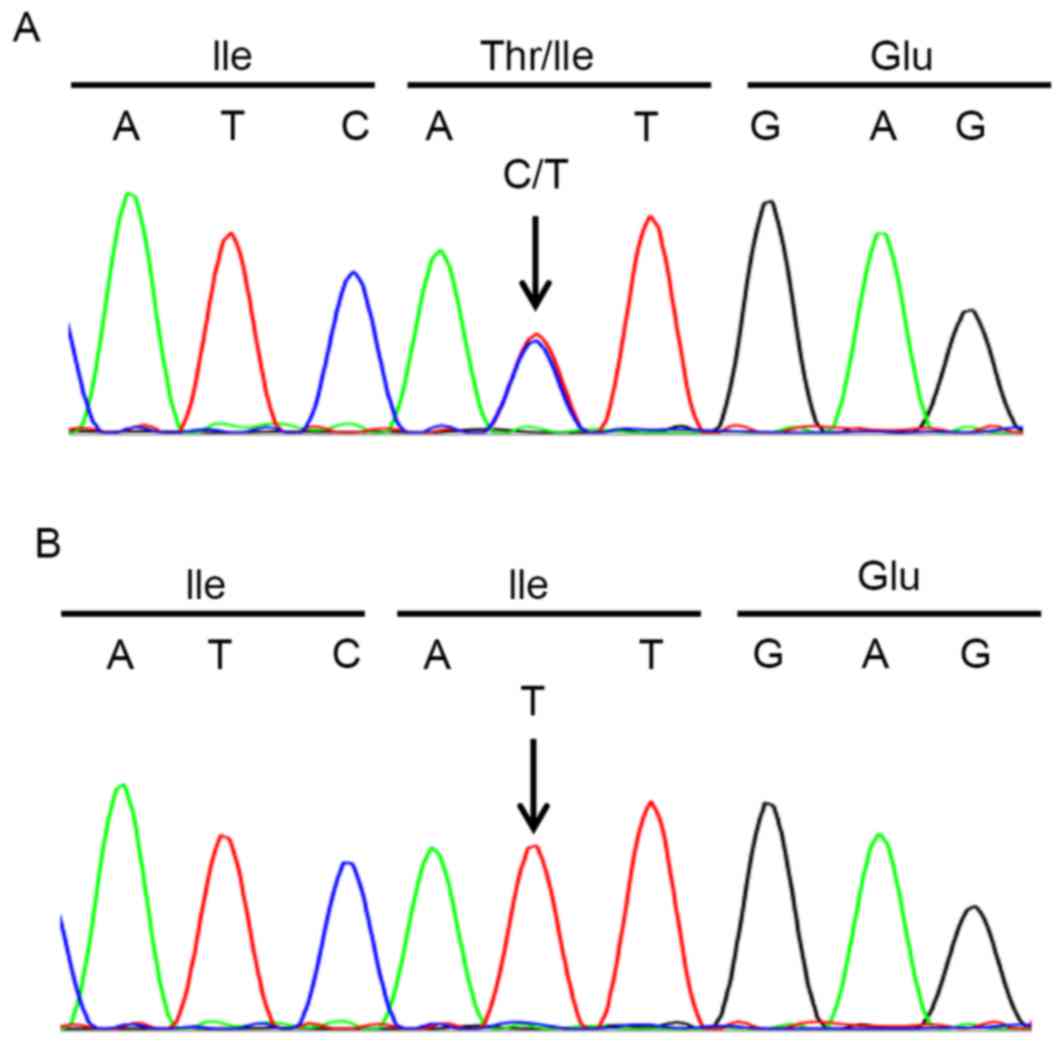
the sequencing chromatogram, peaks of the wild type allele were
revealed to overlap with the T315I mutant allele (Fig. 2), suggesting that almost half of the
leukemia cells lacked the T315I mutation. Administration of
bosutinib at 500 mg per day was prescribed (Fig. 3). Notably, blasts in the PB, as well
as hepatosplenomegaly, disappeared within 2 and 3 weeks,
respectively. Adverse effects experienced by the patient included
diarrhea [CTCAE v4.0 Grade (G) 2], anorexia (G3), jaundice (G3,
bilirubin 4.6 mg/dl), systemic erythema (G2) and papilloedema (G1).
Bosutinib was discontinued on day 8 due to jaundice, yet was
resumed at a dose of 300 mg on day 14. This lower dose was better
tolerated, however the levels of alanine transaminase (ALT) became
elevated (G3, 187 IU/l), which required temporary interruption. The
patient was referred to a collaborating hospital for
transplantation in February 2015. After two months of bosutinib
prescription, the blast level was decreased to 19.4%, and major
BCR-ABL1 level was 3.5×104 copy/µg RNA. The
patient continued to take bosutinib while waiting for the
transplantation. After four months of bosutinib prescription, the
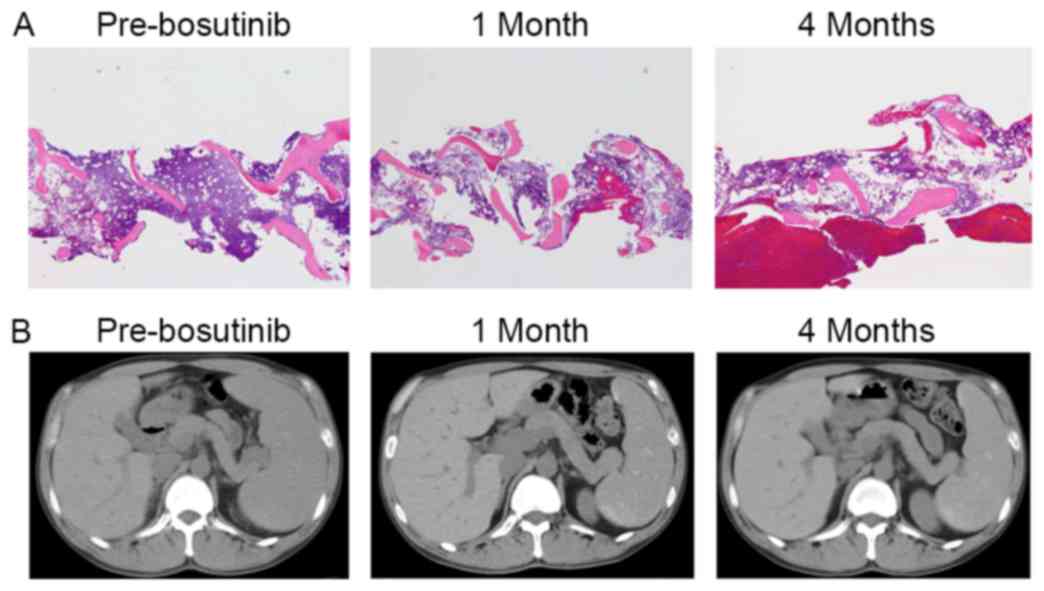
patient's BM became more hypocellular (Fig. 4A) and his spleen did not show regrowth
(Fig. 4B). Blasts in the BM were
reduced to 17.5%, and a cytogenetic analysis revealed a normal
karyotype (46, XY [20/20]). Sequencing of ABL1 of the BM
sample only detected the T315I mutant allele (Fig. 2B). Stem cell transplantation was still
considered necessary due to persistent pancytopenia and transfusion
dependence, despite the absence of disease progression (based on
the percentage of blasts in the PB or splenomegaly status).
Subsequent to taking bosutinib for five months, the patient
underwent cord blood transplantation at the end of April 2015 that
was accompanied by high-dose cytarabine (2 g × 2/day for two days)
followed by a conditioning regimen of fludarabine (180
mg/m2), an intravenous injection of busulfan (12.8
mg/kg) and melphalan (80 mg/m2). Engraftment was
confirmed on day 25. BM aspiration was performed on day 36. The
blast count was 0%, the karyotype was 46,XX [20/20] (as a result of
a female donor), and BCR-ABL1 was not detected by
quantitative PCR. Subsequent to becoming transfusion-independent,
the patient was discharged from the hospital on day 64. Acute
graft-vs.-host disease (GVHD) was observed in skin (stage I), but
not in the liver (stage 0) or gut (stage 0) (Grade I). Major
BCR-ABL1 level in PB was <0.0018% by International Scale
at one month and two months subsequent to transplantation.
Tacrolimus for GVHD treatment was discontinued at four months. At
five months, the patient has no sign of relapse, and follow up was
begun in an outpatient setting.
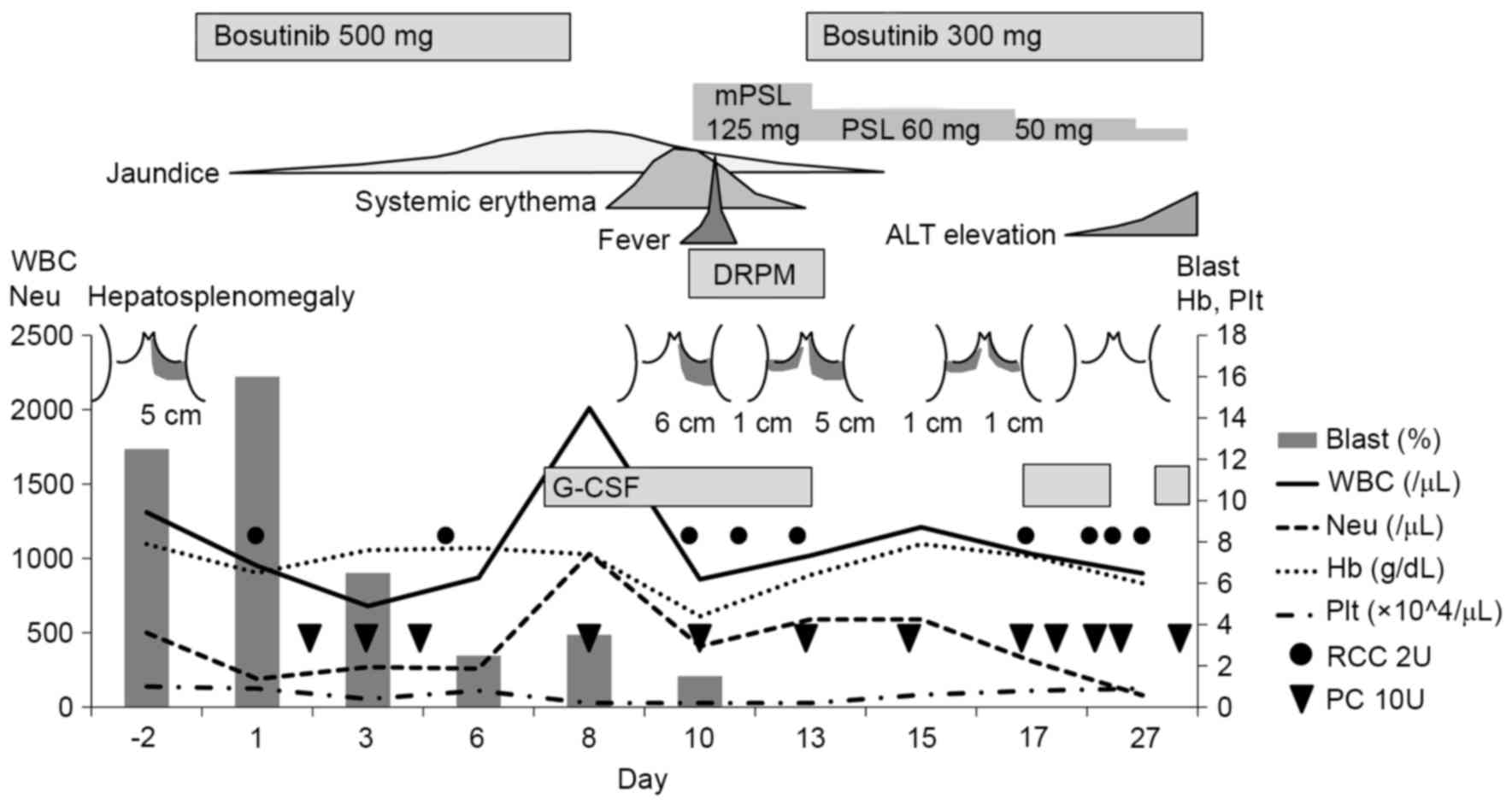 | Figure 3.Summary of the clinical symptoms,
blood analyses, and course of treatment for the present case
report. Hepatosplenomegaly is indicated according to the size below
the costal arches. mPSL, methylprednisolone; ALT, alanine
transaminase; WBC, white blood cells; DRPM, doripenem; Neu,
neutrophils; Hb, hemoglobin; Plt, platelets; G-CSF,
granulocyte-colony stimulating factor (filgrastim); RCC 2U, 2 units
of red cell concentrates; PC 10U, 10 units of platelet
concentrates. |
Discussion
Point mutations in the ABL1 kinase domain
have been associated with resistance to TKIs in patients with CML
(4–6,10,11). In the present case report, a patient
with T315I mutation-positive CML-BC demonstrated a response to
bosutinib over five months, and successfully underwent cord blood
transplantation. Initially, when exhibiting CML-CP, the patient was
intolerant to treatment with dasatinib [due to thrombocytopenia and
low compliance (23). In addition,
the patients CML-BP disease was resistant to chemotherapy combined
with the TKIs, dasatinib or imatinib (22). Correspondingly, the patient was
revealed to carry the T315I mutation. It has been reported that
bosutinib is ineffective against T315I-mutated BCR-ABL1 in
vitro (4,10,11). In
addition, in phase 1 and 2 clinical trials, CML-CP patients with
the T315I mutation exhibited poor responses compared to those
without the mutation (15–17). In Japan, bosutinib became available in
December 2014 (21), whereas
ponatinib (8,9) and the non-TKI, omacetaxine (24), which have been shown to be active
against T315I-positive cases, were not available at this time. In
the present case, the sequencing chromatogram of ABL1
implied that at least half of the leukemia cells carried wild-type
BCR-ABL1 (Fig. 2A). Indeed,
bosutinib was effective in debulking the leukemia cells in the
patient. Furthermore, ABL1 sequencing subsequent to the
administration of bosutinib detected only the T315I peak (Fig. 2B). Thus, the present results
demonstrate that when a patient carries cells with the T315I
mutation, sequencing chromatograms of ABL1 can potentially
provide an estimate of the T315I-positive leukemia cell burden.
Additionally, in cases where resistance/intolerance to multiple
TKIs is a factor, bosutinib may potentially provide an effective
pre-transplant therapy while promising new drugs including
ponatinib (8,24), omacetaxine (24) and axitinib (25) that target the T315I mutation are
becoming widely available. Furthermore, for individual situations,
certain drugs may or may not be suitable due to preexisting
comorbidities and/or pretreatments.
It has been reported that Src family kinases,
including Lyn, Hck, and Fgr, perform critical roles in
BCR-ABL1-independent TKI resistance (5,6) and
disease progression in CML, particularly during the progression to
B lymphoid BP (26–29). In leukemia cells from patients with
CML who exhibited disease progression during imatinib therapy, Hck
and Lyn were revealed to be strongly expressed and/or activated
(26). Correspondingly,
downregulation of Lyn by siRNA induced apoptosis in BCR-ABL1
positive blasts, particularly lymphoid blasts (27). In addition, mouse models have
demonstrated that Lyn, Hck and Fgr are required for the transition
from CML-CP to lymphoid BP (28,29). In
the present case, the percentage of blasts in the BM decreased from
96.1 to 17.5% following treatment with Bosutinib. In addition, the
spleen demonstrated sustained shrinkage over five months of the
bosutinib treatment regimen. The reduction in leukemia load was
more than would be expected just from the killing of T315I-negative
blasts. Thus, it is possible that the dual Src/ABL1 kinase
inhibitor, bosutinib, was able to effectively suppress Src, Lyn and
Hck in the T315I-positive lymphoid BP cells, thereby additionally
inducing anti-leukemia effects. It will be important for future
studies to compare the sensitivity of CML cells from lymphoid BP
vs. myeloid BP to bosutinib. To date, no such data are available
(18,21).
The clinical course in this report suggested that
the interruption of dasatinib for any reasons during CML treatment,
particularly in late-CP or AP/BC, may induce a resistant clone
against TKIs, even if the resistant mutated clone existed in BM
prior to treatment with dasatinib. Indeed, poor adherence may be
the predominant reason for failure to achieve adequate molecular
responses in patients treated with imatinib for >2 years
(23). A management of adverse
effects and a continuation of TKI are essential for CML treatment.
The selection of an appropriate TKI for each patient with CML with
individual situations during TKI treatment as well as at a
diagnosis of CML is the key to the best possible outcome (30).
In conclusion, the present case represents a patient
with T315I-positive CML-BP. The patient responded to bosutinib as a
fourth-line treatment. Bosutinib was also useful as a
pre-transplant therapy for reducing leukemia cell load. Thus,
patients who are resistant or intolerant to multiple TKIs, unless
the leukemia cells are uniformly T315I-positive, may benefit from a
bosutinib treatment regimen.
Acknowledgments
The authors would like to thank Dr. Shinya Kimura
(Department of Hematology and Oncology, Saga University) and Dr.
Chiaki Nakaseko (Department of Hematology, Chiba University
Hospital) for their help.
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 4th Edition.
International Agency for Research on Cancer; Lyon: pp. 130–139.
2008
|
|
2
|
Wilson MB, Schreiner SJ, Choi HJ, Kamens J
and Smithgall TE: Selective pyrrolo-pyrimidine inhibitors reveal a
necessary role for Src family kinases in Bcr-Abl signal
transduction and oncogenesis. Oncogene. 21:8075–8088. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lionberger JM, Wilson MB and Smithgall TE:
Transformation of myeloid leukemia cells to cytokine independence
by Bcr-Abl is suppressed by kinase-defective Hck. J Biol Chem.
275:18581–18585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baccarani M, Deininger MW, Rosti G,
Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes
JE, Guilhot F, et al: European LeukemiaNet recommendations for the
management of chronic myeloid leukemia: 2013. Blood. 122:872–884.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Hare T, Zabriskie MS, Eiring AM and
Deininger MW: Pushing the limits of targeted therapy in chronic
myeloid leukaemia. Nat Rev Cancer. 12:513–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eide CA and O'Hare T: Chronic myeloid
leukemia: Advances in understanding disease biology and mechanisms
of resistance to tyrosine kinase inhibitors. Curr Hematol Malig
Rep. 10:158–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azam M, Seeliger MA, Gray NS, Kuriyan J
and Daley GQ: Activation of tyrosine kinases by mutation of the
gatekeeper threonine. Nat Struct Mol Biol. 15:1109–1118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortes JE, Kantarjian H, Shah NP, Bixby D,
Mauro MJ, Flinn I, O'Hare T, Hu S, Narasimhan NI, Rivera VM, et al:
Ponatinib in refractory Philadelphia chromosome-positive leukemias.
N Engl J Med. 367:2075–2088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cortes JE, Kim DW, Pinilla-Ibarz J, le
Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ,
Talpaz M, et al: A phase 2 trial of ponatinib in Philadelphia
chromosome-positive leukemias. N Engl J Med. 369:1783–1796. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Redaelli S, Piazza R, Rostagno R,
Magistroni V, Perini P, Marega M, Gambacorti-Passerini C and
Boschelli F: Activity of bosutinib, dasatinib, and nilotinib
against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol.
27:469–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Redaelli S, Mologni L, Rostagno R, Piazza
R, Magistroni V, Ceccon M, Viltadi M, Flynn D and
Gambacorti-Passerini C: Three novel patient-derived BCR/ABL mutants
show different sensitivity to second and third generation tyrosine
kinase inhibitors. Am J Hematol. 87:E125–E128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrett AJ and Ito S: The role of stem
cell transplantation for chronic myelogenous leukemia in the 21st
century. Blood. 125:3230–3235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saussele S and Silver RT: Management of
chronic myeloid leukemia in blast crisis. Ann Hematol. 94 Suppl
2:S159–S165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rix LL Remsing, Rix U, Colinge J,
Hantschel O, Bennett KL, Stranzl T, Müller A, Baumgartner C, Valent
P, Augustin M, et al: Global target profile of the kinase inhibitor
bosutinib in primary chronic myeloid leukemia cells. Leukemia.
23:477–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cortes JE, Kantarjian HM, Brummendorf TH,
Kim DW, Turkina AG, Shen ZX, Pasquini R, Khoury HJ, Arkin S,
Volkert A, et al: Safety and efficacy of bosutinib (SKI-606) in
chronic phase Philadelphia chromosome-positive chronic myeloid
leukemia patients with resistance or intolerance to imatinib.
Blood. 118:4567–4576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gambacorti-Passerini C, Brümmendorf TH,
Kim DW, Turkina AG, Masszi T, Assouline S, Durrant S, Kantarjian
HM, Khoury HJ, Zaritskey A, et al: Bosutinib efficacy and safety in
chronic phase chronic myeloid leukemia after imatinib resistance or
intolerance: Minimum 24-month follow-up. Am J Hematol. 89:732–742.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khoury HJ, Cortes JE, Kantarjian HM,
Gambacorti-Passerini C, Baccarani M, Kim DW, Zaritskey A,
Countouriotis A, Besson N, Leip E, et al: Bosutinib is active in
chronic phase chronic myeloid leukemia after imatinib and dasatinib
and/or nilotinib therapy failure. Blood. 119:3403–3412. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kantarjian HM, Cortes JE, Kim DW, Khoury
HJ, Brümmendorf TH, Porkka K, Martinelli G, Durrant S, Leip E,
Kelly V, et al: Bosutinib safety and management of toxicity in
leukemia patients with resistance or intolerance to imatinib and
other tyrosine kinase inhibitors. Blood. 123:1309–1318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cortes JE, Kim DW, Kantarjian HM,
Brümmendorf TH, Dyagil I, Griskevicius L, Malhotra H, Powell C,
Gogat K, Countouriotis AM and Gambacorti-Passerini C: Bosutinib
versus imatinib in newly diagnosed chronic-phase chronic myeloid
leukemia: Results from the BELA trial. J Clin Oncol. 30:3486–3492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brümmendorf TH, Cortes JE, de Souza CA,
Guilhot F, Duvillié L, Pavlov D, Gogat K, Countouriotis AM and
Gambacorti-Passerini C: Bosutinib versus imatinib in newly
diagnosed chronic-phase chronic myeloid leukaemia: Results from the
24-month follow-up of the BELA trial. Br J Haematol. 168:69–81.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakaseko C, Takahashi N, Ishizawa K,
Kobayashi Y, Ohashi K, Nakagawa Y, Yamamoto K, Miyamura K, Taniwaki
M, Okada M, et al: A phase 1/2 study of bosutinib in Japanese
adults with Philadelphia chromosome-positive chronic myeloid
leukemia. Int J Hematol. 101:154–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strati P, Kantarjian H, Thomas D, O'Brien
S, Konoplev S, Jorgensen JL, Luthra R, Abruzzo L, Jabbour E,
Quintas-Cardama A, et al: HCVAD plus imatinib or dasatinib in
lymphoid blastic phase chronic myeloid leukemia. Cancer.
120:373–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marin D, Bazeos A, Mahon FX, Eliasson L,
Milojkovic D, Bua M, Apperley JF, Szydlo R, Desai R, Kozlowski K,
et al: Adherence is the critical factor for achieving molecular
responses in patients with chronic myeloid leukemia who achieve
complete cytogenetic responses on imatinib. J Clin Oncol.
28:2381–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cortes J, Lipton JH, Rea D, Digumarti R,
Chuah C, Nanda N, Benichou AC, Craig AR, Michallet M, Nicolini FE,
et al: Phase 2 study of subcutaneous omacetaxine mepesuccinate
after TKI failure in patients with chronic-phase CML with T315I
mutation. Blood. 120:2573–2580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pemovska T, Johnson E, Kontro M, Repasky
GA, Chen J, Wells P, Cronin CN, McTigue M, Kallioniemi O, Porkka K,
et al: Axitinib effectively inhibits BCR-ABL1(T315I) with a
distinct binding conformation. Nature. 519:102–105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donato NJ, Wu JY, Stapley J, Gallick G,
Lin H, Arlinghaus R and Talpaz M: BCR-ABL independence and LYN
kinase overexpression in chronic myelogenous leukemia cells
selected for resistance to STI571. Blood. 101:690–698. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ptasznik A, Nakata Y, Kalota A, Emerson SG
and Gewirtz AM: Short interfering RNA (siRNA) targeting the Lyn
kinase induces apoptosis in primary, and drug-resistant,
BCR-ABL1(+) leukemia cells. Nat Med. 10:1187–1189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Liu Y, Pelletier S, Buchdunger E,
Warmuth M, Fabbro D, Hallek M, Van Etten RA and Li S: Requirement
of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced
B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat
Genet. 36:453–461. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Swerdlow S, Duffy TM, Weinmann R,
Lee FY and Li S: Targeting multiple kinase pathways in leukemic
progenitors and stem cells is essential for improved treatment of
Ph+ leukemia in mice. Proc Natl Acad Sci USA. 103:16870–16875.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larson RA: Is there a best TKI for chronic
phase CML? Blood. 126:2370–2375. 2015. View Article : Google Scholar : PubMed/NCBI
|