Introduction
Clinical statistics show that the bone is often
involved in tumor metastasis during the late stages of most tumors
(1). For example, the tumor cells of
breast cancer, colon cancer, and lung cancer can be found in bone
tissue (2). Clinical statistics also
show that the osseous tumor metastasis would cause clinical
symptoms (3) such as osteoporosis and
pathological fracture by influencing sclerotin and joints (4). The incidence of osteosarcoma covers
45.3% of all types of bone tumors, and is the main morbidity among
teenagers (5). Although research on
bone tumors has made some progress, its nosogenesis remains to be
elucidated, although its occurrence is influenced by genetic and
environmental factors, such as nutrition, and exercise (6).
Previous findings showed that fatty acid synthase
(FASN) plays an important role in fatty acid synthesis and its
inhibition prevents the proliferation of malignant cells and
promotes apoptosis (7). Moreover,
tumors express high amounts of acid such as fatty acid, suggesting
a key role of FASN in cancer cell metabolism. In addition, the
mitogen-activated protein kinase (MAPK)/P53 signaling pathway is
activated in many tumors (8). For
example, the expression level of MAPK/P53 is significantly
increased in tumor cells from breast and colon cancer. The MAPK/P53
signaling pathway regulates the expression of FASN in breast cancer
cells to control cell proliferation (9). However, the role of FASN and MAPK/P53
signaling in bone tumor and the functional relationship of these
molecules are currently unknown.
The aim of the present study was to determine the
correlation between FASN and MAPK/P53 signaling and the incidence
of bone tumors. The aim was to provide theoretical and experimental
support to understand the role of these molecules in the
pathogenesis of bone tumors.
Materials and methods
Cell culture and treatment
The SH081 bone tumor cell line was established and
maintained in our laboratory. We cultured the SH081 cell line at
3°C, 5% CO2, and collected the cells at 90% confluence.
The cells were centrifuged for 10 min at 1,500 × g at 4°C, and then
the fluid was added and the cells were preserved at −80°C. MAPK/P53
signaling pathway (100 µl, 100 ml) blocker (Calbiochem, Billerica,
MA, USA) was added in observation group 2 during culture, and the
cells were cultured at 37°C, 5% CO2. Other materials
used were: dimethyl sulphoxide, fetal bovine serum and trypsin.
RT-qPCR
RNA extraction: We cultured the bone tumor cells at
37°C, 5% CO2, extracted total RNA and measured the
amount of RNA (2). For qPCR the
expression of MAPK/P53 and FASN mRNA in cells was analyzed. This
study generated cDNA by reverse transcription of RNA as template
for qPCR (Applied Biosystems Life Technologies, Foster City, CA,
USA). The primer sequences are shown in Table I. Other materials used were:
Transilluminator (Bio-Rad, Munchen, Germany) and high speed and low
temperature refrigerated centrifuge (Hitachi Ltd., Tokyo,
Japan).
 | Table I.qPCR primers. |
Table I.
qPCR primers.
| Primers | Primer sequences |
|---|
| MAPK/P53-F |
GTCGATCGTCGATCGCTACGC |
| MAPK/P53-R |
CGTAGCTAGTCGATCGACTAGC |
| FASN-F |
TGCTAGCTGATCGATCGATCGTCG |
| FASN-R |
CGTAGCTGATCGATGCTAGCTAGC |
| GAPDH-F |
TGCTAGGCTAGGACGCTAGCTAC |
| GAPDH-R |
CTGGGCTAGATCGACGAGAGCTC |
Enzyme-linked immunosorbent assay
(ELISA) response
We followed the manufacturer's recommendation using
the ELISA kit (Takara Bio, Dalian, China) with minor modifications
(10). The standard protein sample
was diluted in assay buffer at 1:50 and the standard curve was made
according to the instruction. The samples were diluted in PBS (pH
7.2) at 1:100. We added 100 µl of the sample mix in each well, and
added 50 µl of detection solution. TMB chromogenic substrate was
used after the sample was incubated at room temperature for 2 h.
Light absorption was measured at 495 nm, and the concentration of
MAPK/P53 and FASN in each sample was calculated according to the
standard curve.
Western blot analysis
The expression of APC in different samples was
detected by western blot analysis. Total protein extracted with the
animal cell total protein extraction kit was performed according to
the ‘Guide of Molecular Clone’, 3rd edition. The primary antibody
used was diluted at 1:800 and the secondary antibody was diluted at
1:500. Monoclonal and polyclonal, and animal origin are referred to
a previous study (2).
Detection of cell proliferation by
MTT
The procedure was performed as previously described
(10,11).
Statistical analysis
Data were processed and analyzed by SPSS 20.0
(Chicago, IL, USA). When the test level was α=0.05, the difference
was significant at P<0.05. When test level was α=0.01, the
difference was significant at P<0.01.
Results
MAPK/P53 and FASN mRNA expression in
normal and bone tumor cells
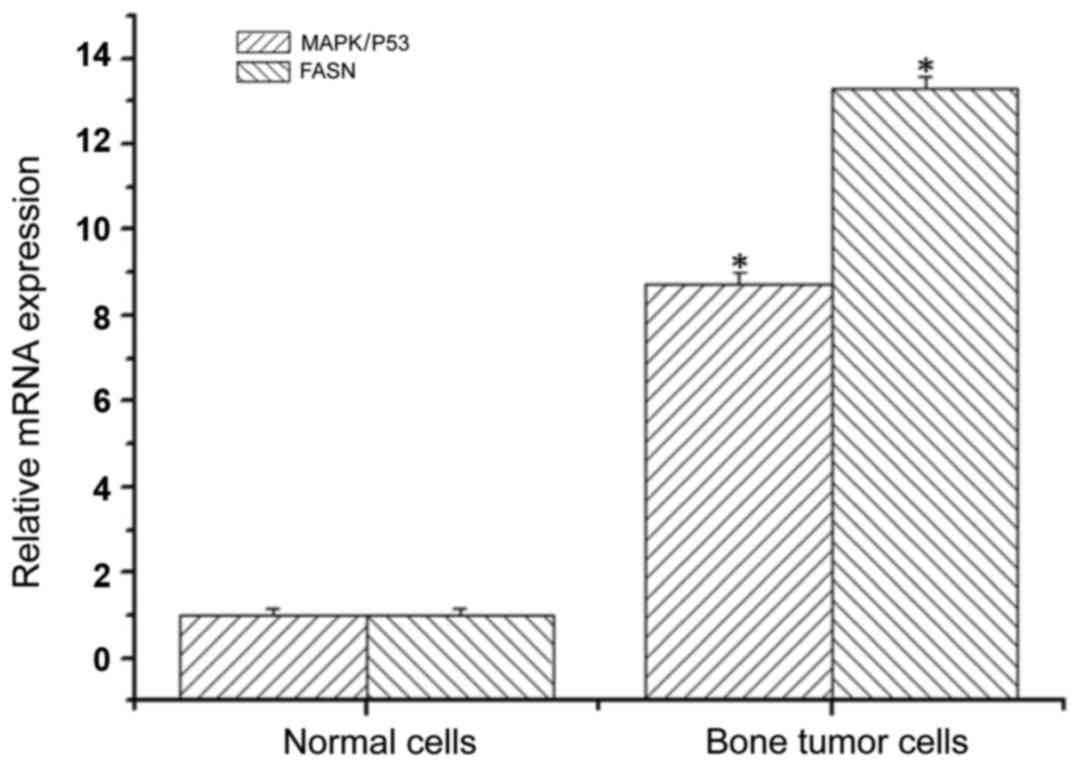
Using SH081 bone tumor cells and normal cells, total
RNA was extracted to determine the expression levels of MAPK/P53
and FASN mRNA by qPCR. The expression level of MAPK/P53 mRNA in
bone tumor cells was 8.74-fold higher than that in the normal
cells, and the difference was statistically significant (Fig. 1). The expression levels of FASN mRNA
in bone tumor cells was 13.3 times higher compared to normal cells,
and statistically significant (Fig.
1). This result indicated robust upregulation of MAPK/P53 and
FASN in bone tumors.
MAPK/P53 and FASN mRNA expression
following MAPK/P53 inhibition
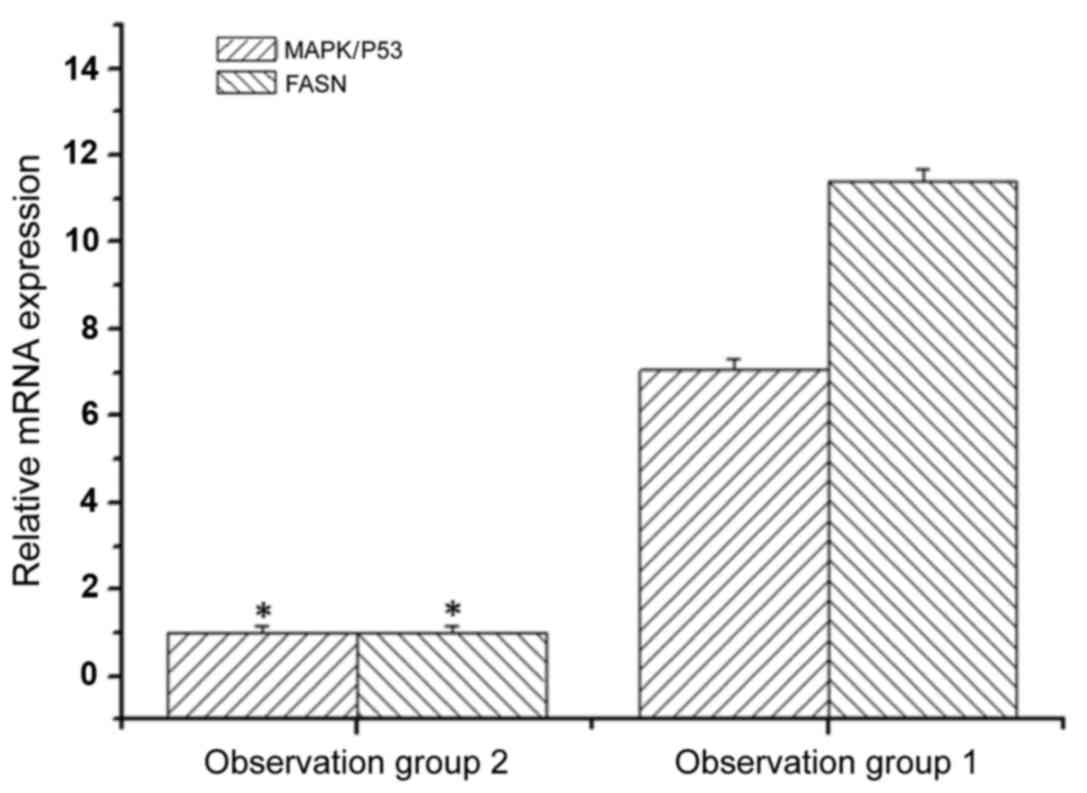
We then examined the consequence of inhibiting
MAPK/P53 in bone tumor cells. MAPK/P53 and FASN mRNA levels were
analyzed in treated and non-treated SH081 bone tumor cells by qPCR.
The expression level of MAPK/P53 mRNA in the non-treated bone tumor
cells was 7.05-fold higher than the level in bone tumor cells
treated with MAPK/P53 inhibitor (Fig.
2). The expression levels of FASN mRNA in the non-treated cells
was 11.4-fold higher compared to the SH081 cells treated with
MAPK/P53 inhibitor (Fig. 2). These
results indicated that MAPK/P53 regulates the expression of FASN
gene in bone tumor cells.
Determination of MAPK/P53 and FASN
expression using western blot analysis
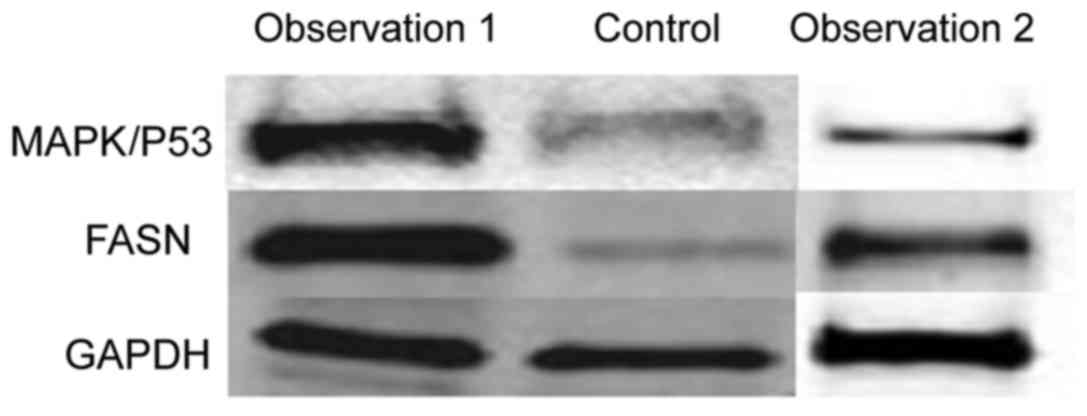
To verify the changes in mRNA, we extracted total
protein extracted from normal cells and SH081 bone tumor cells and
measured the expression of the MAPK/P53 and FASN proteins by
western blot analysis. The expression levels of MAPK/P53 and FASN
proteins in normal cells were significantly lower than in the bone
tumor cells (Figs. 3 and 4). This result was in accordance with the
results observed by qPCR.
Expression of MAPK/P53 and FASN by
ELISA
To verify the changes in MAPK/P53 and FASN proteins
by a more quantitative technique, we measured the expression by
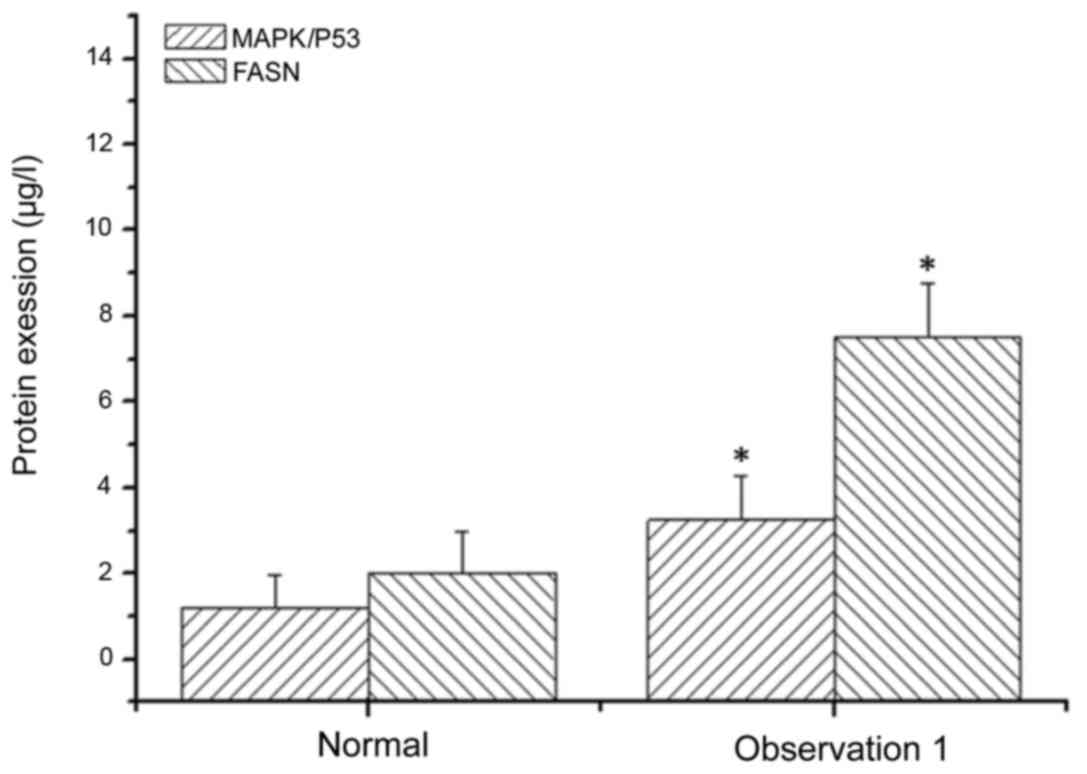
ELISA. The expression level of MAPK/P53 in SH081 bone tumor cells
was 3.25±1.03 µl/l, but significantly higher in normal cells
(1.21±0.76 µl/l) (Fig. 5). The
expression level FASN in normal cells was 2.03±0.96 µl/l, which was
significantly lower than in bone tumor cells (7.52±1.25 µl/l)
(Fig. 5).
Expression of MAPK/P53 and FASN
proteins after MAPK/P53 inhibition by ELISA
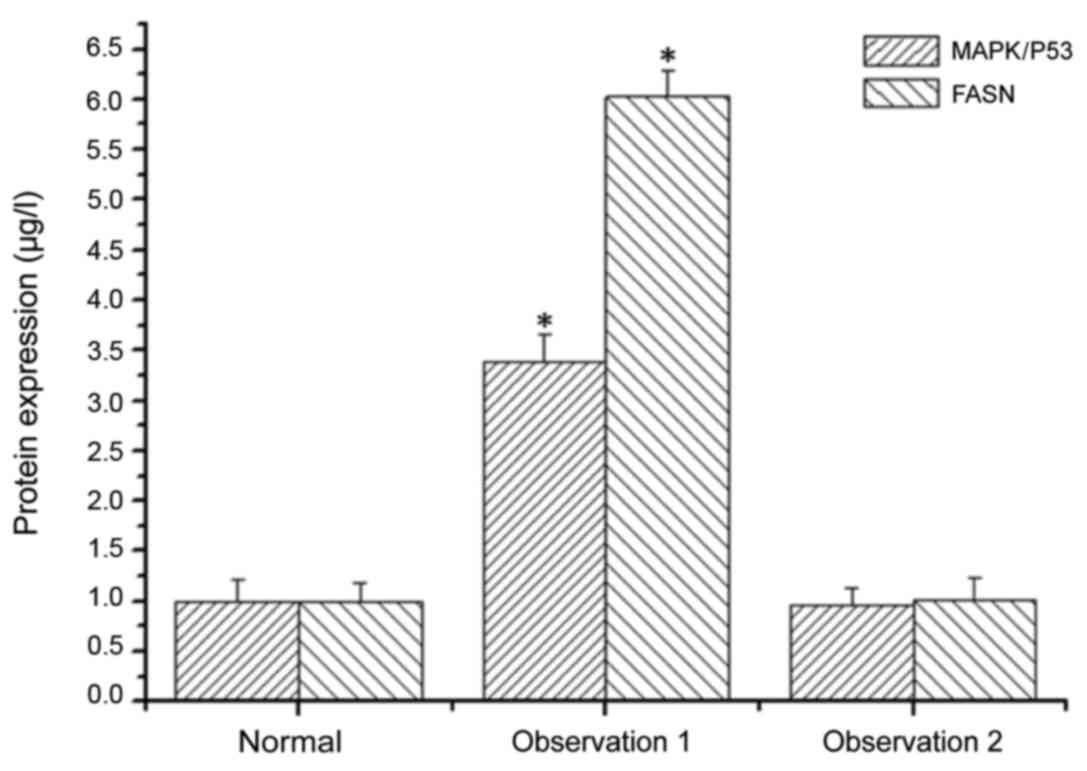
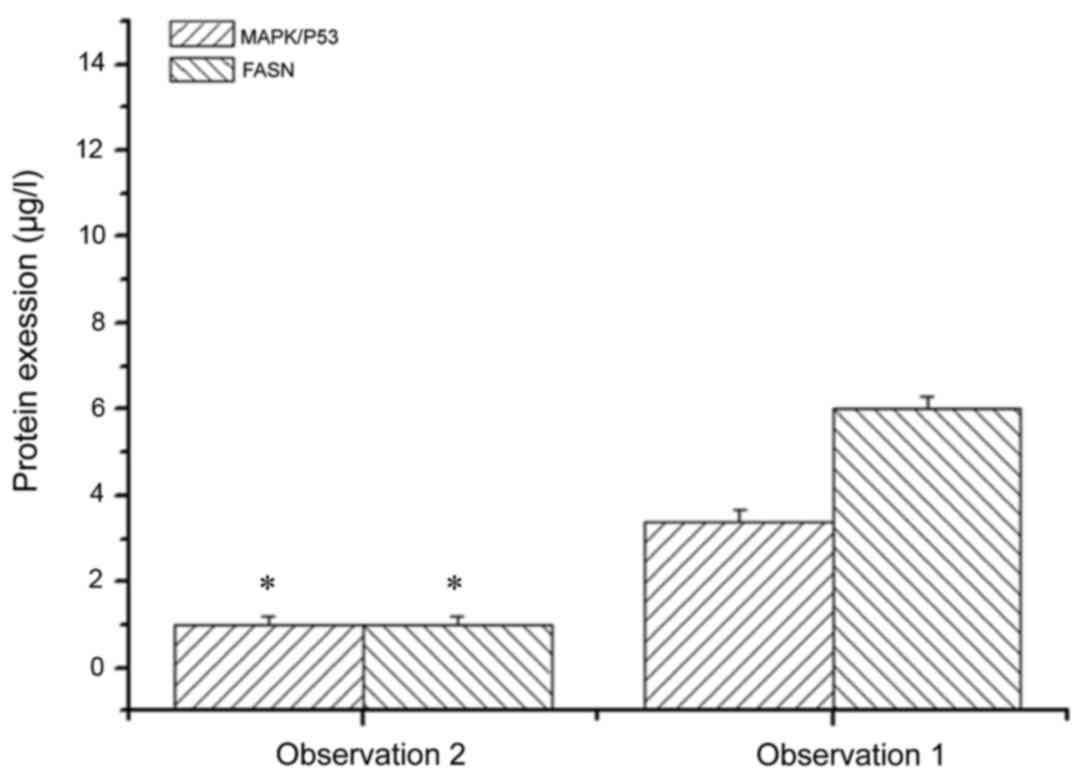
The expression levels of MAPK/P53 and FASN in SH081
bone tumor cells treated and not treated with MAPK/P53 inhibitor
were examined. The expression level of MAPK/P53 in bone tumor cells
not treated with MAPK/P53 inhibitor was 3.39-fold higher than bone
tumor cells treated with MAPK/P53 inhibitor (Fig. 6). The expression levels of FASN in
bone tumor cells not treated with MAPK/P53 inhibitor was 6.03-fold
higher than in bone tumor cells treated with MAPK/P53 inhibitor
(Fig. 6). These results indicated
that MAPK/P53 regulates the expression of FASN in bone tumor cells,
which was consistent with the qPCR and western blot results.
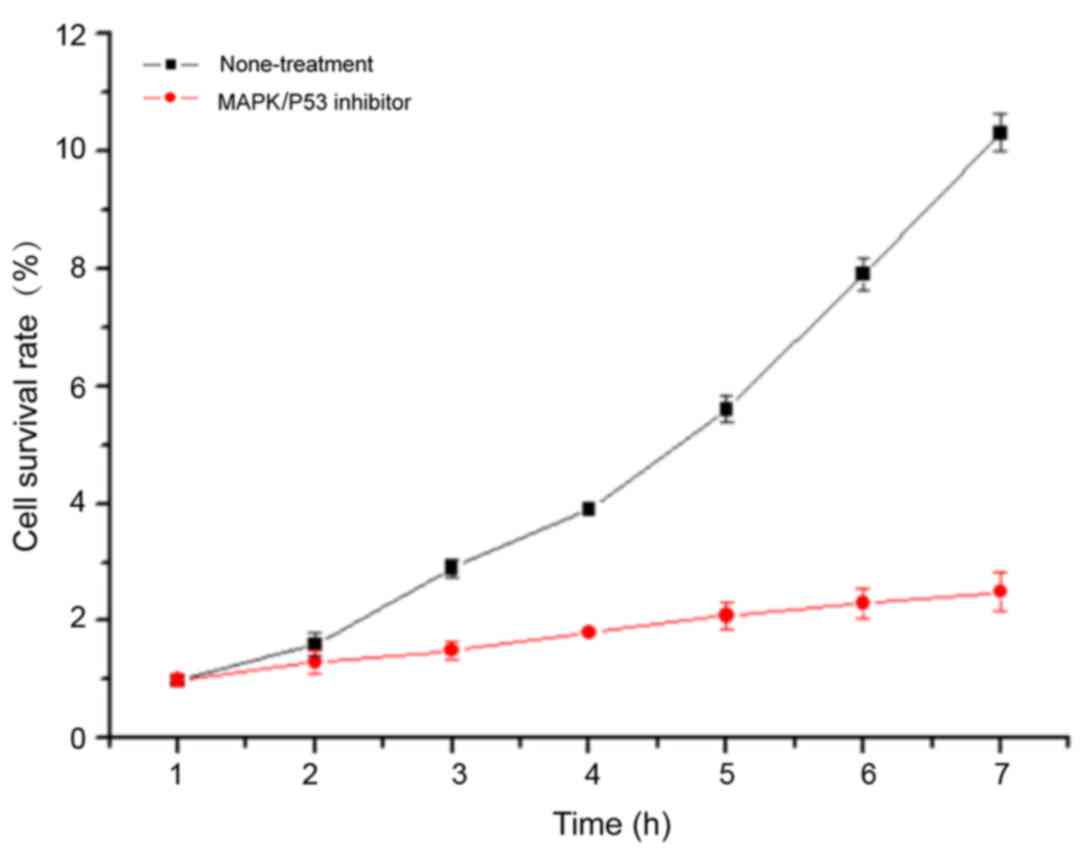
Proliferation of bone tumor cells
treated with MAPK/P53 inhibitor
The cell proliferation of bone tumor cells treated
and not treated with MAPK/P53 inhibitor was assessed to determine
the functional relevance of the activation of the MAPK/P53
signaling pathway. Compared with the SH081 cells not treated with
MAPK/P53 inhibitor, the cell proliferation of bone tumor cells
treated with MAPK/P53 inhibitor was significantly reduced (Fig. 7). This result suggested that the
MAPK/P53 signaling pathway promotes the proliferation of bone tumor
cells.
Discussion
FASN in cells mainly participates in the metabolic
process of acidic materials such as fatty acid (12). Moreover, FASN has various enzymatic
activities such as condensation, dehydration, and polymerization in
plants. Previously, it has been found that the pH of the bone tumor
fluid was significantly lower than that in normal tissues. Analysis
of the main constituents of bone tumor fluids shows that the
content of acidic materials such as fatty acid is elevated,
suggesting that in bone tumor the metabolism and synthesis of fatty
acids and other acidic materials undergo pathological changes. The
expression level of FASN in tumor cells such as breast and bladder
cancer cells was apparently higher than that in normal cells
(13). Breast cancer cells treated
with FASN inhibitor exhibit a lower rate of proliferation and a
higher ratio of apoptosis, which suggests that FASN regulates
proliferation and apoptosis in breast cancer. Further research
shows that in colon cancer cells FASN inhibits cell growth cycle
transition from S to G2/M stage (10), providing theoretical support for FASN
inhibition of cancer cell proliferation.
The MAPK signaling pathway is a main signal
transduction pathway closely connected with several stress
reactions, and physical and chemical reactions within the cells.
For example, MAPK signaling was involved in the cell response to
outside stimuli by activating and regulating the client protein,
such as P38MAPK (14). Additionally,
the expression level of MAPK in tumor cells was apparently higher
than that in normal cells (15,16).
Furthermore, inhibition of MAPK signaling in breast cancer impairs
proliferation and promotes apoptosis (17). To the best of our knowledge, in the
present study, we have shown for the first time that the expression
level of FASN was significantly elevated in bone tumor cells
compared to normal cells with the expression level of MAPK/P53. The
expression level of FASN in tumor cells treated with MAPK/P53
inhibitor significantly decreased, and the proliferation rate was
reduced. This indicates that FASN participates in bone tumor
proliferation by regulating MAPK/P53 activity, and as a result
participate in the occurrence and progress of bone tumors.
References
|
1
|
Zhao Q, Liu ZD, Xue Y, Wang JF, Li H, Tang
QJ, Wang YM, Dong P and Xue CH: Ds-echinoside A, a new triterpene
glycoside derived from sea cucumber, exhibits antimetastatic
activity via the inhibition of NF-κB-dependent MMP-9 and VEGF
expressions. J Zhejiang Univ Sci B. 12:534–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li N, Bu X, Wu P, Wu P and Huang P: The
‘HER2-PI3K/Akt-FASN Axis’ regulated malignant phenotype of
colorectal cancer cells. Lipids. 47:403–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toegel S, Wu SQ, Otero M, Goldring MB,
Leelapornpisid P, Chiari C, Kolb A, Unger FM, Windhager R and
Viernstein H: Caesalpinia sappan extract inhibits
IL1β-mediated overexpression of matrix metalloproteinases in human
chondrocytes. Genes Nutr. 7:307–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie X, Li W, Lan T, Liu W, Peng J, Huang
K, Huang J, Shen X, Liu P and Huang H: Berberine ameliorates
hyperglycemia in alloxan-induced diabetic C57BL/6 mice through
activation of Akt signaling pathway. Endocr J. 58:761–768. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu W, Zhang X, Liu P, Shen X, Lan T, Li
W, Jiang Q, Xie X and Huang H: Effects of berberine on matrix
accumulation and NF-kappa B signal pathway in alloxan-induced
diabetic mice with renal injury. Eur J Pharmacol. 638:150–155.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu S, Yu Y, Liu L, Wang X, Lu S, Liang Y,
Liu X, Xie L and Wang G: Increased plasma exposures of five
protoberberine alkaloids from Coptidis Rhizoma in
streptozotocin-induced diabetic rats: is P-GP involved? Planta Med.
76:876–881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tahara A, Tsukada J, Tomura Y, Yatsu T and
Shibasaki M: Effects of high glucose on AVP-induced hyperplasia,
hypertrophy, and type IV collagen synthesis in cultured rat
mesangial cells. Endocr Res. 37:216–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponchiardi C, Mauer M and Najafian B:
Temporal profile of diabetic nephropathy pathologic changes. Curr
Diab Rep. 13:592–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv ZM, Wang Q, Wan Q, Lin JG, Hu MS, Liu
YX and Wang R: The role of the p38 MAPK signaling pathway in high
glucose-induced epithelial-mesenchymal transition of cultured human
renal tubular epithelial cells. PLoS One. 6:e228062011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oh D, Lee Y, La B, Yeo J, Chung E, Kim Y
and Lee C: Fatty acid composition of beef is associated with exonic
nucleotide variants of the gene encoding FASN. Mol Biol Rep.
39:4083–4090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Souza FR, Chiquitelli MG, da Fonseca
LF, Cardoso DF, da Silva Fonseca PD, de Camargo GM, Gil FM, Boligon
AA, Tonhati H, Mercadante ME, et al: Associations of FASN gene
polymorphisms with economical traits in Nellore cattle (Bos
primigenius indicus). Mol Biol Rep. 39:10097–10104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeon SH, Lee SH, Choi BH, Lee HJ, Jang GW,
Lee KT, Kim KH, Lee JH and Chung HY: Genetic variation of FASN is
associated with fatty acid composition of Hanwoo. Meat Sci.
94:133–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alim MA, Wang P, Wu XP, Li C, Cui XG,
Zhang SL, Zhang Q, Zhang Y and Sun DX: Effect of FASN gene on milk
yield and milk composition in the Chinese Holstein dairy
population. Anim Genet. 45:111–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu JJ, Luo J, Wang W, Yu K, Wang HB, Shi
HB, Sun YT, Lin XZ and Li J: Inhibition of FASN reduces the
synthesis of medium-chain fatty acids in goat mammary gland.
Animal. 8:1469–1478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohammadi H, Shahrebabak MM and Sadeghi M:
Association between single nucleotide polymorphism in the ovine
DGAT1 gene and carcass traits in two Iranian sheep breeds. Anim
Biotechnol. 24:159–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nafikov RA, Schoonmaker JP, Korn KT, Noack
K, Garrick DJ, Koehler KJ, Minick-Bormann J, Reecy JM, Spurlock DE
and Beitz DC: Sterol regulatory element binding transcription
factor 1 (SREBF1) polymorphism and milk fatty acid composition. J
Dairy Sci. 96:2605–2616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin YC, Li ZH, Hong ZS, Xu CX, Han JA,
Choi SH, Yin JL, Zhang QK, Lee KB, Kang SK, et al: Conjugated
linoleic acid synthesis-related protein proteasome subunit α 5
(PSMA5) is increased by vaccenic acid treatment in goat mammary
tissue. J Dairy Sci. 95:4286–4297. 2012. View Article : Google Scholar : PubMed/NCBI
|