Introduction
Cervical cancer is the third most commonly diagnosed
malignancy and the fourth leading cause of cancer-associated
mortality in women worldwide, with ~530,000 new cases and 275,100
cervical cancer-associated mortalities occurring in females in 2008
(1). The initiation and progression
of cervical cancer is a multi-step process, involving multiple
factors and the transformation of normal cervical epithelium into
cervical intraepithelial neoplasia, which subsequently transforms
into invasive cervical cancer (2,3). A number
of studies have demonstrated that persistent infection with
high-risk human papillomavirus (HPV) serves an important role in
the initiation and progression of cervical cancer (4–6). However,
previous studies have shown that HPV infection alone is
insufficient to induce malignant changes and that other factors
must contribute to cervical carcinogenesis and progression
(7,8).
Accumulated studies reported that abnormal expression or activity
of specific genes is responsible for the pathogenesis of cervical
cancers (9–11). Currently, surgery, radiotherapy and
chemotherapy are the main therapies for patients with cervical
cancer (12). However, subsequent to
these treatments, ~30% of patients developed cancer recurrence,
lymph node recurrence or distant metastasis and eventually obtained
an unfavorable prognosis (13).
Therefore, it is of great significance to fully understand the
molecular mechanisms underlying the biology, genetics, causes and
cellular origin of cervical cancer, which are important for
developing novel therapeutic strategies for patients with cervical
cancer.
MicroRNAs (miRNAs) are a sizable group of
endogenous, non-protein-coding and short RNAs of 21–23 nucleotides,
which negatively regulate their target mRNAs through binding the 3′
untranslated regions (3′UTRs) of mRNAs, causing mRNA degradation or
inhibiting translation (14,15). miRNAs regulate the activity of >30%
of human genes, and therefore perform important roles in a variety
of physiological and pathological processes, including cell
proliferation, apoptosis, metastasis, glucose and lipid metabolism,
and infection and immune responses (16–20).
Previous studies have demonstrated that miRNAs are deregulated in
numerous types of human cancers, and are associated with
tumorigenesis and development by regulation of oncogenes or tumor
suppressors (21–23). These previous findings indicated the
important functions of miRNAs in the initiation and progression of
human cancers, and demonstrate the potential of miRNAs as efficient
therapeutic targets for cancer treatment.
In the present study, miR-10b was identified as a
tumor suppressor miRNA in cervical cancer. The present data
revealed that miR-10b was significantly downregulated in cervical
cancer tissues and cell lines. It was also revealed that miR-10b
overexpression inhibited cervical cancer cell proliferation,
migration and invasion, while miR-10b under-expression had the
opposite effect. Furthermore, the present study demonstrated that
insulin-like growth factor-1 receptor (IGF-1R) was directly
regulated by miR-10b in cervical cancer, and subsequent
downregulation of IGF-1R mimicked the inhibitory effects of miR-10b
on cervical cancer.
Materials and methods
Tumor specimens
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Guilin Medical University
(Guangxi, China). A total of 46 cases of cervical cancer tissues
and adjacent normal cervical epithelial tissues were collected from
patients who were newly diagnosed with cervical cancer between
March 2012 and August 2014 in the Affiliated Hospital of Guilin
Medical University. No radiotherapy or chemotherapy was performed
in any patients prior to surgery. Fresh tissues were stored in
liquid nitrogen prior to RNA extraction.
Cell culture and transfection. A total of 5 human
cervical cancer cell lines (HeLa, CaSki, HT-3, C-33A and SiHa), a
normal human cervix epithelial cell line (Ect1/E6E7) and the
HEK293T cell line were all purchased from American Type Culture
Collection (Manassas, VA, USA). All cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) and 1% antibiotic/antimycotic
(Thermo Fisher Scientific, Inc.) in a humidified air atmosphere of
5% CO2 at 37°C.
The miR-10b mimics, corresponding negative controls
(NC), miR-10b inhibitor, NC inhibitor and luciferase report vectors
[pGL3-IGF-1R-3′UTR wild type (Wt) and pGL3-IGF-1R-3′UTR mutant
(Mut)] were obtained from GenePharma (Shanghai, China). IGF-1R
small interfering RNA (siRNA) and NC siRNA were chemically
synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Transfection of mimics, inhibitors, siRNAs and luciferase report
vectors was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from tissues and cells using
the mirVana miRNA isolation kit (Ambion; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Reverse
transcription was performed with the PrimeScript™ RT reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China), followed by RT-PCR
conducted using SYBR® Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.). The expression level of miR-10b was
normalized to the expression level of U6 small nuclear RNA. U6
small nuclear RNA was used as a loading control. All reactions were
performed on the Applied Biosystems 7500 real-time PCR system
(Thermo Fisher Scientific, Inc.) and run in triplicate.
Cell proliferation assay. Cervical cancer cells
(3×103 cells/100 µl) were seeded onto 96-well plates.
Following incubation for 6–8 h, cells were transfected with mimics,
inhibitor or siRNAs and cultured at 37°C for 24, 48, 72 and 96 h.
Cellular proliferation was measured using MTT assay (Sigma Chemical
Co., St Louis, MO, USA). Briefly, 20 µl MTT solution (5 mg/ml) was
added to each well and incubated at 37°C for an additional 4 h.
Culture medium was then removed and formazan crystals were
dissolved in dimethyl sulfoxide. The optical density (OD) at 490 nm
was detected with an enzyme linked immunosorbent assay reader
(Dasit Group S.p.A, Milan, Italy).
Migration and invasion assay. Migration and invasion
assays were performed using Transwell chambers (pore size, 8 µm;
Costar; Corning Incorporated, Corning, NY, USA). For the migration
assay, 5×104 cells in 100 µl of FBS-free culture medium
were added to the upper chambers, and 500 µl of DMEM medium
supplemented with 20% FBS was added to the lower chambers as a
chemoattractant. Following incubation at 37°C for 24 h, cells
remaining on the upper membrane of the Transwell chamber were
removed using a cotton swab. The migrated cells were fixed, stained
at room temperature with 0.1% crystal violet, washed and then dried
in air. For the invasion assay, 5×104 cells in 100 µl of
FBS-free culture medium were added to the upper chambers, which
were pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA).
The subsequent steps were similar to the migration assay, but the
Transwell chambers were incubated at 37°C for 48 h. Images of five
randomly selected fields of the migrated/invaded cells were
captured and the cells were counted, under an inverted microscope
(magnification, ×200; CKX41; Olympus Corporation, Tokyo,
Japan).
Bioinformatics analysis and luciferase report assay.
To predict the potential targets of miR-10b, bioinformatics
analysis was conducted using microRNA.org
(http://www.microrna.org/microrna/)
and TargetScan (http://www.targetscan.org/).
HEK293T cells were seeded onto 24-well plates, and
were co-transfected with pGL3-IGF-1R-3′UTR Wt or pGL3-IGF-1R-3′UTR
Mut, and miR-10b mimics or NC. The transfected cells were cultured
for 48 h, and luciferase activities were detected using the
Dual-Luciferase® reporter assay system (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol. Renilla luciferase activity was normalized to Firefly
luciferase activity. Results were obtained from three independent
experiments.
Western blot analysis
Cells were washed twice with cold PBS (Thermo Fisher
Scientific, Inc.) and lysed with radioimmunoprecipitation assay
lysis buffer. Equal amounts of protein were dissolved in 10%
SDS-PAGE and blotted onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then blocked
with 5% non-fat milk in TBS with Tween-20 (TBST), followed by
incubation with the following primary antibodies: Mouse anti-human
monoclonal IGF-1R (dilution, 1:1,000; catalog no. sc-81464; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse anti-human
monoclonal GADPH (dilution, 1:1,000; catalog no. sc-59540; Santa
Cruz Biotechnology, Inc.). Following incubation overnight at 4°C,
the membranes were washed three times with TBST, incubated with
goat anti-mouse horseradish peroxidase-conjugated secondary
antibodies (dilution, 1:3,000; catalog no. sc-2005; Santa Cruz
Biotechnology, Inc.) and visualized by enhanced chemiluminescence
(EMD Millipore), according to the manufacturers protocol. GADPH was
used as a loading control.
Statistical analysis
All values were presented as the mean ± standard
deviation. Differences between groups were assessed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-10b is downregulated in cervical
cancer
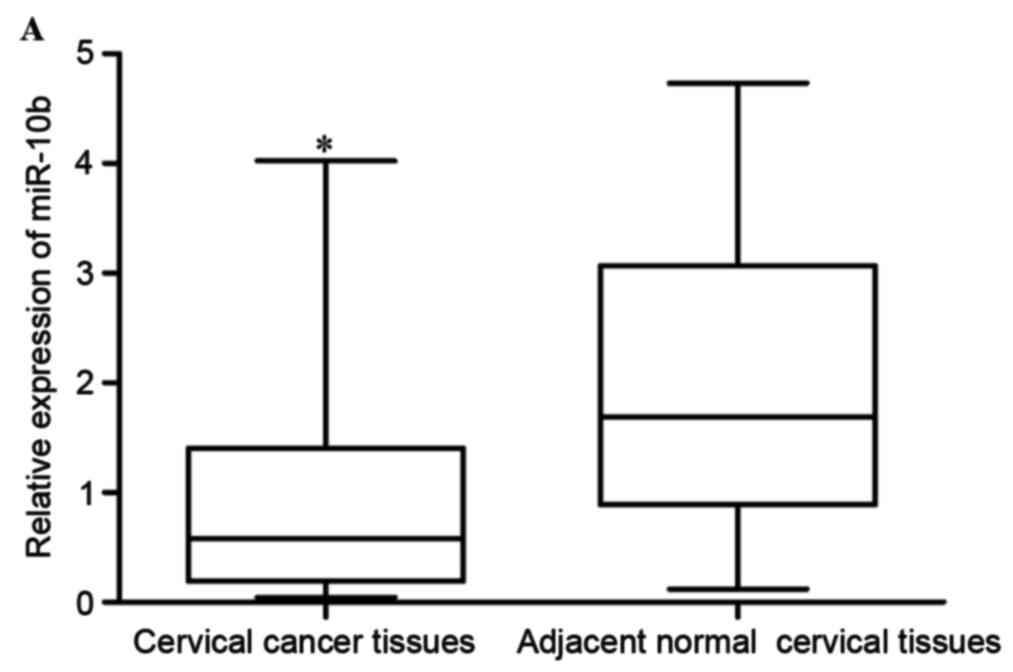
As first step of the present study, the expression
levels of miR-10b in cervical cancer tissues and adjacent normal
cervical epithelial tissues were measured. The results revealed
that miR-10b expression was significantly lower in cervical cancer
tissues compared with adjacent normal cervical epithelial tissues,
indicating that miR-10b may act as a tumor suppressor in cervical
cancer (P<0.05; Fig. 1A).
The expression of miR-10b was then examined in five
human cervical cancer cell lines (HeLa, CaSki, HT-3, C-33A and
SiHa). The results revealed that miR-106 was lower in all five
examined cell lines, compared with the normal human cervix
epithelial Ect1/E6E7 cell line (P<0.05; Fig. 1B).
miR-10b negatively regulates the
proliferation, migration and invasion of cervical cancer cells
The expression of miR-10b in HeLa cells was the
highest in the five cell lines, while SiHa had the lowest miR-10b
expression levels. Considering the present results, HeLa and SiHa
cells were selected for subsequent experiments. SiHa cells were
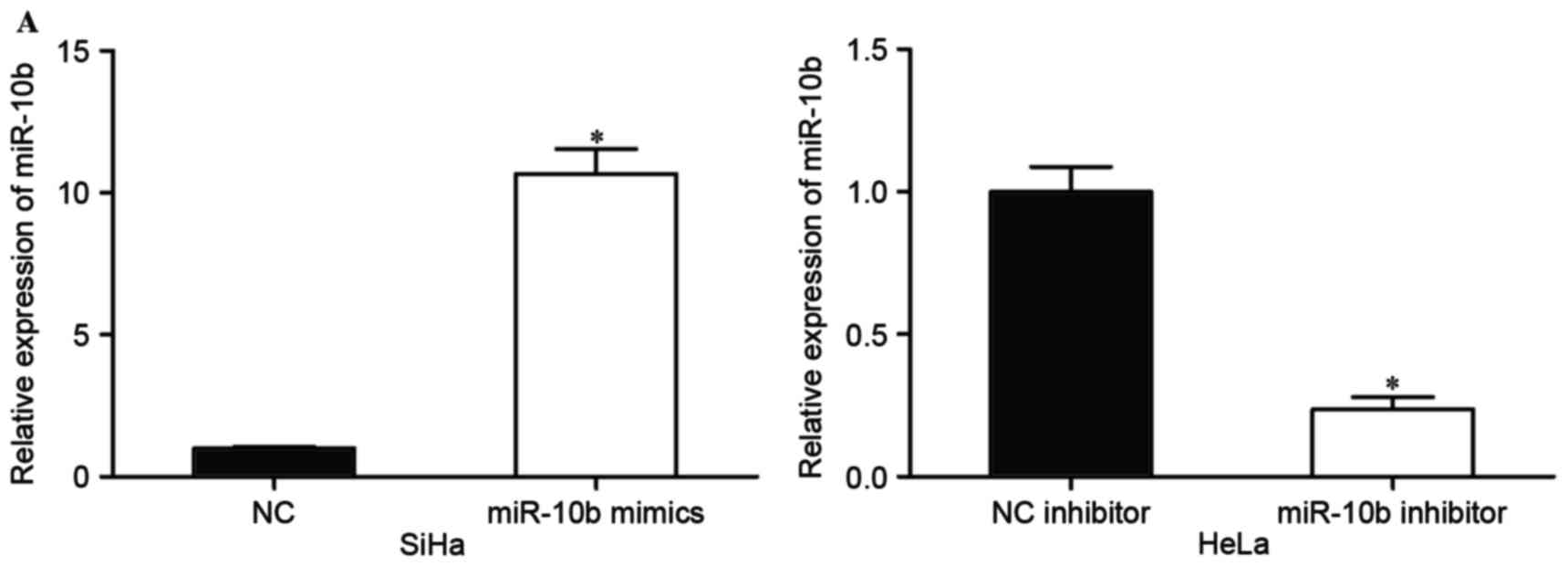
transfected with miR-10b mimics or NC, and HeLa cells were
transfected with miR-10b inhibitor or NC inhibitor. At 48 h
following transfection, the transfection efficiency was measured by
RT-PCR. The results revealed that the miR-10b mimic significantly
upregulated expression levels in SiHa cells, and the miR-10b
inhibitor downregulated miR-10b expression in HeLa cells
(P<0.05; Fig. 2A).
To investigate the effect of miR-10b on cervical
cancer cell proliferation, cell proliferation assays (MTT assays)
were performed. Compared with the control groups, miR-10b mimics
inhibited the proliferation of SiHa cells, while the miR-10b
inhibitor prompted proliferation of HeLa cells (P<0.05; Fig. 2B). Migration and invasion assays were
performed to explore the effects of miR-10b on metastasis of
cervical cancer cells. As shown in Fig.
2C, the miR-10b mimic reduced SiHa cell migration and invasion
abilities (P<0.05). HeLa cell migration and invasion abilities
were increased following transfection with miR-10b inhibitor
compared with that in cells transfected with NC inhibitor
(P<0.05). These results indicated that miR-10b may act as a
tumor suppressor in cervical cancer.
IGF-1R is a direct target of miR-10b
in cervical cancer
It is generally accepted that miRNAs exert their
functions through binding to the 3′UTR of target mRNAs and
regulating their expression. Therefore, bioinformatics analysis was
performed with microRNA.org and TargetScan. As shown
in Fig. 3A, IGF-1R was predicated to
be a potential target of miR-10b.
To investigate whether IGF-1R was a genuine target
of miR-10b, luciferase report assay was conducted.
pGL3-IGF-1R-3′UTR Wt or pGL3-IGF-1R-3′UTR Mut, along with miR-10b
mimics or NC, were co-transfected into HEK293T cells. As shown in
Fig. 3B, the luciferase activities of
pGL3-IGF-1R-3′UTR Wt were significantly suppressed when miR-10b
mimics were co-transfected (P<0.05). By contrast, the luciferase
activities of the pGL3-IGF-1R-3′UTR Mut were unaffected by
transfection of miR-10b mimics (P>0.05). These results indicated
that miR-10b directly targeted the 3′UTR of IGF-1R.
The effect of miR-10b on the expression levels of
IGF-1R was also measured. The results revealed that miR-10b mimics
decreased IGF-1R expression in SiHa cells, while miR-10b inhibitor
improved IGF-1R levels in HeLa cells (P<0.05). IGF-1R was a
direct target of miR-10b in cervical cancer.
IGF-1R is involved in miR-10b-mediated
proliferation, migration and invasion of cervical cancer cells
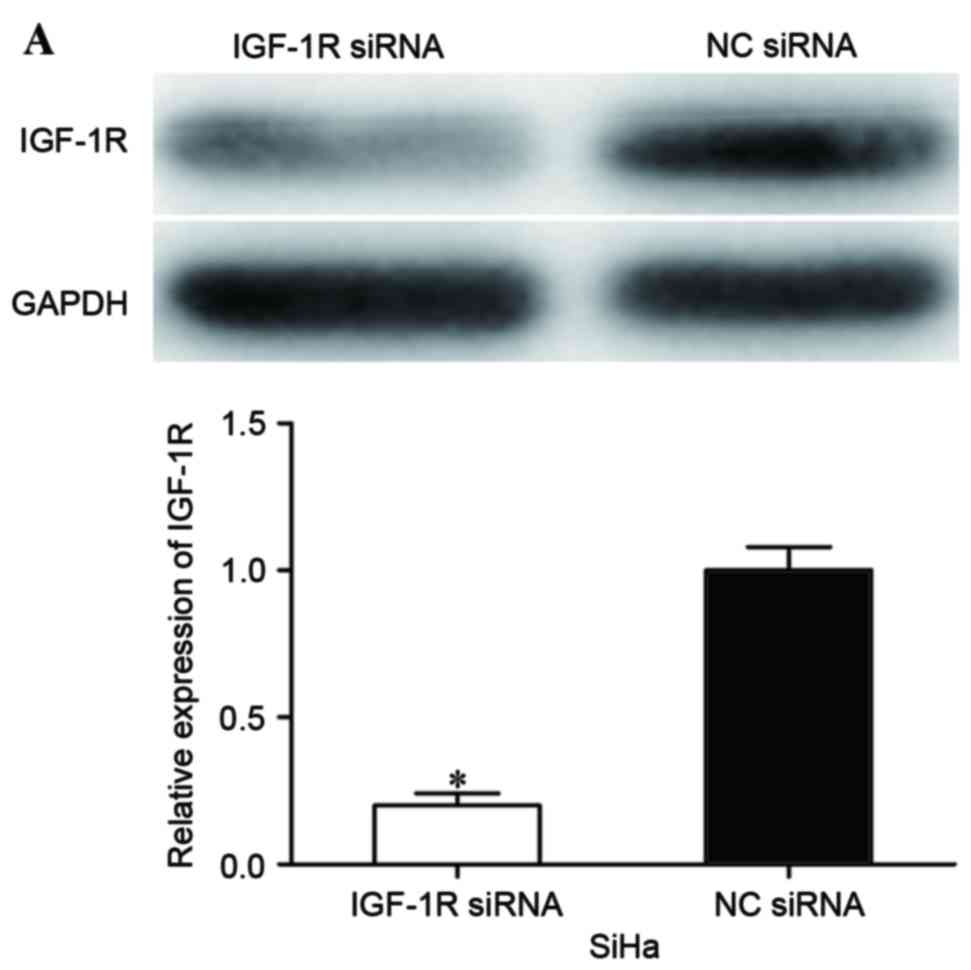
To evaluate whether IGF-1R was involved in
miR-10b-mediated proliferation, migration and invasion of cervical
cancer cells, IGF-1R siRNA was adopted to reduce IGF-1R expression.
At 72 h following transfection, western blot analysis was performed
to assess its transfection efficiency. As shown in Fig. 4A, IGF-1R siRNA significantly decreased
IGF-1R expression in SiHa cells when compared with cells
transfected with NC siRNA (P<0.05).
Subsequently, cell proliferation, migration and
invasion assays were performed to investigate the effects of IGF-1R
siRNA on cell proliferation, migration and invasion. The present
data revealed that IGF-1R significantly inhibited proliferation
(P<0.05; Fig. 4B), migration
(P<0.05; Fig. 4C) and invasion
(P<0.05; Fig. 4C) of cervical
cancer cells, indicating that IGF-1R acted as a downstream effector
in the miR-10b-mediated proliferation, migration and invasion of
cervical cancer cells.
Discussion
The main factors affecting the clinical prognosis of
metastatic and recurrent cervical cancer have not been fully
investigated. It is important to explore the molecular mechanisms
underlying the initiation and development of cervical cancer. A
growing amount of evidence has demonstrated that miRNAs are
important regulators of various types of biological processes in
cancers, including cell proliferation, cell cycle, apoptosis,
invasion and migration (24–26). Furthermore, the abnormal expression of
miRNAs is associated with carcinogenesis and progression of cancer
(27–29). Therefore, investigating the expression
and functions of miRNAs in cervical cancer may benefit the
development of improved strategies for refractory cervical cancer
treatment.
The present data revealed that expression levels of
miR-10b were lower in cervical cancer tissues compared with
adjacent normal cervical epithelial tissues. Consistently, five
cervical cancer cell lines also expressed lower miR-10b. In
functional studies, miR-10b overexpression significantly inhibited
proliferation, migration and invasion of cervical cancer cells,
while miR-10b under-expression had the opposite effects. To the
best of our knowledge, it was demonstrated for the first time that
IGF-1R was a direct target of miR-10b in cervical cancer. Thus, the
present findings indicated that in cervical cancer, miR-10b may act
as a tumor suppressor miRNA that is commonly downregulated in
cancer tissues, and its overexpression may inhibit cervical cancer
growth and metastasis.
Previously, miR-10b was revealed to be upregulated
in numerous types of human cancers, including melanoma (30), gastric cancer (31), non-small-cell lung cancer (32), glioma (33), colorectal cancer (34), bladder cancer (35), breast cancer (36), nasopharyngeal carcinoma (37), pancreatic cancer (38) and hepatocellular carcinoma (39). In addition, miR-10b expression levels
were revealed to be associated with clinicopathological factors. In
gastric cancer, miR-10b expression levels were associated with the
size of tumor, Lauren classification, depth of invasion, lymph node
and distant metastasis, TNM stage and prognosis (40). Zhang et al (41) reported that the relative expression
levels of miR-10b in non-small-cell lung cancer were significantly
positively associated with TNM stage and regional lymph node
involvement. Kaplan-Meier analysis revealed that patients with
increased levels of miR-10b had significantly poorer survival rate
than those with lower expression of miR-10b. Nishida et al
(42) revealed that high level
miR-10b were associated with high incidence of lymphatic invasion
and poor prognosis in patients with colorectal cancer. These
studies indicated that miR-10b may be a prognostic target for
cancers.
Functionally, miR-10b was validated as an oncogene
(31,32). Previous studies demonstrated that
ectopic miR-10b expression improved migration and invasion
abilities of bladder cancer, nasopharyngeal carcinoma and gastric
cancer cells (31,35,37). Liao
et al revealed that restoration of miR-10b expression
enhanced proliferation, migration and invasion of hepatocellular
carcinoma (39,43). In non-small-cell lung cancer, miR-10b
overexpression prompted cell proliferation and invasion, and
inhibited apoptosis (32,44). However, the expression and functions
of miRNAs are tissue specific. miR-10b was reported to be
downregulated in clear-cell renal cell carcinoma (45) and was associated with metastasis and
progression of clear-cell renal cell carcinoma (46). Increased miR-10b expression inhibited
cell proliferation, migration and invasion of clear-cell renal cell
carcinoma (45). In the present
study, it was verified that miR-10b was downregulated in cervical
cancer, and acted as a tumor suppressor. These contradictory
results may be explained by the imperfect complementarity of the
interactions between miRNAs and target genes (47).
Different cancers have different target genes of
miR-10b, including Hoxd10 (31) in
gastric cancer, E-cadherin (41) and
Krüppel-like factor 4 (44) in
non-small-cell lung cancer, ras homolog family member C (RhoC)
(34) in colorectal cancer and RhoC,
urokinase-type plasminogen activator receptor matrix
metalloproteinases (39) and cell
adhesion molecule 1 (43) in
hepatocellular carcinoma. In the present study, IGF-1R was
identified as a novel target gene of miR-10b in cervical cancer. Of
numerous potential target genes for miR-10b, predicted by
bioinformatics analysis, IGF-1R was selected for the present study.
IGF-1R was previously revealed to be upregulated in cervical cancer
(48). Luciferase report assay
results indicated that miR-10b may directly target the 3′UTR of
IGF-1R. In vitro miR-10b overexpression significantly
decreased IGF-1R expression, whereas inhibition of miR-10b resulted
in increased IGF-1R. In addition, IGF-1R siRNA may mimic the
effects of miR-10b overexpression on proliferation, migration and
invasion of cervical cancer cells. Thus, the present results
suggested that miR-10b acted as a tumor suppressor in cervical
cancer, at least in part, through negative regulation of
IGF-1R.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that miR-10b is downregulated in
cervical cancer. miR-10b acted as a tumor suppressor in cervical
cancer by inhibiting cell proliferation, migration and invasion,
and directly regulating IGF-1R expression via binding to its 3′UTR.
This provides new insights into the mechanisms of initiation and
progression of cervical cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howell LP, Zhou H, Wu W and Davis R:
Significance of subclassifying high-grade squamous intraepithelial
lesions into moderate dysplasia/CIN II versus severe dysplasia/CIN
III/CIS in the bethesda system terminology. Diagn Cytopathol.
30:362–366. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng K, Zheng W, Mo X, Liu F, Li M, Liu Z,
Zhang W and Hu X: Dysregulated microRNAs involved in the
progression of cervical neoplasm. Arch Gynecol Obstet. 292:905–913.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wardak S: Human Papillomavirus (HPV) and
cervical cancer. Med Dosw Mikrobiol. 68:73–84. 2016.PubMed/NCBI
|
|
5
|
Syrjänen KJ and Syrjänen SM: Human
papillomavirus (HPV) typing as an adjunct to cervical cancer
screening. Cytopathology. 10:8–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wen Y, Pan XF, Zhao ZM, Chen F, Fu CJ, Li
SQ, Zhao Y, Chang H, Xue QP and Yang CX: Knowledge of human
papillomavirus (HPV) infection, cervical cancer, and HPV vaccine
and its correlates among medical students in Southwest China: A
multi-center cross-sectional survey. Asian Pac J Cancer Prev.
15:5773–5779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildesheim A and Wang SS: Host and viral
genetics and risk of cervical cancer: A review. Virus Res.
89:229–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin CM, Astbury K and O'Leary JJ:
Molecular profiling of cervical neoplasia. Expert Rev Mol Diagn.
6:217–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertelsen BI, Steine SJ, Sandvei R, Molven
A and Laerum OD: Molecular analysis of the PI3K-AKT pathway in
uterine cervical neoplasia: Frequent PIK3CA amplification and AKT
phosphorylation. Int J Cancer. 118:1877–1883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheung TH, Lo KW, Yim SF, Chan LK, Heung
MS, Chan CS, Cheung AY, Chung TK and Wong YF: Epigenetic and
genetic alternation of PTEN in cervical neoplasm. Gynecol Oncol.
93:621–627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang PH, Yang SF, Chen GD, Han CP, Chen
SC, Lin LY and Ko JL: Human nonmetastatic clone 23 type 1 gene
suppresses migration of cervical cancer cells and enhances the
migration inhibition of fungal immunomodulatory protein from
Ganoderma tsugae. Reprod Sci. 14:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pareja R, Rendón GJ, Sanz-Lomana CM,
Monzón O and Ramirez PT: Surgical, oncological, and obstetrical
outcomes after abdominal radical trachelectomy-a systematic
literature review. Gynecol Oncol. 131:77–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kogo R, How C, Chaudary N, Bruce J, Shi W,
Hill RP, Zahedi P, Yip KW and Liu FF: The microRNA-218~Survivin
axis regulates migration, invasion and lymph node metastasis in
cervical cancer. Oncotarget. 6:1090–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sevignani C, Calin GA, Siracusa LD and
Croce CM: Mammalian microRNAs: A small world for fine-tuning gene
expression. Mamm Genome. 17:189–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Wang Q and Pan X: MicroRNAs and
their regulatory roles in animals and plants. J Cell Physiol.
210:279–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fei B and Wu H: MiR-378 inhibits
progression of human gastric cancer MGC-803 cells by targeting
MAPK1 in vitro. Oncol Res. 20:557–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Yin B, Wang B, Ma Z, Liu W and Lv
G: MicroRNA-210 promotes proliferation and invasion of peripheral
nerve sheath tumor cells targeting EFNA3. Oncol Res. 21:145–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang WH, Gui JH, Wang CZ, Chang Q, Xu SP,
Cai CH, Li YN, Tian YP, Yan L and Wu B: The identification of
miR-375 as a potential biomarker in distal gastric adenocarcinoma.
Oncol Res. 20:139–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Misawa A, Katayama R, Koike S, Tomida A,
Watanabe T and Fujita N: AP-1-Dependent miR-21 expression
contributes to chemoresistance in cancer stem cell-like SP cells.
Oncol Res. 19:23–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohdaira H, Sekiguchi M, Miyata K and
Yoshida K: MicroRNA-494 suppresses cell proliferation and induces
senescence in A549 lung cancer cells. Cell Prolif. 45:32–38. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho WC, Chow AS and Au JS: Restoration of
tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung
adenocarcinoma patients with epidermal growth factor receptor
mutation. Eur J Cancer. 45:2197–2206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Zhou Y, Pan H, Zhou J, Fan Y and Qu
P: microRNA-99a inhibiting cell proliferation, migration and
invasion by targeting fibroblast growth factor receptor 3 in
bladder cancer. Oncol Lett. 7:1219–1224. 2014.PubMed/NCBI
|
|
24
|
Li E, Zhang J, Yuan T and Ma B: MiR-145
inhibits osteosarcoma cells proliferation and invasion by targeting
ROCK1. Tumour Biol. 35:7645–7650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gururajan M, Josson S, Chu GC, Lu CL, Lu
YT, Haga CL, Zhau HE, Liu C, Lichterman J, Duan P, et al: miR-154*
and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate
epithelial to mesenchymal transition and bone metastasis of
prostate cancer. Clin Cancer Res. 20:6559–6569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Ma X, Cai Q, Wang X, Yu B, Cai Q,
liu B, Zhu Z and Li C: MiR-199a-3p promotes gastric cancer
progression by targeting ZHX1. FEBS Lett. 588:4504–4512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu T, Hou L and Huang Y: EZH2-specific
microRNA-98 inhibits human ovarian cancer stem cell proliferation
via regulating the pRb-E2F pathway. Tumour Biol. 35:7239–7247.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiu F, Sun R, Deng N, Guo T, Cao Y, Yu Y,
Wang X, Zou B, Zhang S, Jing T, et al: miR-29a/b enhances cell
migration and invasion in nasopharyngeal carcinoma progression by
regulating SPARC and COL3A1 gene expression. PLoS One.
10:e01209692015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alizadeh S, Kaviani S, Soleimani M, Abroun
S, Kashani-Khatib Z, Asgharzadeh A, Dargahi H and Mousavi R: Mir-55
inhibition can reduce cell proliferation and induce apoptosis in
Jurkat (Acute T cell Leukemia) cell line. Iran J Ped Hematol Oncol.
4:141–150. 2014.PubMed/NCBI
|
|
30
|
Saldanha G, Elshaw S, Sachs P, Alharbi H,
Shah P, Jothi A and Pringle JH: microRNA-10b is a prognostic
biomarker for melanoma. Mod Pathol. 29:112–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang YY, Li L, Ye ZY, Zhao ZS and Yan ZL:
MicroRNA-10b promotes migration and invasion through Hoxd10 in
human gastric cancer. World J Surg Oncol. 13:2592015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang J, Sun C, Wang S, He Q and Li D:
microRNA miR-10b inhibition reduces cell proliferation and promotes
apoptosis in non-small cell lung cancer (NSCLC) cells. Mol Biosyst.
11:2051–2059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji Y, Wei Y, Wang J, Gong K, Zhang Y and
Zuo H: Correlation of microRNA-10b upregulation and poor prognosis
in human gliomas. Tumour Biol. 36:6249–6254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang YF, Li Z, Zhao XH, Zuo XM, Zhang Y,
Xiao YH, Li J and Peng ZH: MicroRNA-10b is upregulated and has an
invasive role in colorectal cancer through enhanced Rhoc
expression. Oncol Rep. 33:1275–1283. 2015.PubMed/NCBI
|
|
35
|
Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K,
Zeng J, He W, Zeng G, Ye Z and Xu H: MicroRNA-10b promotes
migration and invasion through KLF4 and HOXD10 in human bladder
cancer. Oncol Rep. 31:1832–1838. 2014.PubMed/NCBI
|
|
36
|
Han X, Yan S, Weijie Z, Feng W, Liuxing W,
Mengquan L and Qingxia F: Critical role of miR-10b in transforming
growth factor-β1-induced epithelial-mesenchymal transition in
breast cancer. Cancer Gene Ther. 21:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun XJ, Liu H, Zhang P, Zhang XD, Jiang ZW
and Jiang CC: miR-10b promotes migration and invasion in
nasopharyngeal carcinoma cells. Asian Pac J Cancer Prev.
14:5533–5537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-β actions.
Oncogene. 33:4664–4674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao CG, Kong LM, Zhou P, Yang XL, Huang
JG, Zhang HL and Lu N: miR-10b is overexpressed in hepatocellular
carcinoma and promotes cell proliferation, migration and invasion
through RhoC, uPAR and MMPs. J Transl Med. 12:2342014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang YY, Ye ZY, Zhao ZS, Li L, Wang YX,
Tao HQ, Wang HJ and He XJ: Clinicopathologic significance of
miR-10b expression in gastric carcinoma. Hum Pathol. 44:1278–1285.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Xu L, Yang Z, Lu H, Hu D, Li W,
Zhang Z, Liu B and Ma S: MicroRNA-10b indicates a poor prognosis of
non-small cell lung cancer and targets E-cadherin. Clin Transl
Oncol. 17:209–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishida N, Yamashita S, Mimori K, Sudo T,
Tanaka F, Shibata K, Yamamoto H, Ishii H, Doki Y and Mori M:
MicroRNA-10b is a prognostic indicator in colorectal cancer and
confers resistance to the chemotherapeutic agent 5-fluorouracil in
colorectal cancer cells. Ann Surg Oncol. 19:3065–3071. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li QJ, Zhou L, Yang F, Wang GX, Zheng H,
Wang DS, He Y and Dou KF: MicroRNA-10b promotes migration and
invasion through CADM1 in human hepatocellular carcinoma cells.
Tumour Biol. 33:1455–1465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Li M, Zhang G and Pang Z:
MicroRNA-10b overexpression promotes non-small cell lung cancer
cell proliferation and invasion. Eur J Med Res. 18:412013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He C, Zhao X, Jiang H, Zhong Z and Xu R:
Demethylation of miR-10b plays a suppressive role in ccRCC cells.
Int J Clin Exp Pathol. 8:10595–10604. 2015.PubMed/NCBI
|
|
46
|
Wu X, Weng L, Li X, Guo C, Pal SK, Jin JM,
Li Y, Nelson RA, Mu B, Onami SH, et al: Identification of a
4-microRNA signature for clear cell renal cell carcinoma metastasis
and prognosis. PLoS One. 7:e356612012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y,
Yu W, Wu X, Ye J, Yang S, et al: Identification of miR-7 as an
oncogene in renal cell carcinoma. J Mol Histol. 44:669–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Steller MA, Delgado CH, Bartels CJ,
Woodworth CD and Zou Z: Overexpression of the insulin-like growth
factor-1 receptor and autocrine stimulation in human cervical
cancer cells. Cancer Res. 56:1761–1765. 1996.PubMed/NCBI
|