Introduction
Periostin is an extracellular matrix (ECM) protein
involved in the regulation of intercellular adhesion via
interactions with other ECM proteins including fibronectin,
tenascin-C, collagen V and periostin itself (1,2). Periostin
is highly expressed in early osteoblastic cells in vitro and
in the periosteum and periodontal ligaments in vivo and has
been revealed to serve a role in bone and tooth formation and the
maintenance of structural integrity of these tissues (3). In addition, it has been reported that
the expression of periostin correlates with the severity of heart
failure (4) and that mechanical
stress induces the expression of periostin in fibroblasts of the
heart (5) and periodontal ligaments
(6). Periostin has been frequently
reported to be overexpressed in various types of human cancer cell
lines and tissues, including breast, colon, ovarian and gastric
cancer, non-small cell lung carcinoma and neuroblastoma (7). The overexpression of periostin by cancer
stroma and/or neoplastic epithelium is generally associated with
malignant phenotypes (7). However,
this association was not identified in bladder cancer (8). Previously, we revealed that the
downregulation of periostin mRNA expression was associated
with higher grade human bladder cancer and that ectopic expression
of periostin using a retroviral vector suppressed the in
vitro invasiveness of human bladder cancer cells. We also
demonstrated that periostin suppresses cell invasiveness in human
bladder cancer cell lines via the downregulation of E-cadherin by
suppressing protein kinase B (Akt) phosphorylation and Twist
(9).
The present study investigated the in vivo
tumor suppressor function of periostin in a mouse orthotopic model
of bladder cancer and revealed that periostin suppressed the in
vivo invasiveness of bladder cancer via the
phosphoinositide-dependent kinase-1 (PDK1)/Akt/mammalian target of
rapamycin (mTOR) signaling pathway.
Materials and methods
Orthotopic implantation of bladder
cancer cells
All procedures were performed in compliance with the
Ethical Guidelines for Animal Experimentation and Care and Use of
Laboratory Animals at Shiga University of Medical Science (Otsu,
Japan). The protocol was approved by the Committee on the Ethics of
Research Center for Animal Life Science at Shiga University of
Medical Science. The animals were housed in a specific
pathogen-free room with controlled temperature (20–22°C), humidity
(50–60%) and a pre-set light/dark cycle (12:12 h). Mice were
allowed ad libitum access to food (CE-2; CLEA Japan, Inc.,
Tokyo, Japan) and water. A mouse orthotopic model of bladder cancer
was used as described previously (10,11). A
total of 16, 8-week-old BALB/c-nu-nu female nude mice (ca 20 g;
CLEA Japan, Inc.) were used for the present study. A 24-gauze
catheter was transurethrally inserted into the bladder of nude
mice. In total, 100 µl 0.2% trypsin in 0.02% EDTA was infused and
retained in the bladder for 30 min. Subsequent to trypsinization,
bladders were washed with PBS (−). Subsequently, 100 µl suspensions
of serum-free medium containing 5×106 cancer cells were
instilled into bladders. The urethra was ligated with a 4–0 nylon
suture to ensure the retention of cancer cells. After 3 h, the
suture was removed, and bladder was evacuated by spontaneous
voiding. A total of four weeks subsequent to the instillation of
bladder cancer cells, mice were sacrificed under anesthesia and
weighed. Bladders were excised and weighed. Opened bladder walls
were then longitudinally cut, divided into four equal parts and
histopathologically evaluated. Resected tumors that developed on
the back of the mice were fixed with 10% formalin in PBS for 4 h
and embedded in paraffin. Serial 3-µm sections were used for
histological evaluation using hematoxylin-eosin staining.
Deparaffinization and rehydration of the sections was performed
using 4 incubation steps of xylene and a series of 100% ethanol,
95% ethanol and twice 70% ethanol. Each step was performed for 5
min at room temperature. The sections were deionized using water
for 15 min at room temperature. Subsequently, the sections were
stained with hematoxylin (Merck KGaA, Darmstadt, Germany) for 4 min
and eosin (Eosin Y; Merck KGaA) for 4 min at room temperature. To
visualize these sections, we used a microscope (Eclipse E400; Nikon
Corporation, Tokyo, Japan). Photos were captured using a digital
camera system (DS-2Mv-L2; Eclipse E400; Nikon Corporation).
Mitotic counts in tumors developed in
mouse urinary bladders
Mitotic counts were performed using a standard
laboratory microscope (magnification, ×400; Nikon ECLIPSE E400;
Nikon Corporation, Tokyo, Japan). The mitotic activity index,
mitotic figures/3 high-power fields (hpfs), of mouse orthotopic
bladder tumors was determined using the number of mitotic figures
in three randomly-selected hpfs.
Cell lines
The human bladder cancer UMUC-3 (American Type
Culture Collection, Manassas, VA, USA) cell line was previously
used in an experiment as the mouse orthotopic model of bladder
cancer (11). UMUC-3 and 293T
(American Type Culture Collection) were cultured in RPMI-1640
medium and Dulbecco's modified Eagle's medium (both from Nacalai
Tesque, Inc., Kyoto, Japan), respectively, supplemented with 10%
fetal calf serum (Sigma-Aldrich; Merck KGaA), penicillin (100 U/ml)
and streptomycin (100 µg/ml) at 37°C in a humidified 5%
CO2 atmosphere. The 293T cell line was used for the
preparation of recombinant retroviruses.
Preparation of recombinant retrovirus
and virus infection
The pCXbsr retrovirus vector carrying human
periostin complementary DNA (cDNA) and pCXbsr control vector
(12) were transfected into 293T
cells with the helper plasmid, pCL-ampho (13), using Lipofectamine-Plus reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The amphotropic
retroviruses in the culture medium were collected 48 h subsequent
to transfection, filtered and stored at −80°C until use. For viral
infection, 2×105 cells were seeded onto 60-mm dishes and
cultured overnight at 37°C. Following polybrene treatment (2 µg/ml)
for 30 min, retroviruses were added to cell cultures and incubated
for 1 h at 37°C. Blasticidin-resistant colonies were selected
subsequent to a 7-day incubation in media containing 5 µg/ml
blasticidin (Invitrogen; Thermo Fisher Scientific, Inc.) prior to
pooling and being used in additional assays.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
For RT-PCR, total RNA was isolated from cultured
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Isolated RNA was used for first-strand cDNA synthesis using
Superscript™ II Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) and an Oligo (dT)12–18 primer
(Invitrogen; Thermo Fisher Scientific, Inc.) for 50 min at 42°C.
cDNA was amplified by Taq DNA polymerase (Takara, Otsu, Japan) with
primers specific for periostin: HP16S forward,
5′-GTGGTAGCACCTTCAAAGAAATCC-3′ and HP22A reverse,
5′-GCAACTTCCTCACGGGTGTGTC-3′ (12).
PCR was performed with 30 cycles consisting of denaturation at 94°C
for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for
30 sec, followed by a final extension for 7 min. GAPDH primers were
GAPDH-5F, 5′-ACCACAGTCCATGCCATCAC-3′ and GAPDH-3R,
5′-TCCACCACCCTGTTGCTTGTA-3′. For GAPDH, 30 cycles of PCR were
performed with an annealing temperature of 60°C. PCR products were
separated by electrophoresis on 1.5% agarose gels containing
ethidium bromide prior to imaging using an ultraviolet
transilluminator and printgraph (AE6915; Atto, Co., Tokyo,
Japan).
Pharmacological treatments
IL-6-hydroxymethyl-chiro-inositol
2(R)-2-O-methyl-3-O-octadecylcarbonate (Akt inhibitor; Calbiochem;
EMD Millipore, Billerica, MA, USA), 7-hydroxystaurosporine (UNC-01,
PDK1 inhibitor, Sigma-Aldrich; Merck KGaA) and rapamycin (mTOR
inhibitor, LC Laboratories, Woburn, MA, USA) were prepared in
dimethyl sulfoxide (DMSO) and stored at −20°C. Cells were seeded at
1×106 cells per 60-mm dish and incubated at 37°C for 16
h. Inhibitors (0.1% of culture medium) were then added to culture
media (PDK, 0.1 and 1 µM; Akt, 10 and 25 µM; mTOR, 5 and 20 nM),
and cells were incubated at 37°C for 6 h. In all experiments using
inhibitors, the same volume of DMSO was added to control samples.
Recombinant human periostin protein (r-periostin, SinoBiological,
Inc., Beijing, China) was prepared in PBS(−) and stored at −20°C.
In experiments using r-periostin, the same volume of PBS(−) was
added to control samples.
In vitro invasion assay
The in vitro invasive potential of cells was
determined using Matrigel™ basement membrane matrix
invasion chambers (chamber size, 6.4 mm; membrane surface area, 0.3
cm2; pore size, 8 µm; BD Biosciences, Bedford, MA, USA)
following the manufacturer's protocol. In total, 500 µl cell
suspension (3×104 cells/ml) was added to each chamber.
Chambers containing cells were incubated at 37°C for 2 days in a
humidified 5% CO2 atmosphere. Noninvasive cells were
removed from membrane upper surfaces using cotton swabs. Invasive
cells on membrane undersides were stained with
Diff-Quik™ stain (Kokusai-Shiyaku, Kobe, Japan) and
counted under a light microscope (TMS; Nikon Corporation). Each
sample was assayed in triplicate in three independent
experiments.
Tumorigenicity in nude mice with
subcutaneous injection
The tumorigenicity of cancer cells was determined by
injecting 3×106 cells (in 0.2 ml) subcutaneously into
6-week-old BALB/c-nu/nu female mice. Tumorigenic potential was
evaluated 3 weeks subsequent to inoculation. Mice were sacrificed
under anesthesia, and tumors were excised and weighed.
Antibodies
Anti-α-tubulin (clone, DM1A; #T9026) and anti-Flag
(clone, M2; #F3165) monoclonal antibodies were purchased from
Sigma-Aldrich; Merck KGaA. Anti-phospho Akt (T308 and S473, #13038;
D25E6 and D9E, #4060) rabbit monoclonal, anti-Akt (#9272) rabbit
polyclonal, anti-phospho phosphatidylinositol 3 kinase [PI3K; p85
(Y458)/p55 (Y199)] rabbit polyclonal, anti-PI3K (p85; clone, 19H8;
#4257) rabbit monoclonal, anti-phospho phosphoinositide-dependent
protein kinase 1 (PDK1; S241; #3061) rabbit monoclonal, anti-PDK1
(#3062) rabbit polyclonal, anti-phospho S6 ribosomal protein
(S240/244, clone, D68F8; #5364) rabbit monoclonal, anti-S6
ribosomal protein (clone, 5G10; #2217) rabbit monoclonal and
anti-Snail (clone, C15D3; #3879) rabbit monoclonal antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The anti-E-cadherin (#13116) mouse monoclonal antibody was
purchased from BD Biosciences. The anti-Twist (clone H81;
#sc-15393) rabbit polyclonal antibody was purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). All antibodies were uses at
a dilution of 1:1,000.
Immunoblotting
Cells were lysed in Laemmli-SDS buffer containing
62.5 mM Tris-HCl (pH 6.8), 10% glycerol, 5%2-mercaptoethanol, 2%
SDS, 0.01% bromophenol blue and 5 mM EDTA. SDS-PAGE was performed
on cell lysate samples and separated proteins were
electrotransferred to membrane filters (Immobilon-P; EMD
Millipore). Subsequent to blocking with TBS-T (10 mM Tris-HCl, pH
7.6, 150 mM sodium chloride 0.1% Tween-20) containing 5% bovine
serum albumin (BSA), filters were incubated with primary antibodies
in TBS-T containing 2% BSA overnight. Filters were washed with
TBS-T and incubated for 1 h in horseradish peroxidase-conjugated
anti-mouse or anti-rabbit IgG (NA931 and NA934, respectively; GE
Healthcare Life Sciences, Chalfont, UK) diluted 1:10,000 in TBS-T
containing 2% BSA. Subsequent to several washes with TBS-T,
immunoreactivity was detected using an enhanced chemiluminescence
system (GE Healthcare Life Sciences) according to the
manufacturer's protocol.
Statistical analysis
All quantitative data are presented as means ±
standard deviation estimated from ≥3 replicates per experiment.
Student's t-test and Fisher's exact probability test were used for
statistical analyses. All statistical analyses were performed using
the R statistical software package, version 2.6.2 (R foundation for
Statistical Computing, Vienna, Austria). P<0.05 was considered
to indicate a statistically significant result.
Results
Periostin suppresses the in vitro
invasiveness of the human bladder cancer UMUC-3 cell line
To investigate the ability of periostin to suppress
the in vivo malignant phenotypes of bladder cancer cells in
a mouse orthotopic model, the human bladder cancer UMUC-3 cell line
was used. An amphotropic retrovirus containing the
blasticidin-resistant gene and the periostin gene
tagged with Flag at its C-terminus (pCXbsr/Per) and the control
vector virus (pCXbsr) were introduced into UMUC-3 cells. RT-PCR and
immunoblot analyses demonstrated that UMUC-3 cells infected with
pCXbsr/Per expressed exogenous periostin mRNA and protein
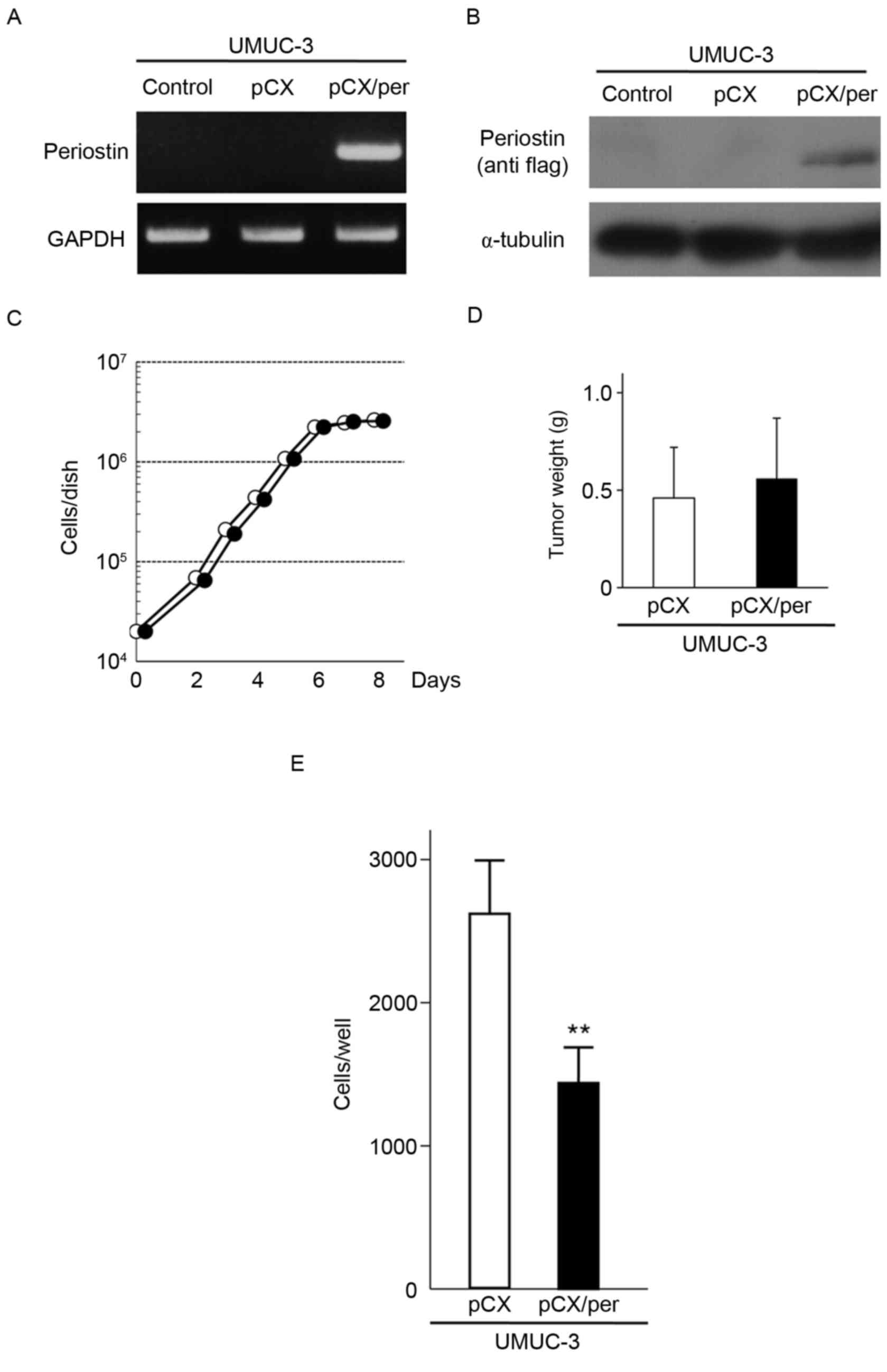
(Fig. 1A and B). The growth rate of
UMUC-3 cells expressing exogenous periostin was similar to that of
control cells infected with the vector virus (Fig. 1C). The tumorigenicity of the two cell
lines was assayed by subcutaneous injection into nude mice. As
shown in Fig. 1D, three weeks
following inoculation, the mean tumor weight from three mice
inoculated with periostin-expressing UMUC-3 cells (0.56±0.31 g)
were almost similar to that from three mice inoculated with vector
control UMUC-3 cells (0.46±0.26 g; P=0.691). However, the in
vitro cell invasiveness of UMUC-3 cells expressing exogenous
periostin was markedly lower compared with control cells infected
with the vector virus (Fig. 1E).
These results indicate that periostin is able to suppress the in
vitro cell invasiveness of UMUC-3 bladder cancer cells without
affecting cellular proliferation and subcutaneous tumor growth in
nude mice.
Periostin suppresses in vivo
invasiveness of UMUC-3 cells in a mouse orthotopic model of bladder
cancer
To assess the ability of periostin to suppress the
in vivo invasiveness and tumorigenicity of UMUC-3 cells,
vector control and periostin-expressing UMUC-3 cells were instilled
into the bladders of seven (group A) and nine (group B) nude mice,
respectively. As shown in Table I, no
differences in body weight were observed between the two groups
(P=0.335) 4 weeks subsequent to the instillation of bladder cancer
cells. The mean weight of bladders instilled with
periostin-expressing UMUC-3 cells (group B, 39.4±11.0 mg) was
significantly lower than bladders instilled with vector control
cells (group A, 61.7±20.0 mg, P=0.013). Tumor formation was
detected in six of seven (85.7%) and five of nine (55.6%) bladders
in vector control UMUC-3 cells (group A) and periostin-expressing
UMUC-3 cells (group B), respectively. A total of five (83.3%) of 6
UMUC-3 bladder tumors containing the vector virus exhibited
histopathological evidence of muscle invasion (Fig. 2A), while none of the five
periostin-expressing UMUC-3 bladder tumors revealed muscle invasion
(P=0.031, Fig. 2B). Thick edematous
lesions were observed in the submucosa of all periostin-expressing
UMUC-3 bladder tumors (Fig. 2B-D).
Tumors were separated from muscular layers by these edematous
lesions in periostin-expressing UMUC-3 bladder tumors. Even in the
bladder tumor-formed mice, the mean weight of bladders instilled
with periostin-expressing UMUC-3 cells (46.6±9.7 mg) was
significantly lower compared with bladders instilled with vector
control cells (66.7±16.3 mg, P=0.039). As no difference in body
weights was observed between the two groups, the differences in
bladder weight were likely a result of bladder tumor size. No
differences in the mitotic activity index, an indicator of
proliferation, were observed between control vector and
periostin-expressing UMUC-3 bladder tumor tissues (Table I, Fig. 2E
and F). These results indicate that periostin is able to
suppress the in vivo invasiveness and tumor growth of UMUC-3
bladder tumors without affecting proliferative activity in a mouse
orthotopic model of bladder cancer.
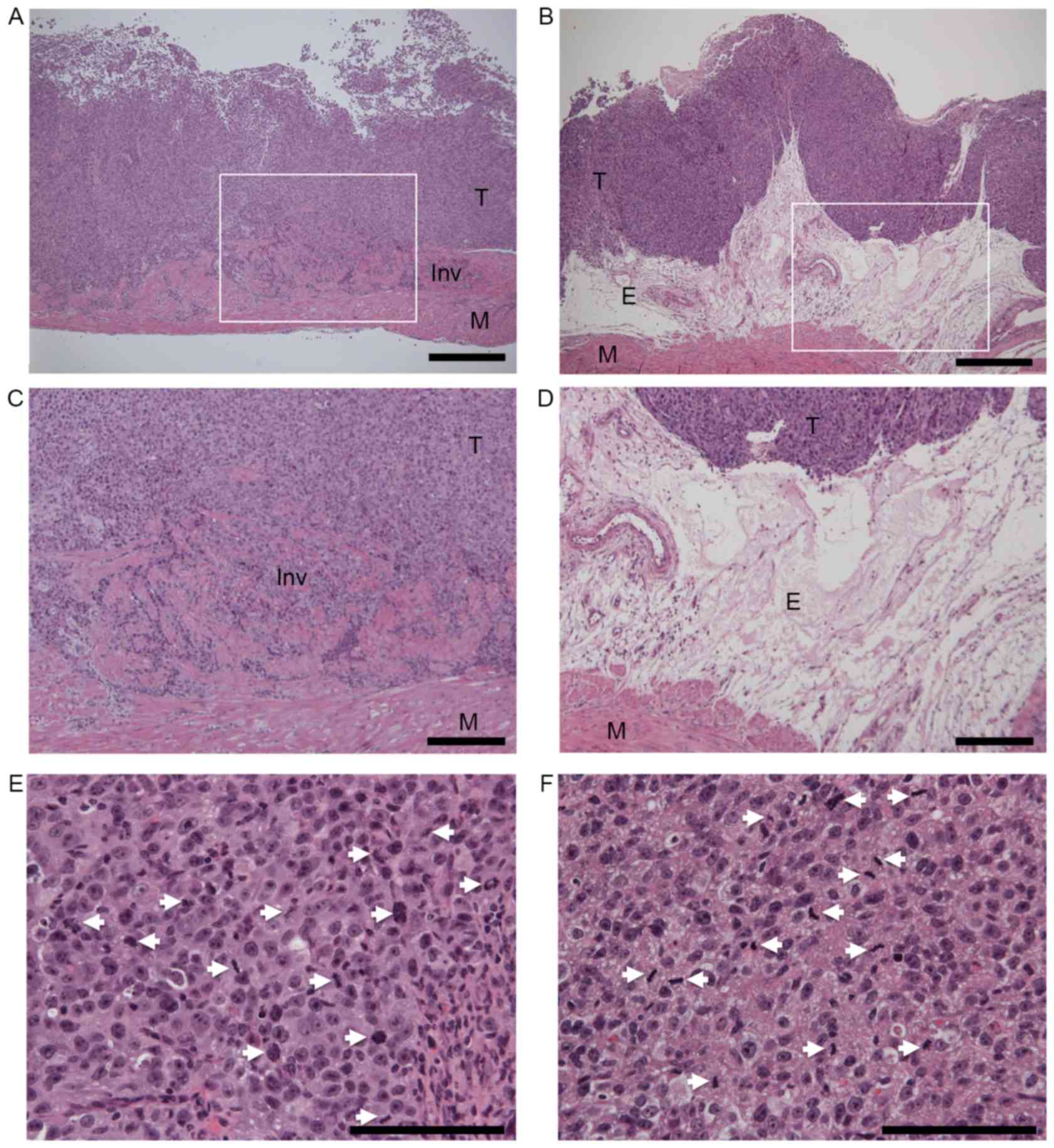 | Figure 2.Histopathological examinations of (A)
vector control and (B) periostin-expressing UMUC-3 bladder tumors
in a mouse orthotopic model of bladder cancer. Hematoxylin and
eosin (HE) stain, magnification ×40. Scale bars represent 500 µm.
(C and D) are higher magnification of (A and B), respectively.
White squares in (A and B) indicate the area of (C and D),
respectively. (C) Invasive growth of vector control UMUC-3 bladder
tumor. (D) Edematous lesions of periostin-expressing UMUC-3 bladder
tumor (magnification, ×100). Scale bars represent 200 µm. (E)
Mitotic figures in vector control and (F) periostin-expressing
UMUC-3 bladder tumors (magnification, ×400). Scale bars represent
100 µm. The white arrows indicate mitotic cells. Mitotic counts
were performed using a standard laboratory microscope
(magnification, ×40). Mitotic activity index (mitotic figures/3
hpfs) of mouse orthotopic bladder tumors was determined using the
number of mitotic figures in three randomly-selected hpfs. T,
tumor; M, muscular layer; E, edematous lesion; Inv, Invasive
growth; hpfs, high power fields. |
 | Table I.Results of the mouse orthotopic model
of bladder cancer using UMUC-3 cells. |
Table I.
Results of the mouse orthotopic model
of bladder cancer using UMUC-3 cells.
| Characteristics | Control | Periostin | P-value |
|---|
| n | 7 | 9 |
|
| Body weight,
ga | 23.8±1.6 | 24.4±1.0 | 0.335 |
| Bladder weight,
mga | 61.7±20.0 | 39.4±11.0 | 0.013 |
| Tumor
formationb | 6/7 (85.7%) | 5/9 (55.6%) | 0.455 |
| Bladder weight of
tumor formed mouse, mga | 66.7±16.3 | 46.6±9.7 | 0.039 |
| Muscle invasion of
bladder tumorb | 5/6 (83.3%) | 0/5 (0%) | 0.031 |
| Mitotic activity
indexa | 12.0±3.0 | 11.9±3.5 | 0.974 |
Effect of periostin on Akt and mTOR
signaling in UMUC-3 cells
To investigate the underlying mechanism of periostin
to suppress invasion, the expression of E-cadherin was examined. As
shown in Fig. 3A, E-cadherin
expression was not detected in periostin-expressing and vector
control UMUC-3 cells. No differences in the expression of Snail or
Twist, known to negatively regulate E-cadherin transcription, were
observed between vector control and periostin-expressing UMUC-3
cells. The association between the expression of periostin and
signaling of Akt-mTOR in bladder cancer was also determined. As
shown in Fig. 3B, exogenous
expression of periostin decreased Akt phosphorylation in UMUC-3
cells. Measurement of phosphorylation of S6 ribosomal protein, a
downstream protein of mTOR kinase, was used to determine mTOR
activity. S6 phosphorylation was also found to be decreased in
periostin-expressing UMUC-3 cells compared with control cells
(Fig. 3B). Phosphorylation of PDK1,
an upstream kinase of Akt, was also suppressed in
periostin-expressing UMUC-3 cells (Fig.
3B). However, phosphorylation of PI3 K, another upstream kinase
of Akt, was not detected in periostin-expressing and control UMUC-3
cells, although PI3 K protein was faintly expressed in the two
types of cell (Fig. 3B). Together,
these results indicate that periostin is able to suppress the
activity of the PDK1/Akt/mTOR pathway in UMUC-3 cells.
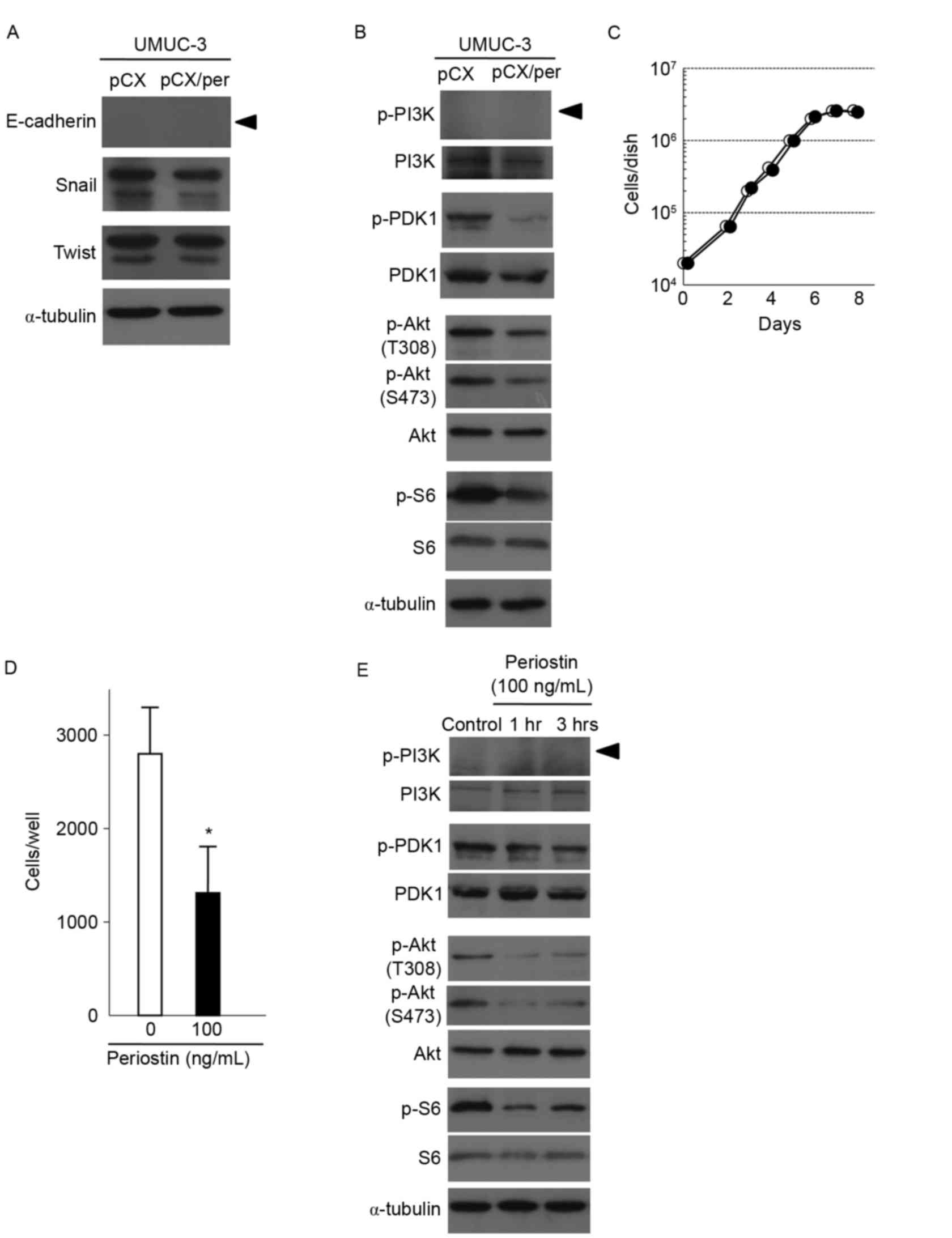 | Figure 3.Effects of the expression of periostin
on PDK1/Akt/mTOR signaling and expression of proteins regulating
epithelial-mesenchymal transition in UMUC-3 cells. (A) Immunoblot
analyses of the EMT-associated proteins E-cadherin, Snail and Twist
in vector control and periostin-expressing UMUC-3 cells. α-tubulin
expression was used as an internal control. The arrowhead indicates
the E-cadherin band. (B) Immunoblot analyses of total and
phosphorylated forms of PDK1, Akt and S6, a downstream protein of
mTOR, in vector control and periostin-expressing UMUC-3 cells.
α-tubulin expression was used as an internal control. The arrowhead
indicates the p-PI3K band. (C) Growth curves of UMUC-3 cells
treated with 100 ng/ml periostin and vector control cells.
Experiments were performed in triplicate. SD was too small to show
as error bars. White and black circles indicate the control and
periostin treatment (100 ng/ml), respectively. (D) Suppression of
cell invasiveness of UMUC-3 cells following treatment with 100
ng/ml periostin for 6 h. Number of cells invading through the
Matrigel is shown. Bar, ± SD of triplicate chambers for each
experiment. *P=0.046 vs. control cells. (E) Immunoblot analyses of
total and phosphorylated forms of PDK1, Akt and S6 in control
UMUC-3 cells and cells treated with 100 ng/ml periostin for 1 and 3
h. α-tubulin expression was used as an internal control. The
arrowhead indicated the p-PI3K band. PDK1,
phosphoinositide-dependent kinase-1; Akt, protein kinase B; mTOR,
mammalian target of rapamycin; SD, standard deviation. |
Treatment with r-periostin suppresses
cell invasiveness and PDK1/Akt/mTOR signaling in UMUC-3 cells
The present study assessed the effect of treatment
with r-periostin on cell invasiveness and PDK1/Akt/mTOR signaling.
The UMUC-3 cells were treated with 100 ng/ml of r-periostin. As
revealed in Fig. 3C and D,
r-periostin treatments suppressed in vitro cell invasiveness
without affecting cellular proliferation. As shown in Fig. 3E, phosphorylation of PDK1, Akt and S6
was also suppressed following treatment with r-periostin for 1 and
3 h. These results are consistent with the results of experiments
using periostin-expressing UMUC-3 cells (Figs. 1, 3A and
B).
Effects of PDK1, Akt, and mTOR
inhibition on in vitro cell invasiveness of UMUC-3 cells
To assess the contribution of PDK1/Akt/mTOR
signaling to the suppression of cell invasiveness by periostin, the
present study evaluated the effects of inhibitors of PDK1 (0.1 and
1 µM), Akt (10 and 25 µM) and mTOR (5 and 20 nM) on the
invasiveness of UMUC-3 cells. Immunoblot analysis demonstrated that
respective inhibitors effectively suppressed the phosphorylation of
PDK1, Akt and S6 without affecting PDK1, Akt and S6 protein levels
(Fig. 4A-C). Akt inhibitors also
suppressed the phosphorylation of S6. Following incubation with
inhibitors for 6 h, cells were inoculated into Matrigel chambers
without the inhibitors. As shown in Fig.
4D-F, treatment with each of the inhibitors significantly
suppressed the in vitro cell invasiveness of UMUC-3 cells.
No effect on cellular proliferation of UMUC-3 cells was observed
with any of the inhibitors (Fig. 4G).
These results indicate that PDK1/Akt/mTOR signaling is required for
the suppression of cell invasiveness by periostin in UMUC-3 cells
(Fig. 4H).
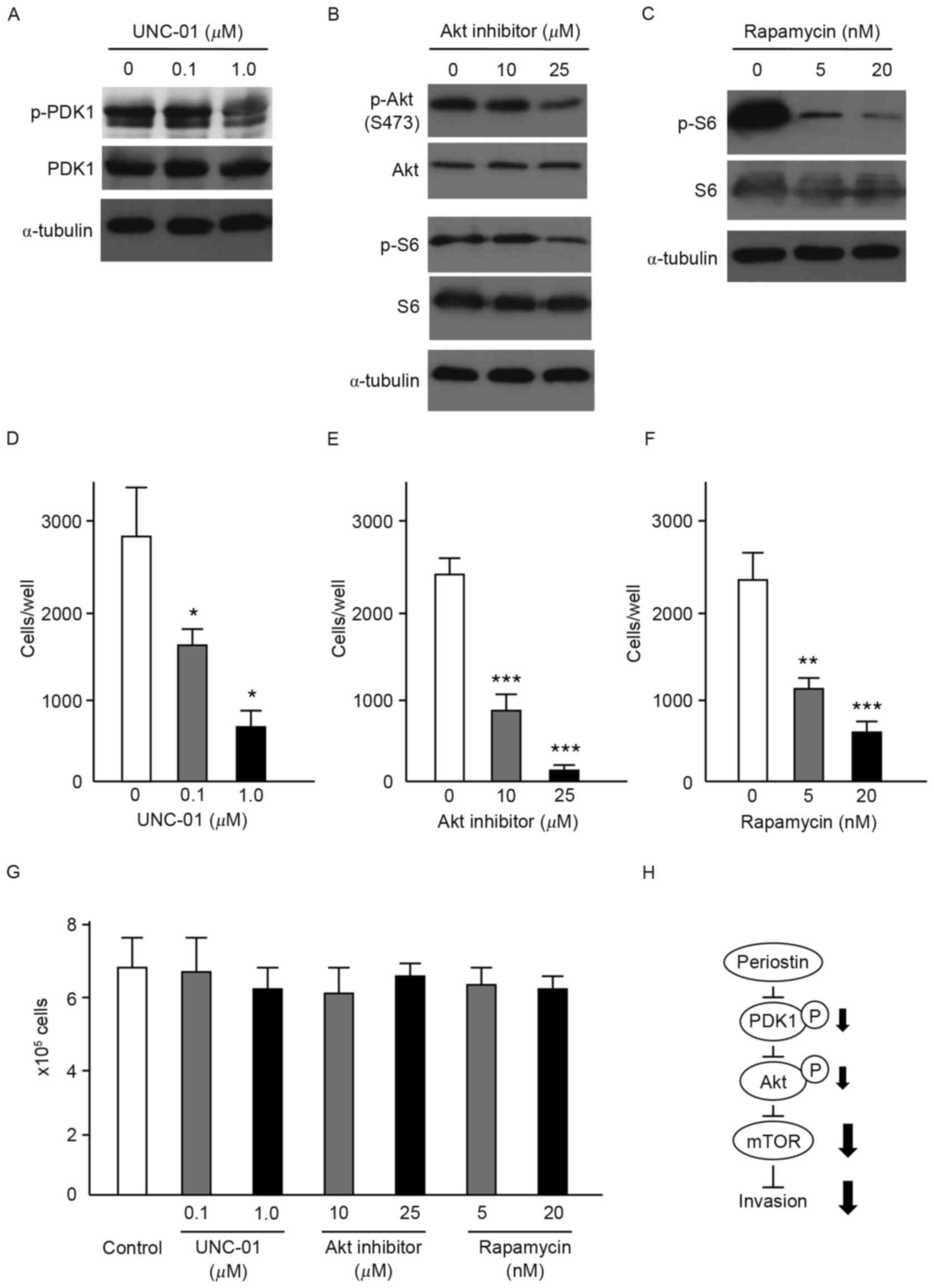 | Figure 4.Effects of PDK1, Akt and mTOR
inhibitors on the in vitro cell invasiveness of UMUC-3
cells. (A) Immunoblot analysis of UMUC-3 cells treated with (A) the
PDK1 inhibitor, UNC-01 (0.1 and 1 µM), (B) Akt inhibitor (10 and 25
µM) and (C) the mTOR inhibitor, rapamycin (5 and 20 nM). Expression
of α-tubulin was used as an internal control. Effect of (D) PDK1
inhibitor, (E) Akt inhibitor and (F) mTOR inhibitor on the cell
invasiveness of UMUC-3 cells. Number of cells invading through the
Matrigel is shown. Each sample was assayed in triplicate. Bars, ±
SD of triplicate chambers for each experiment. *P<0.05,
**P=0.003, ***P<0.001 vs. untreated control cells. (G) Cell
proliferation of untreated control UMUC-3 cells and UMUC-3 cells
treated with PDK1, Akt and mTOR inhibitors. Cells grown in
triplicate plates were trypsinized and counted. Bars, ± SD of
triplicate culture dishes for each experiment. (H) Schematic
illustration of the signaling pathway responsible for the
suppressive effect of periostin on the invasiveness of UMUC-3
bladder cancer cells. PDK1, phosphoinositide-dependent kinase-1;
Akt, protein kinase B; mTOR, mammalian target of rapamycin; SD,
standard deviation. |
Discussion
The present study demonstrated that periostin is
able to suppress the in vivo invasiveness of bladder cancer
cells in a mouse orthotopic model system. Although the difference
in in vitro and in vivo proliferation was not
observed between the control vector and periostin-expressing UMUC-3
cells, periostin-expressing UMUC-3 bladder tumors were smaller
compared with the control UMUC-3 bladder tumors. The suppression of
tumorigenicity by periostin may be due to decreased numbers of
cancer cells implanting into the bladder surface, as
periostin-expressing UMUC-3 bladder tumors exhibited reduced
invasive ability. Notably, thick edematous lesions were observed in
the submucosa of periostin-expressing UMUC-3 bladder tumors. The
development of thick edematous lesions in response to periostin may
contribute to the suppression of tumor growth and invasion.
However, the mechanism underlying the formation of edematous
lesions in the submucosa of periostin-expressing UMUC-3 bladder
tumors has yet to be elucidated. Edematous lesions were not
detected in periostin-expressing UMUC-3 subcutaneous tumors in nude
mice, and no difference in tumorigenicity was observed between the
control vector and periostin-expressing UMUC-3 subcutaneous tumors
in nude mice. Opposing effects of periostin on differing cancer
types suggest tissue-specific functions of periostin (9). Organ-specific fibroblasts may contribute
to the suppressive effects of periostin on in vivo tumor
invasiveness.
Furthermore, the present study revealed that the
PDK1/Akt/mTOR signaling pathway is required for the suppressive
effects of periostin on the invasiveness of UMUC-3 cells. We
previously identified the suppression of in vitro cell
invasiveness by periostin in the human bladder cancer cell lines,
SBT31A and T24. In these cells, periostin upregulated the
expression of E-cadherin via the suppression of Akt phosphorylation
and Twist (9). However, ectopic
expression of periostin in UMUC-3 bladder cancer cells did not
induce E-cadherin expression. Suppression of PI3K activity via a
negative feedback mechanism may be responsible for the differing
responses between T24 and UMUC-3 cells as a result of the
homozygous null deletion of PTEN in UMUC-3 cells (14). Alterations of PTEN, encompassing
homozygous gene deletion, loss of heterozygosity, and gene
mutation, have been identified in ~30% of patients with bladder
cancer (15). However, the effect of
PTEN inactivation on downstream signal cascades, such as mTOR, in
bladder cancer has not yet been fully evaluated. UMUC-3 cells
exhibit utility in elucidating the molecular mechanisms underlying
carcinogenesis in bladder cancer cells deficient in PTEN. The
PI3K/Akt/mTOR signaling pathway has recently been identified as a
candidate pathway in bladder cancer progression by
clinicopathological studies (14,16).
mTOR-containing multiprotein complex 1 (mTORC1) regulates cell
growth, proliferation and survival, whereas mTORC2 controls
cytoskeletal remodeling and cancer cell adhesion and migration
(17,18). Previous studies have demonstrated that
rapamycin-sensitive mTORC1 serves a critical role in the regulation
of cell motility and invasion (19–21). In
the present study, rapamycin clearly suppressed the invasiveness of
UMUC-3 cells, indicating that mTORC1 is required for the
suppressive effects of periostin on the cell invasiveness of
bladder cancer. The PDK1/Akt/mTOR signaling pathway may constitute
a targetable signaling pathway for therapies against bladder cancer
invasion.
In clinical cases, non-muscle invasive bladder
cancer is predominantly treated by transurethral resection of
bladder tumor (TUR-Bt). However, the intravesical recurrence rate
of bladder cancer following TUR-Bt has been revealed to be 50–70%
(22,23). The majority of the recurrence of
bladder cancer is recognized as the result of intraluminal seeding.
Surgical injury of TUR-Bt disseminates a large number of cancer
cells, and forms the mucosal defect, resulting in the implantation
of cancer cells in the bladder surface (24,25).
Therefore, the prevention of early seeding following TUR-Bt is
crucial. Indeed, a single intravesical instillation of a
chemotherapeutic agent immediately following TUR-Bt significantly
decreased the risk of recurrence in patients with bladder cancer
(26,27). In the present study, cancer cells were
instilled into bladders following trypsin treatment of the bladder
surface in a mouse orthotopic model of bladder cancer. This system
resembles the implantation of cancer cells subsequent to TUR-Bt.
The invasiveness of cancer cells is closely associated with tumor
cell seeding. In the present study, periostin was revealed to
suppress the invasiveness and tumorigenicity of cancer cells in a
mouse orthotopic model of bladder cancer. This suppression may
result from the decreased number of the implanted cancer cells on
bladder surface by the function of periostin. Treatment with
r-periostin (100 ng/ml) was able to suppress the in vitro
invasiveness of bladder cancer cells via modulation of
PDK1/Akt/mTOR signaling. Periostin may exhibit utility as a potent
chemotherapeutic agent for use in intravesical treatment following
TUR-Bt by suppressing bladder cancer invasiveness.
Acknowledgements
The authors would like to thank Ms. Akiyo Ushio
(Division of Microbiology and Infectious Diseases, Shiga University
of Medical Science) for her technical assistance. The present study
was supported by a Grant-in Aid for Scientific Research (C) (grant
nos. 25462476 and 24590480) from the Ministry of Education,
Science, Sports, and Culture of Japan.
References
|
1
|
Hwang EY, Jeong MS, Park EK, Kim JH and
Jang SB: Structural characterization and interaction of periostin
and bone morphogenetic protein for regulation of collagen
cross-linking. Biochem Biophys Res Commun. 449:425–431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Norris RA, Borg TK, Butcher JT, Baudino
TA, Banerjee I and Markwald RR: Neonatal and adult cardiovascular
pathophysiological remodeling and repair: Developmental role of
periostin. Ann N Y Acad Sci. 1123:30–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periostium and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsuragi N, Morishita R, Nakamura N,
Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T
and Sugimura K: Periostin as a novel factor responsible for
ventricular dilation. Circulation. 110:1806–1813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang D, Oparil S, Feng JA, Li P, Perry G,
Chen LB, Dai M, John SW and Chen TF: Effects of pressure overload
on extracellular matrix expression in the heart of the atrial
natriuretic peptide-null mouse. Hypertension. 42:88–95. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilde J, Yokozeki M, Terai K, Kudo A and
Moriyama K: The divergent expression of periostin mRNA in the
periodontal ligament during experimental tooth movement. Cell
Tissue Res. 312:345–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim CJ, Yoshioka N, Tambe Y, Kushima R,
Okada Y and Inoue H: Periostin is down-regulated in high grade
human bladder cancers and suppresses in vitro cell invasiveness and
in vivo metastasis of cancer cells. Int J Cancer. 117:51–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim CJ, Sakamoto K, Tambe Y and Inoue H:
Opposite regulation of epithelial-to-mesenchymal transition and
cell invasiveness by periostin between prostate and bladder cancer
cells. Int J Oncol. 38:1759–1766. 2011.PubMed/NCBI
|
|
10
|
Watanabe T, Shinohara N, Sazawa A,
Harabayashi T, Ogiso Y, Koyanagi T, Takiguchi M, Hashimoto A,
Kuzumaki N, Yamashita M, et al: An improved intravesical model
using human bladder cancer cell lines to optimize gene and other
therapies. Cancer Gene Ther. 7:1575–1580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka M, Gee JR, De La Cerda J, Rosser
CJ, Zhou JH, Benedict WF and Grossman HB: Noninvasive detection of
bladder cancer in an orthotopic murine model with green
fluorescence protein cytology. J Urol. 170:975–978. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim CJ, Isono T, Tambe Y, Chano T, Okabe
H, Okada Y and Inoue H: Role of alternative splicing of periostin
in human bladder carcinogenesis. Int J Oncol. 32:161–169.
2008.PubMed/NCBI
|
|
13
|
Naviaux RK, Costanzi E, Haas M and Verma
IM: The pCL vector system: Rapid production of helper-free,
high-titer, recombinant retroviruses. J Virol. 70:5701–5705.
1996.PubMed/NCBI
|
|
14
|
Hansel DE, Platt E, Orloff M, Harwalker J,
Sethu S, Hicks JL, De Marzo A, Steinle RE, His ED, Theodorescu D,
et al: Mammalian target of rapamycin (mTOR) regulates cellular
proliferation and tumor growth in urothelial carcinoma. Am J
Pathol. 176:3062–3072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gildea JJ, Herlevsen M, Harding MA,
Gulding KM, Moskaluk CA, Frierson HF and Theodorescu D: PTEN can
inhibit in vitro organotypic and in vivo orthotopic invasion of
human bladder cancer cells even in the absence of its lipid
phosphatase activity. Oncogene. 23:6788–6797. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan X, Mendoza A, Khanna C and Helman LJ:
Rapamycin inhibits ezrin-mediated metastatic behavior in a murine
model of osteosarcoma. Cancer Res. 65:2406–2411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Busch S, Renaud SJ, Schleussner E, Graham
CH and Markert UR: mTOR mediates human trophoblast invasion through
regulation of matrix-remodeling enzymes and is associated with
serine phosphorylation of STAT3. Exp Cell Res. 315:1724–1733. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berven LA, Willard FS and Crouch MF: Role
of the p70(S6K) pathway in regulating the actin cytoskeleton and
cell migration. Exp Cell Res. 296:183–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Li F, Cardelli JA, Martin KA,
Blenis J and Huang S: Rapamycin inhibits cell motility by
suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene.
25:7029–7040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Chen L, Chung J and Huang S:
Rapamycin inhibits F-actin reorganization and phosphorylation of
focal adhesion proteins. Oncogene. 27:4998–5010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lutzeyer W, Rübben H and Dahm H:
Prognostic parameters in superficial bladder cancer: An analysis of
315 cases. J Urol. 127:250–252. 1982.PubMed/NCBI
|
|
23
|
Herr HW, Laudone VP and Whitmore WF Jr: An
overview of intravesical therapy for superficial bladder tumors. J
Urol. 138:1363–1368. 1987.PubMed/NCBI
|
|
24
|
Soloway MS and Masters S: Urothelial
susceptibility to tumor cell implantation: Influence of
cauterization. Cancer. 46:1158–1163. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
See WA, Miller JS and Williams RD:
Pathophysiology of transitional tumor cell adherence to sites of
urothelial injury in rats: Mechanisms mediating intravesical
recurrence due to implantation. Cancer Res. 49:5414–5418.
1989.PubMed/NCBI
|
|
26
|
Sylvester RJ, Oosterlinck W and van der
Meijden AP: A single immediate postoperative instillation of
chemotherapy decreases the risk of recurrence in patients with
stage Ta T1 bladder cancer: A meta-analysis of published results of
randomized clinical trials. J Urol. 171:2186–2190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamura K, Ono Y, Kinukawa T, Matsuura O,
Yamada S, Ando T, Fukatsu T, Ohno Y and Ohshima S: Nagoya
University Urological Oncology Group: Randomized study of a single
early instillation of (2′'R)-4′-O-tetrahydropyranyl-doxorubicin for
a single superficial bladder carcinoma. Cancer. 94:2363–2368. 2002.
View Article : Google Scholar : PubMed/NCBI
|