Introduction
Osteosarcoma is a malignant and potentially
metastatic primary bone tumor, and occurs most often in children
and young adults, typically after age 10 years (1–3). In ~20%
of patients with osteosarcoma, clinically detectable metastatic
disease is present at the time of diagnosis, and up to 40% of
patients present with advanced metastases (4–6). Despite
great advances in multimodal treatment during the previous few
decades, patients with metastatic osteosarcoma have a poor
prognosis, with 5-year survival rates of <20% (7,8). Thus, it
is highly desirable to seek a novel strategy for the treatment of
metastatic osteosarcoma.
Interleukin-24 (IL-24), also known as melanoma
differentiation-associated 7 due to its initial discovery from
human melanoma cells by combined treatment with interferon-β and
mezerein, is a unique member of the IL-10 family that exhibits
almost ubiquitous cancer-specific toxicity, with no harmful effects
on normal cells or tissues (9–12). The
overproduction of IL-24 may selectively inhibit cancer cell growth
and induce apoptosis in a broad spectrum of human cancer types, and
additionally induces indirect antitumor activity through inhibition
of angiogenesis, activation of the antitumor immune response and
sensitization of cancer cells to radiation, chemotherapy and
antibody-induced killing (12–14).
Previous studies have mainly focused on the role of
IL-24 tumor-suppressive effects by prevention of proliferation and
promotion of apoptosis in a wide variety of tumors (11,14).
However, it has been observed that IL-24 inhibits neuroblastoma
cell migration and invasion by regulating numerous
invasion-associated molecules in vitro (15,16). These
observations are consistent with the results of other studies,
which reported that IL-24 inhibited migration and invasion of human
ovarian, liver and lung cancer cells in vitro (17–19).
Fisher et al (20) have
demonstrated that IL-24 is safe and promotes significant clinical
activity, particularly in the context of patients with metastatic
melanoma (20). In the present study,
we investigated the effects of IL-24 on migration and invasion in
spontaneously metastasizing human osteosarcoma 143B cells in
vitro, and attempted to identify the associated molecular
mechanisms of metastasis suppression. It was demonstrated that
IL-24 is able to inhibit migration and invasion in spontaneously
metastasizing human 143B osteosarcoma cells via inhibiting c-Jun
N-terminal kinase (JNK) and c-Jun phosphorylation to downregulate
matrix metalloproteinase (MMP)-2 and MMP-9.
Materials and methods
Virus production, cell line and
antibodies
Adenovirus (Ad).IL-24 (a replication-deficient
adenovirus with IL-24 gene) and Ad.green fluorescent protein (GFP)
(a replication-deficient adenovirus with reporter gene GFP) were
maintained in our laboratory as previously described (16). The human highly metastatic
osteosarcoma cell line 143B was purchased from the American Type
Culture Collection (Manassas, VA, USA) and maintained in Dulbecco's
modified Eagle's medium (DMEM) and Ham's F12 (1:1 mixture; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified 37°C incubator with 5% CO2. Anti-JNK rabbit
antibody (dilution, 1:500; catalog no., sc-572),
anti-phosphorylated (p)-JNK rabbit antibody (dilution, 1:400;
catalog no., sc-12882) and anti-β-actin rabbit antibody (dilution,
1:400; catalog no., sc-7210) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Anti-extracellular
signal-regulated kinase (Erk)1/2 rabbit antibody (dilution,
1:1,000; catalog no., #4695), anti-p-Erk rabbit antibody (dilution,
1:1,000; catalog no., #4376), anti-p38 rabbit antibody (dilution,
1:1,000; catalog no., #8690), anti-p-p38 rabbit antibody (dilution,
1:1,000; catalog no., #4511), anti-c-Jun rabbit antibody (dilution,
1:500; catalog no., #9165), anti-p-c-Jun rabbit antibody (dilution,
1:500; catalog no., #2361), anti-MMP-2 rabbit antibody (dilution,
1:1,000; catalog no., #13132) and anti-MMP-9 rabbit antibody
(dilution, 1:1,000; catalog no., #13667) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell viability assay
Cell viability was detected by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as described previously (16).
Briefly, the cells were plated in 96-well plates (1×104
cells per well) and infected with Ad.IL-24 or Ad.GFP at the
indicated time point (24, 48, 72 or 96 h) or multiplicity of
infection (MOI; 1, 5, 10, 50 or 100), the medium was removed and
fresh medium containing MTT (0.5 mg/ml) was added to each well. The
cells were incubated at 37°C for 4 h, and an equal volume of
solubilization solution (0.01 N HCl in 10% sodium dodecyl sulfate)
was added to each well and mixed thoroughly. The absorbance at 490
nm was measured using an ELx-800 spectrometer reader (Bio-Tek
Instruments, Inc., Winooski, VT USA). All MTT assays were prepared
in triplicate and repeated three times.
Cell migration and invasion assay
143B cells were seeded at a density of
5×105 cells/well in 6-well tissue culture plates. The
following day, cells were infected with Ad.IL-24 and Ad.GFP
(control) at MOI=10. At 8 h subsequent to infection, the cells were
trypsinized, washed in phosphate-buffered saline (PBS) and
resuspended in serum-free DMEM. The cell migration or invasion
assay was performed in a 24-well Transwell unit (Sigma-Aldrich; EMD
Millipore, Billerica, MA, USA) or a 24-well Transwell unit coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA),
respectively. The lower chambers of the Transwell units were filled
with serum-free medium, and the upper chambers were seeded with
1×105 cells from each group in triplicate wells.
Following 24- and 48-h incubations, the cells that had passed
through the filter into the lower wells were counted. The
experiments were performed four times, and the results were
recorded as the mean of these experiments.
Western blotting
Cells were harvested and incubated in lysis buffer
containing 20 mmol/l Tris-HCl (pH 7.5), 1% Triton X-100, 150 mmol/l
NaCl, 10% glycerol, 1 mmol/l Na3VO4, 50
mmol/l NaF, 100 mmol/l phenylmethylsulfonyl fluoride and a
commercial protease inhibitor mixture (Roche Diagnostics, Basel,
Switzerland) for 20 min on ice. After insoluble debris was pelleted
by centrifugation at 14,000 × g for 15 min at 4°C, the supernatants
were collected and determined for protein content using the
Bradford method (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Proteins (50 µg) were resolved under denaturing conditions by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto nitrocellulose membranes. Following blocking for 2
h in PBS with 0.1% Tween 20 and 3% bovine serum albumin (Beyotime
Institute of Biotechnology, Haimen China), the membranes were
incubated overnight at 4°C with the appropriate primary antibodies.
Membranes were then washed with PBS and incubated with alkaline
phosphatase-conjugated goat anti-rabbit secondary antibody
(dilution, 1:10,000; catalog no., A3687; Sigma-Aldrich; EMD
Millipore) for 2 h. Membranes were developed using the nitro-blue
tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate
p-toluidine salt color substrate (Promega Corporation, Madison, WI,
USA). The density of the bands on the membrane was scanned and
analyzed using an image analyzer. The bands on the membranes were
visualized and quantified with an Odyssey® CLx Infrared
Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Use of SP600125 in cell culture
SP600125 (Sigma-Aldrich; EMD Millipore) was
dissolved in dimethyl sulfoxide (Sigma-Aldrich; EMD Millipore), as
a 20 mM stock solution, and further diluted (1:1,000) in culture
medium to administer to treatment group cells. Following treatment
with 20 μM SP600125, 143B cells were infected with Ad.IL-24 or
Ad.GFP at MOI=10 for 48 h.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis of the results was performed using a one-way
analysis of variance or Student's t-test. The experimental data
were analyzed using SPSS version 16.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
IL-24 inhibits osteosarcoma 143B cell
proliferation
In the present study, the expression of IL-24 in
143B cells was investigated via adenoviral infection. As shown in
Fig. 1A and B, IL-24 protein levels
were efficiently augmented following infection with Ad.IL-24 for 48
h compared to Ad.GFP as assayed by western blotting. Subsequently,
the effects on 143B cells proliferative ability were assessed by
MTT assay following infection with Ad.IL-24 or Ad.GFP at various
MOI or at various time points. As shown in Fig. 1C, overexpression of IL-24 markedly
inhibited the growth of the infected 143B cells in a dose-dependent
manner. Simultaneously, the proliferation of 143B cells was
significantly inhibited at 72 h following infection with Ad.IL-24
(Fig. 1D; P=0.0036). This indicated
that IL-24 was able to effectively inhibit the proliferation of
143B cells.
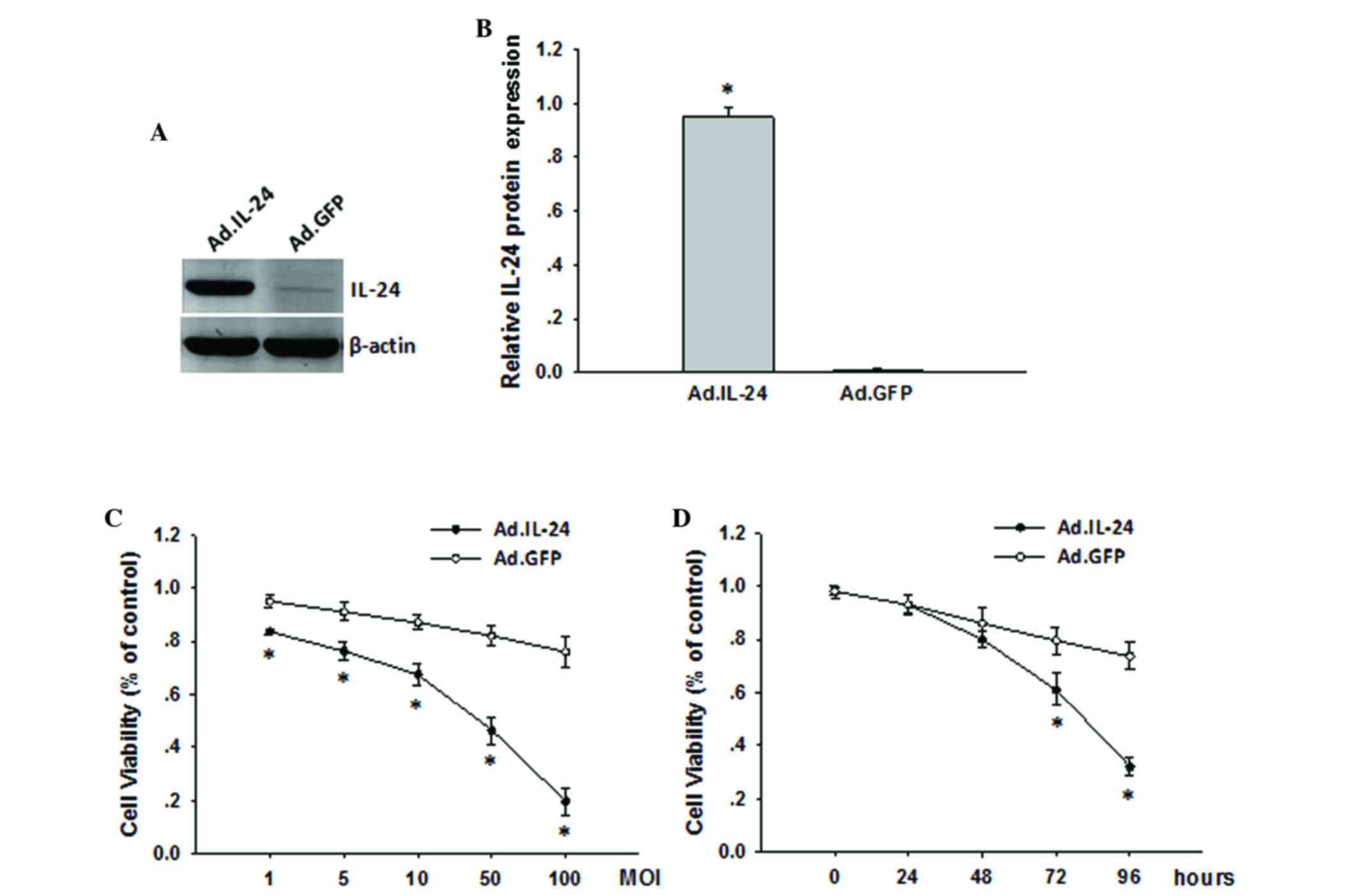 | Figure 1.Overexpression of IL-24 suppresses
osteosarcoma 143B cell proliferation. (A) Overexpression of IL-24
in 143B cells was analyzed by western blotting. β-actin was used as
an internal control. (B) Quantitative representation of the
expression of IL-24 protein. Data are presented as the mean ± SD
from three independent experiments (n=3). (C) 143B cells were
seeded into 96-well plates and treated with Ad.IL-24 and Ad.GFP,
respectively, at MOI=1, 5, 10, 50 and 100. Following 72 h of
infection, the cell viability was measured by MTT assays. (D) 143B
cells were seeded into 96-well plates and treated with Ad.IL-24 and
Ad.GFP, respectively, at MOI=10. The cell viability was measured by
MTT assays at 24, 48, 72 and 96 h following treatment. Following
infection for 48 h, no significant inhibition of tumor cell
proliferation was observed in Ad.IL-24 treated cells compared to
Ad.GFP treated cells. Data are presented as the mean ± SD from
three independent experiments (n=3). *P<0.05 compared with
Ad.GFP. IL, interleukin; Ad, adenovirus; GFP, green fluorescent
protein; MOI, multiplicity of infection; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SD,
standard deviation. |
IL-24 inhibits osteosarcoma 143B cell
migration and invasion
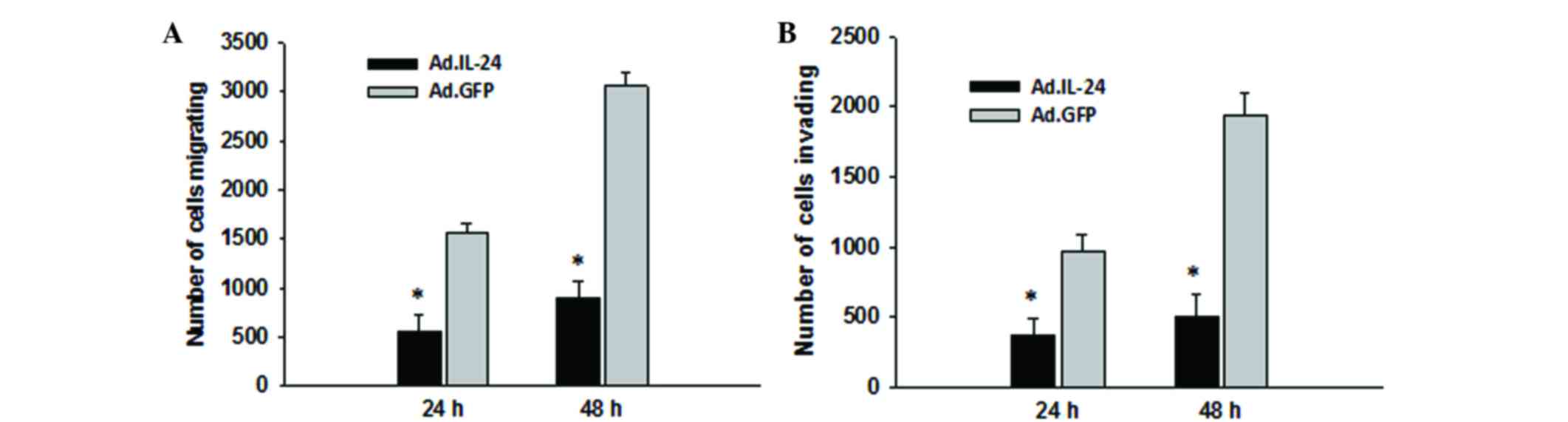
Subsequently, the present study detected the effects
on 143B cells migration and invasion ability following infection
with Ad.IL-24 or Ad.GFP at MOI=10 for 48 h by using Transwell
assays. It was observed that overexpression of IL-24 was able to
significantly decrease 143B cell migration ability (Fig. 2A; P=0.0006), and this effect was
consistent across the invasion assay (Fig. 2B; P=0.0003). 143B cell viability was
investigated at 48 h following infection with Ad.IL-24 and the
results are presented in Fig. 1D.
Treatment with Ad.IL-24 at 48 h did not significantly inhibit the
proliferation of 143B cells (P=0.7426). This indicated that IL-24
inhibits 143B cell migration and invasion independently of IL-24
cytotoxicity.
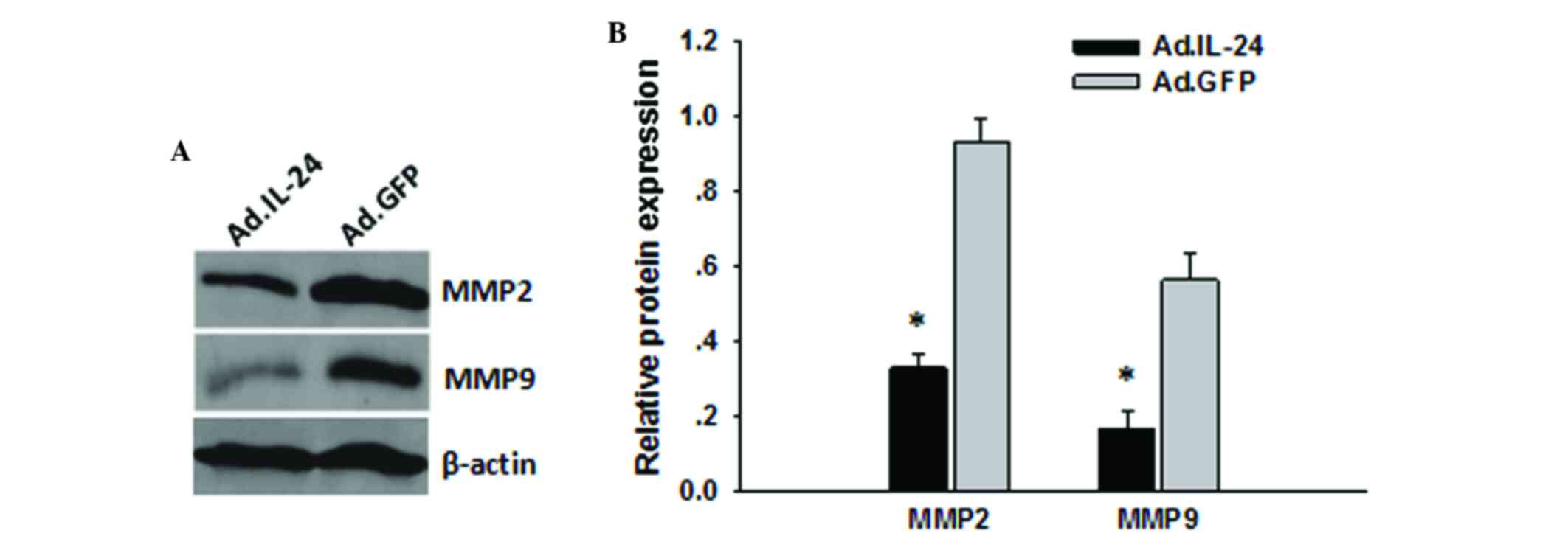
IL-24 reduces MMP-2 and MMP-9
expression
To determine the underlying molecular mechanism of
IL-24 inhibition of migration and invasion, the level of migration-
and invasion-associated proteins in 143B cells was detected
following treatment with Ad.IL-24. MMP-2 and MMP-9 have a critical
role in osteosarcoma cell migration and invasion (21). To investigate whether MMP-2 and MMP-9
are involved in IL-24 inhibition of the migration and invasion of
osteosarcoma cells, the present study assessed the levels of MMP-2
and MMP-9 in osteosarcoma cells treated with Ad.IL-24 at MOI=10 for
48 h by western blotting. Results presented in Fig. 3A and B indicated that the levels of
MMP-2 and MMP-9 were reduced in the Ad.IL-24 group compared with
the control group. This reduction may have led to the inhibition of
migration and invasion in 143B cells following treatment with
Ad.IL-24.
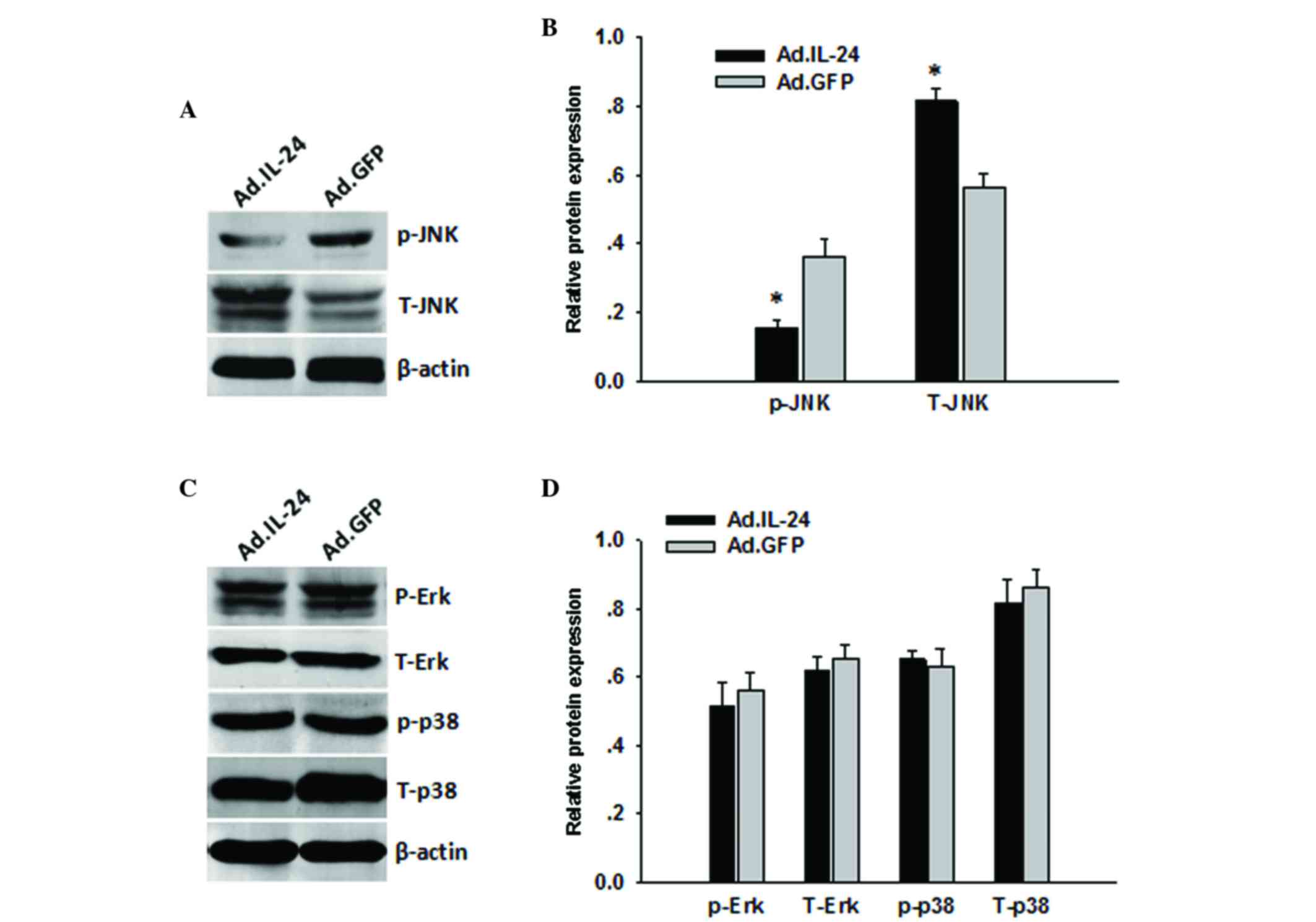
JNK/c-Jun signaling pathway is
associated with IL-24-induced cell migration and invasion
The mitogen-associated protein kinase (MAPK)
signaling pathway is closely associated with osteosarcoma cell
migration and invasion. Therefore, to determine whether IL-24
regulates 143B cell migration and invasion via the MAPK signaling
pathway, the present study investigated the signaling pathways that
were activated by IL-24. 143B cells were pre-treated with Ad.IL-24
at MOI=10 for 48 h and compared with the Ad.GFP treatment group.
Following treatment, total protein was extracted and analyzed by
western blotting. As shown in Fig. 4,
p-JNK was significantly decreased in the 143B cells following
Ad.IL-24 treatment (P<0.0001). However, there were no marked
changes in the levels of p-Erk (P=0.0689) or p-p38 (P=0.0732)
caused by IL-24 overexpression (Fig.
4).
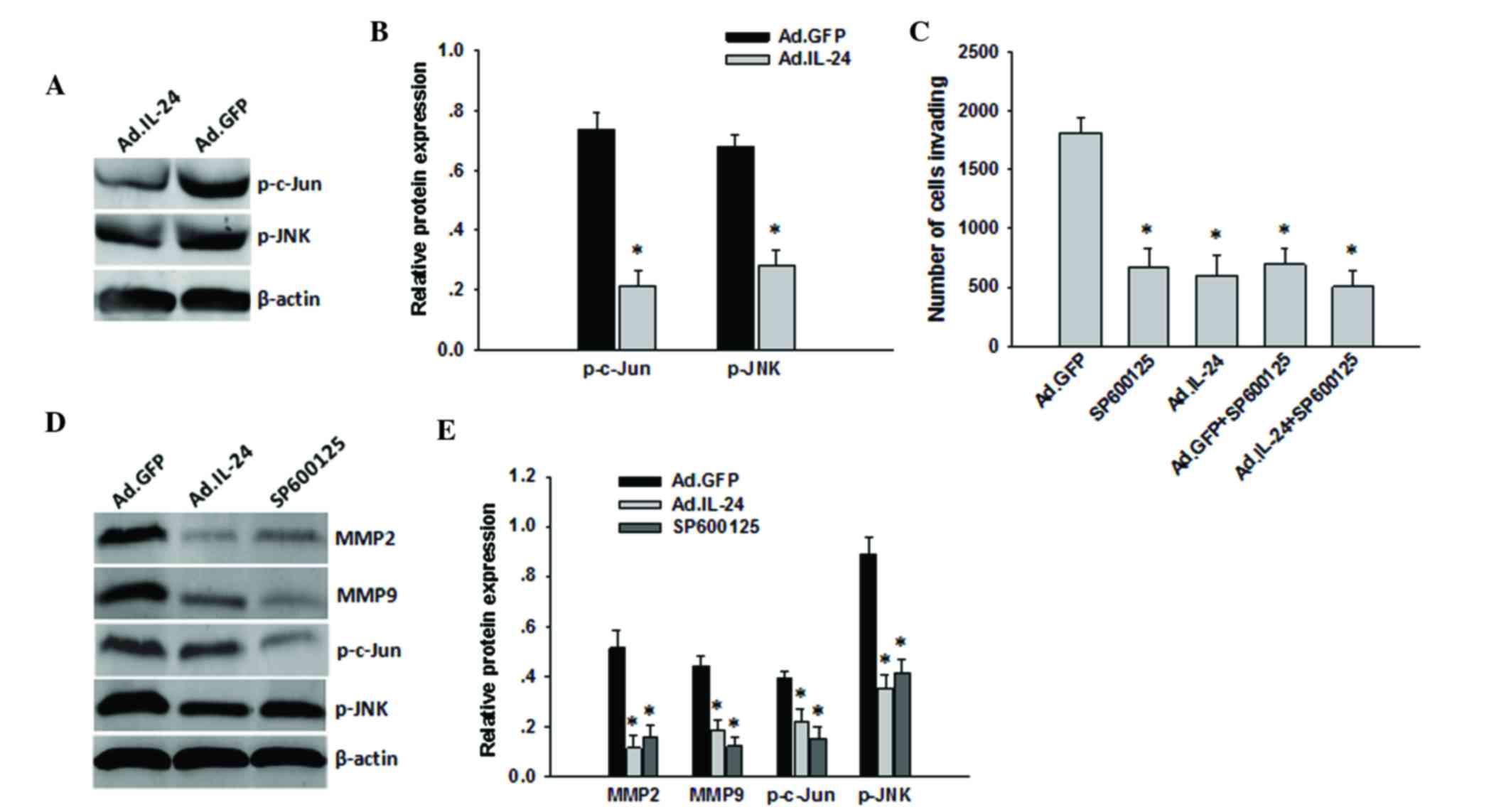
Subsequently, the present study additionally
determined the association between the migration function of IL-24
and the JNK signaling pathway. Western blotting revealed that when
p-JNK levels were decreased by IL-24 overexpression in the 143B
cells, p-c-Jun levels were also decreased (Fig. 5A and B). These results suggest that
IL-24 may regulate p-c-Jun through p-JNK in osteosarcoma cells. To
further determine whether IL-24 regulates 143B cell invasion
through the JNK/c-Jun signaling pathways, the JNK pathways were
blocked with the specific inhibitor SP600125 in the 143B cells and
subsequently infected with Ad.IL-24 or Ad.GFP at MOI=10 for 48 h.
As shown in Fig. 5C, 143B cells
pre-treated with SP600125 and infected with Ad.IL-24 did not
demonstrate a marked difference in inhibition of invasion compared
with cells treated with SP600125 or Ad.IL-24 alone. Furthermore,
when p-JNK levels were decreased by SP600125 treatment in the 143B
cells, p-c-Jun, MMP-2 and MMP-9 levels were also decreased
(Fig. 5D and E). These results
suggest that the JNK/c-Jun signaling pathways may have important
roles in mediating IL-24 inhibited osteosarcoma cell migration and
invasion.
Discussion
Enforced expression of IL-24 inhibits the growth of
a broad spectrum of cancer cells, without exerting deleterious
effects in normal cells and tissues. Previously, our and others'
data have demonstrated that overexpressed IL-24 may inhibit
migration and invasion in human ovarian, liver and lung cancer
cells and neuroblastoma in vitro (15,17–19). In
the present study, anti-proliferative effects were observed
following overexpression of IL-24 in osteosarcoma cells.
Anti-invasion and anti-migration effects of IL-24 overexpression
were also observed on highly metastatic 143B osteosarcoma cells.
Treatment with Ad.IL-24 at 48 h did not significantly inhibit the
proliferation of 143B cells. However, treatment with Ad.IL-24 at 48
h significantly decreased 143B cell migration and invasion.
Therefore, the results of the present study appear to indicate that
the suppressive role of IL-24 on osteosarcoma cell migration and
invasion is independent of IL-24 cytotoxicity. Thus, the present
study further clarified the associative mechanism by which IL-24
regulates migration and invasion of human osteosarcoma cells.
To facilitate tumor cell migration and invasion,
cells must alter their cell-cell properties, rearrange the
extracellular matrix (ECM) environment and reorganize their
cytoskeletons (22–25). ECM degradation is vital for migration
of the metastatic cell from the primary tumor site and invasion to
the metastatic site (26). MMPs are a
zinc-dependent endopeptidase family known to be responsible for the
degradation of the ECM (27,28). Among the MMP family members, MMP-2 and
MMP-9 have significant roles in tumor metastasis by degrading
collagen and stimulating tumor growth (27). The suppression of MMP-2 and MMP-9 may
be effective approaches for anti-metastasis treatment of cancer.
The results of the present study indicated that the expression
levels of MMP-2 and MMP-9 were suppressed by IL-24. Thus, one
potential mechanism that may explain IL-24 as a migration and
invasion suppressor is negative regulation of the MMP signaling
pathways.
MAPK signaling pathways are associated with
tumorigenesis and metastatic potential in osteosarcoma (29,30). In
addition, MAPKs have a role in major signaling pathways that
control MMPs (25). A number of
studies have demonstrated that stress-activated protein kinases/JNK
and Erk transcriptionally regulate the expression of MMP-2 and
MMP-9, which results in regulation of cell migration and invasion
(31–34). Silibinin is able to suppress
osteosarcoma MG-63 cell invasion by inhibiting c-Jun/activator
protein 1 induction of MMP-2 (35).
Statin reduces osteosarcoma cell migration and invasion though
JNK-c-Jun-MMP-2 signaling pathways to inhibit
3-hydroxy-3-methylglutaryl-coenzyme A reductase (36). In the present study, IL-24 was
overexpressed in osteosarcoma 143B cells, and it was observed that
the activation of JNK/c-Jun was inhibited, which may be responsible
for the downregulation of MMP-2 and MMP-9.
In summary, to the best of our knowledge, the
present study is the first to provide evidence that IL-24 is able
to inhibit osteosarcoma invasion through the JNK/c-Jun signaling
pathway, via decreasing MMP-2/MMP-9 levels. Understanding the
molecular mechanism by which IL-24 inhibits osteosarcoma
progression will not only improve our understanding of the
metastatic mechanisms of osteosarcoma but may also provide a novel
potential therapeutic agent for the treatment of metastatic
osteosarcoma.
References
|
1
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21 Suppl 7:vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terezhalmy GT, Riley CK and Moore WS:
Osteosarcoma. Quintessence Int. 31:592–593. 2000.PubMed/NCBI
|
|
3
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyers PA, Heller G, Healey JH, Huvos A,
Applewhite A, Sun M and LaQuaglia M: Osteogenic sarcoma with
clinically detectable metastasis at initial presentation. J Clin
Oncol. 11:449–453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kager L, Zoubek A, Pötschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, et al Cooperative German-Austrian-Swiss Osteosarcoma
Study Group, : Primary metastatic osteosarcoma: Presentation and
outcome of patients treated on neoadjuvant Cooperative Osteosarcoma
Study Group protocols. J Clin Oncol. 21:2011–2018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasalkar DD, Chu WC, Lee V, Paunipagar BK,
Cheng FW and Li CK: Pulmonary metastases in children with
osteosarcoma: Characteristics and impact on patient survival.
Pediatr Radiol. 41:227–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyers PA, Schwartz CL, Krailo MD, Healey
JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM,
Harris M, et al Children's Oncology Group, : Osteosarcoma: The
addition of muramyl tripeptide to chemotherapy improves overall
survival - a report from the Children's Oncology Group. J Clin
Oncol. 26:633–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
10
|
Caudell EG, Mumm JB, Poindexter N,
Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S
and Grimm EA: The protein product of the tumor suppressor gene,
melanoma differentiation-associated gene 7, exhibits
immunostimulatory activity and is designated IL-24. J Immunol.
168:6041–6046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sauane M, Gopalkrishnan RV, Sarkar D, Su
ZZ, Lebedeva IV, Dent P, Pestka S and Fisher PB: MDA-7/IL-24: Novel
cancer growth suppressing and apoptosis inducing cytokine. Cytokine
Growth Factor Rev. 14:35–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitaker EL, Filippov VA and
Duerksen-Hughes PJ: Interleukin 24: Mechanisms and therapeutic
potential of an anti-cancer gene. Cytokine Growth Factor Rev.
23:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su ZZ, Lebedeva IV, Sarkar D, Emdad L,
Gupta P, Kitada S, Dent P, Reed JC and Fisher PB: Ionizing
radiation enhances therapeutic activity of mda-7/IL-24: Overcoming
radiation- and mda-7/IL-24-resistance in prostate cancer cells
overexpressing the antiapoptotic proteins bcl-xL or bcl-2.
Oncogene. 25:2339–2348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dent P, Yacoub A, Hamed HA, Park MA, Dash
R, Bhutia SK, Sarkar D, Wang XY, Gupta P, Emdad L, et al: The
development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther.
128:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuo B, Wang R, Zhang H, Qin H, Yin Y and
Shi Y: Interleukin-24 inhibits cell migration and invasion in the
neuroblastoma cell line SH-SY5Y. Oncol Rep. 30:2749–2754.
2013.PubMed/NCBI
|
|
16
|
Zhuo B, Wang R, Yin Y, Zhang H, Ma T, Liu
F, Cao H and Shi Y: Adenovirus arming human IL-24 inhibits
neuroblastoma cell proliferation in vitro and xenograft tumor
growth in vivo. Tumour Biol. 34:2419–2426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi H, Wei LL, Yuan CF, Yang JX, Yi FP, Ma
YP and Song FZ: Melanoma differentiation-associated
gene-7/interleukin 24 inhibits invasion and migration of human
cervical cancer cells in vitro. Saudi Med J. 28:1671–1675.
2007.PubMed/NCBI
|
|
19
|
Xiao CW, Xue XB, Zhang H, Gao W, Yu Y,
Chen K, Zheng JW and Wang CJ: Oncolytic adenovirus-mediated
MDA-7/IL-24 overexpression enhances antitumor activity in
hepatocellular carcinoma cell lines. Hepatobiliary Pancreat Dis
Int. 9:615–621. 2010.PubMed/NCBI
|
|
20
|
Fisher PB, Sarkar D, Lebedeva IV, Emdad L,
Gupta P, Sauane M, Su ZZ, Grant S, Dent P, Curiel DT, et al:
Melanoma differentiation associated gene-7/interleukin-24
(mda-7/IL-24): Novel gene therapeutic for metastatic melanoma.
Toxicol Appl Pharmacol. 224:300–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi H, Wyckoff J and Condeelis J:
Cell migration in tumors. Curr Opin Cell Biol. 17:559–564. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carragher NO and Frame MC: Focal adhesion
and actin dynamics: A place where kinases and proteases meet to
promote invasion. Trends Cell Biol. 14:241–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf K, Wu YI, Liu Y, Geiger J, Tam E,
Overall C, Stack MS and Friedl P: Multi-step pericellular
proteolysis controls the transition from individual to collective
cancer cell invasion. Nat Cell Biol. 9:893–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolf K and Friedl P: Extracellular matrix
determinants of proteolytic and non-proteolytic cell migration.
Trends Cell Biol. 21:736–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: Multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khanna C, Wan X, Bose S, Cassaday R, Olomu
O, Mendoza A, Yeung C, Gorlick R, Hewitt SM and Helman LJ: The
membrane-cytoskeleton linker ezrin is necessary for osteosarcoma
metastasis. Nat Med. 10:182–186. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang CY, Lee CY, Chen MY, Yang WH, Chen
YH, Chang CH, Hsu HC, Fong YC and Tang CH: Stromal cell-derived
factor-1/CXCR4 enhanced motility of human osteosarcoma cells
involves MEK1/2, ERK and NF-kappaB-dependent pathways. J Cell
Physiol. 221:204–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iiizumi M, Bandyopadhyay S, Pai SK, Watabe
M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, et
al: RhoC promotes metastasis via activation of the Pyk2 pathway in
prostate cancer. Cancer Res. 68:7613–7620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Q, Lan F, Wang X, Yu Y, Ouyang X,
Zheng F, Han J, Lin Y, Xie Y, Xie F, et al: IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 13:182014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon SK, Kim HM, Lee YC and Kim CH:
Disialoganglioside (GD3) synthase gene expression suppresses
vascular smooth muscle cell responses via the inhibition of ERK1/2
phosphorylation, cell cycle progression, and matrix
metalloproteinase-9 expression. J Biol Chem. 279:33063–33070. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao X, Balan V, Tai G and Raz A:
Galectin-3 induces cell migration via a calcium-sensitive
MAPK/ERK1/2 pathway. Oncotarget. 5:2077–2084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC
and Lu KH: Silibinin suppresses human osteosarcoma MG-63 cell
invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of
MMP-2. Carcinogenesis. 28:977–987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fromigué O, Hamidouche Z and Marie PJ:
Statin-induced inhibition of 3-hydroxy-3-methyl glutaryl coenzyme a
reductase sensitizes human osteosarcoma cells to anticancer drugs.
J Pharmacol Exp Ther. 325:595–600. 2008. View Article : Google Scholar : PubMed/NCBI
|