Introduction
Phosphoglucose isomerase (PGI), also termed
phosphohexose isomerase (PHI), is a glycolytic enzyme that
catalyzes the interconversion of glucose-6-phosphate and
fructose-6-phosphate in the glycolysis process. A secreted version
of PGI/PHI, which has cytokine activity, was originally purified
from the conditioned culture medium of human melanoma cells and was
termed autocrine motility factor (AMF) owing to its ability to
stimulate cell motility (1). Until
the respective protein sequences were determined, AMF was termed
neuroleukin, a maturation factor mediating differentiation of human
myeloid leukemia cells, a myofibril-bound serine proteinase
inhibitor, and sperm antigen-36 (2–5). Although
the secretion of AMF by normal cells has not been observed, its
secretion into culture medium has been identified in various cancer
cells. Clinical studies measuring AMF in the serum or urine of
patients with lung, gastrointestinal and renal cancer, have
suggested that it may be a useful biomarker for certain types of
cancer (6,7). In addition to the cell motility
stimulating activity, AMF is involved in cellular proliferation
(8), cellular survival (9), invasion of malignant cells (10), tumor metastasis (11) and the induction of angiogenesis
(12).
AMF binds to and stimulates the AMF receptor (AMFR),
which is a 78 kDa glycoprotein (gp78) with seven transmembrane
domains (13). When stimulated by
AMF, AMFR activates signal transduction pathways that are involved
in enhancing cell motility and proliferation and suppressing cell
adhesion; these include activation of protein kinase C, the
activation and upregulation of Rho-like GTPases and the
upregulation of SNAIL (11,14,15). A
wide range of clinicopathological studies reviewed by Chiu et
al (16), have demonstrated that
expression of AMFR was significantly elevated in various kinds of
tumor cells compared with adjacent normal tissues.
Studies to date reveal that AMF or AMFR may be a
therapeutic drug target, however anticancer drugs targeting these
cellular molecules have not yet been developed. Several studies
have demonstrated that downregulation of endogenous AMF, using
small interfering RNA, interfered with the normal functions of
cancer cells by inhibiting the EGF-induced invasion of breast
cancer cells (17) or by inducing
mesenchymal-to-epithelial transition of human fibrosarcoma,
osteosarcoma and endometrial cancer cells (18–20),
suggesting that inhibiting AMF activity or downregulating AMF
expression would be a useful approach to treat cancer. In the
present study, monoclonal antibodies specific to the recombinant
human AMF (rhAMF) were selected from a naive human scFv phage
display library and evaluated for their antitumor activity and
effects on the plasma level of endogenous AMF. This is the first
report demonstrating that antibodies targeting AMF have antitumor
activity in human tumor-xenografted mice, as well as causing
decreased concentrations of AMF in the plasma.
Materials and methods
Cell lines
MRC-5, HEK293, HEK293F, K-562, Huh-7, and HepG2
cells were grown in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
(vol/vol) heat inactivated fetal bovine serum (Thermo Fisher
Scientific, Inc.). NCI-N87, A549, and MDA-MB-231 cells were grown
in RPMI-1640 supplemented with 10% heat inactivated fetal bovine
serum. HEK293F cells were grown in Expi293™ Expression
medium (Gibco; Thermo Fisher Scientific, Inc.). All cell lines,
with the exception of Huh-7 and HEK293F, were obtained from the
ATCC (Manassas, VA, USA). Huh-7 cells were obtained from The
Japanese Collection of Research Bioresources Cell Bank (Osaka,
Japan) and HEK293F cells were purchased from Thermo Fisher
Scientific, Inc.
Preparation of recombinant human AMF.
E. coli
BL21 (DE3; #RH217; Real Biotech Corporation,
Banqiao, Taiwan) on ice for 10 min were transformed with the pET22b
(+) vector (#69744; Merck KGaA, Darmstadt, Germany) containing AMF
cDNA, according to the manufacturer's protocol. The transformed
E. coli cells in luria broth (Lennox; Sigma-Aldrich; Merck
KGaA) were incubated for 4 h at 37°C with agitation (250 rpm), and
induced with 0.1 mM isopropyl β-D-1-thiogalactopyranoside. HEK293F
cells were transfected with pcDNA3.1-AMF vector (Thermo Fisher
Scientific, Inc.) containing AMF cDNA, using polyethylenimine (PEI;
Polysciences, Inc., Warrington, PA, USA). The transiently
transfected cells were grown in Expi293™ Expression
medium at 37°C for 4 days. The transformed E. coli lysate
and the transfected HEK293F conditioned culture medium were applied
to HisTrap FF (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)
equilibrated with 50 mM Tris buffer (pH 7.4) containing 0.5 M
sodium chloride. The column-bound fractions were eluted with 50 mM
Tris buffer (pH 7.4) containing 0.5 M sodium chloride and 250 mM
imidazole, and then dialyzed with PBS (pH 7.4) three times at 201 ×
g for 10 min at 4°C using VIVA spin 20 (Sartorius AG, Göttingen,
Germany) with 30 kDa molecular weight threshold.
SDS-PAGE analysis
The cell lysate of E. coli cells transformed
with the pET22b-AMF vector and rhAMF purified from the transformed
E. coli cells were separated by electrophoresis on a 10% SDS
Tris-glycine polyacrylamide gel. The protein bands were stained
with Coomassie brilliant blue R-250 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Western blotting analysis
The E. coli cell lysates and the
rhAMF-containing column chromatography fractions were separated by
10% SDS-PAGE and blotted onto a polyvinylidene difluoride membrane
using iBlot apparatus (Invitrogen; Thermo Fisher Scientific, Inc.).
The blot was blocked with 5% skimmed milk in PBS (pH 7.4) for 2 h
at room temperature. The blocked membrane was incubated for 1 h at
room temperature with a mouse monoclonal antibody recognizing the
His-tag conjugated to rhAMF (#70796-3; dilution, 1:1,000; Novagen;
Merck KGaA), then with a horseradish peroxidase (HRP)-conjugated
anti-mouse immunoglobulin G (IgG) antibody (KPL, Inc.,
Gaithersburg, MD, USA) for 1 h at room temperature. The blot was
incubated in the presence of tetramethylbenzidine substrate (KPL,
Inc.), the reaction was stopped by adding a stop solution
(#50-85-01; KPL, Inc.) and the signal was visualized using ChemiDoc
XRS+ with Image Lab™ Software (Bio-Rad Laboratories,
Inc.).
Measurement of the enzyme activity of
AMF
The enzyme activity of a commercial rhAMF
(MyBioSource, San Diego, CA, USA) and the purified rhAMFs were
measured using a glucose-6-phosphate isomerase activity assay kit
(Abcam, Cambridge, UK), according to the manufacturer's
protocol.
ELISA for AMF quantification
AMF secreted in the culture medium and AMF in the
plasma of Balb/c nude mice were measured using a
glucose-6-phosphate isomerase human ELISA kit (Abcam), according to
the manufacturer's protocol.
FACS analysis
Cells were suspended at a density of
1×106 cells/ml in PBS (pH 7.4) and incubated in the
presence of a fluorescein isothiocyanate-conjugated anti-AMF
receptor (AMFR) antibody (#ab76841; dilution, 1:1,000, Abcam) for 1
h at 4°C in the dark. Subsequent to incubation, the cells were
washed 3 times with PBS and incubated in the presence of
goat-anti-rabbit IgG H&L (Alexa Fluor 488®;
#ab150077, dilution 1:1,000, Abcam) for 1 h at 4 in the dark. The
cells were washed 2 times with stain buffer (#554656; BD
Biosciences, Franklin Lakes, NJ, USA). Subsequently, the cell
surface fluorescence was analyzed by flow cytometry using a
FACScaliber (BD Biosciences).
Migration assay
Cancer cell motility was measured by a modification
of the Boyden chamber assay, using a CytoSelect 96-well cell
migration assay kit (Cell Biolabs Inc., San Diego, SD, USA). The
MDA-MB-231, A549, NCI-N87 and HepG2 cells (1×106
cells/ml) were incubated in the upper chamber, and 10 µg/ml E.
coli-derived rhAMF was added to the lower chamber, which was
separated by an 8 µm pore membrane. After 24 h incubation, cells on
the upper surface of the membrane were removed by scraping, and the
cells on the lower surface were detached using cell detachment
solution. The detached cells were lysed and quantified using
CyQuant GR fluorescent dye (Cell Biolabs, Inc., San Diego, CA,
USA), according to the manufacturer's protocol.
Screening of human monoclonal anti-AMF
antibodies
Human monoclonal antibodies against AMF were
selected from a naive human scFv (single chain fragment variable)
phage display library (a total of ~1011 members, A &
R Therapeutics Co., Daejeon, Korea). A total of 4 rounds of
biopanning were performed and four clones of anti-AMF antibodies of
9A-4H, 7F4, 8F and 4H were isolated. During each round,
AMF-specific phages were selected out of the pool by washing away
non-specific binders using PBS-T (PBS containing 0.05% Tween 20).
Following 4 rounds, a mixture of highly specific phage clones was
separated from a mono clone phage and selected on the basis of
affinity and specificity against AMF using indirect ELISA. Briefly,
putative AMF-specific phages were added into the well of 96-well
plates coated with rh-AMF and incubated for 2 h at room
temperature. The wells were washed three times with PBS-T and then
anti-M13 HRP antibody (#ab50370; Abcam) was added and incubated for
1 h at room temperature. Tetramethylbenzidine substrate was added
and the reaction was stopped at 10 min by adding the
ABTS® Peroxidase stop solution kit (#50-85-01; KPL,
Inc.). The plate was measured at a wavelength of 450 nm. The phage
vector from each clone was subjected to sequencing to determine the
nucleotide sequence of VL and VH, and then the nucleotide sequences
were analyzed and grouped. The VH and VL gene elements of a
selected phage were amplified using polymerase chain reaction and
then subcloned into mammalian expression vectors (pYG300H and
pYG300L, respectively; A & R Therapeutics Co.), each of which
contained a constant region of heavy chain and light chain of human
IgG1 coding sequence for fully human IgG1 expression. Sequencing
was performed using ABI PRISM 3730XL Analyzer (Applied Biosystems;
Thermo Fisher Scientific, Inc.) to confirm in-frame insertion of
each variable region into the expression vectors.
Surface plasmon resonance
The antibody-antigen interaction was examined by
surface plasmon resonance spectrometry (SR7500DC; Reichert, Inc.,
Depew, NY, USA). Prior to antigen immobilization, a Polyethylene
Glycol/Carboxyl sensor chip (#13206061; Reichert, Inc.) was first
activated with a mixture of 0.1 M 1-ethyl-3-
(3-dimethylamminopropryl) carbodiimide hydrochloride and 0.05 M
N-hydroxysuccinimide at a flow rate of 20 µl/min for 10 min.
Following the activation of the chip surface, 50 µg/ml antigen was
flowed over the chip surface. The antigen was immobilized to the
surface of the chip via free amine coupling to the immobilized
succinimide, followed by quenching the remaining activated
succinimide ester with 1 M ethanolamine (pH 8.5). The chip was
equilibrated with PBS-T. For the analysis of antibody association,
solutions (0, 12.5, 25, 50, 100, 200, 400 and 800 nM) of anti-AMF
antibodies, 9A-4H, 4H, 7F4 and 8F, in PBS-T was flowed over the
sensor chip at a rate of 30 µl/min for 5 min. For the molecular
dissociation, washing buffer was flowed over the sensor chip at a
rate of 30 µl/min for 5 min. The chip was regenerated with 20 mM
HCl. The data was analyzed using the Scrubber 2.0 software
(BioLogic Software; Bio-Rad Laboratories, Inc.).
A549 or NCI-N87 xenograft in nude
mice
The six-week-old BALB/c female mice (weighing ~20 g;
Charles River Laboratories International, Inc., Yokohama, Japan)
were housed under specific pathogen free conditions. Food and water
were sterilized prior to use; the light cycles were 12 h light/dark
and the temperature was maintained between 20 and 22°C. The animal
protocol was reviewed by the Korea University Institutional Animal
Care & Use Committee (Seoul, Korea). A549 or NCI-N87 cells
suspended in PBS (pH 7.4) were injected subcutaneously in the right
flank of the mice at a concentration of 2×107 cells/ml.
The intravenous administration of sunitinib (4 or 40 mg/kg, orally
administered, once a day for 4 weeks; Sigma-Aldrich; Merck MGaA),
cetuximab (4 or 40 mg/kg, intraperitoneal, twice a week for 4
weeks; Namyang Pharm Co., Ltd., Seoul, Korea), trastuzumab (15
mg/kg, intravenously twice a week for 2 weeks; Namyang Pharm Co.,
Ltd., Seoul, Korea) or human monoclonal anti-AMF antibodies (9A-4H,
7F4, 8F and 4H; 15 mg/kg, intravenously twice a week for 2 weeks)
against E. coli-derived rhAMF, began when the average volume
(V0) of the tumors reached ~100 mm3. Tumor
volumes (Vt) were measured 3 times a week for 3 weeks
using a vernier caliper, and then calculated by the formula 0.5 ×
height × length × width. At the end of the experiment the mice were
sacrificed by CO2 asphyxiation.
Statistical analysis
GraphPad Prism (GraphPad Software, Inc., La Jolla,
CA, USA) was used for statistical analysis. One-way ANOVA and
Dunnett's post hoc t-test were used for multiple comparisons.
Spearman correlation analysis was used to compare the correlation
between plasma AMF concentration and tumor weight in
A549-xenografted mice. P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation and validation of
rhAMF
To select the best material for the experiments,
rhAMFs from 3 different sources were analyzed and compared. The
enzyme activity of rhAMFs purified from the E. coli lysate
transformed with the pET22b-AMF vector, rhAMFs present in the
culture medium of HEK293F cells transiently transfected with the
human AMF cDNA in the pcDNA3.1-AMF vector, and commercial rhAMF
were 4.64, 3.50 and 3.64 mU/mg (Table
I), respectively. The enzyme activity of rhAMFs prepared from
E. coli and HEK293F cells were similar to that of the
commercial rhAMF, which was included in the assay for comparison.
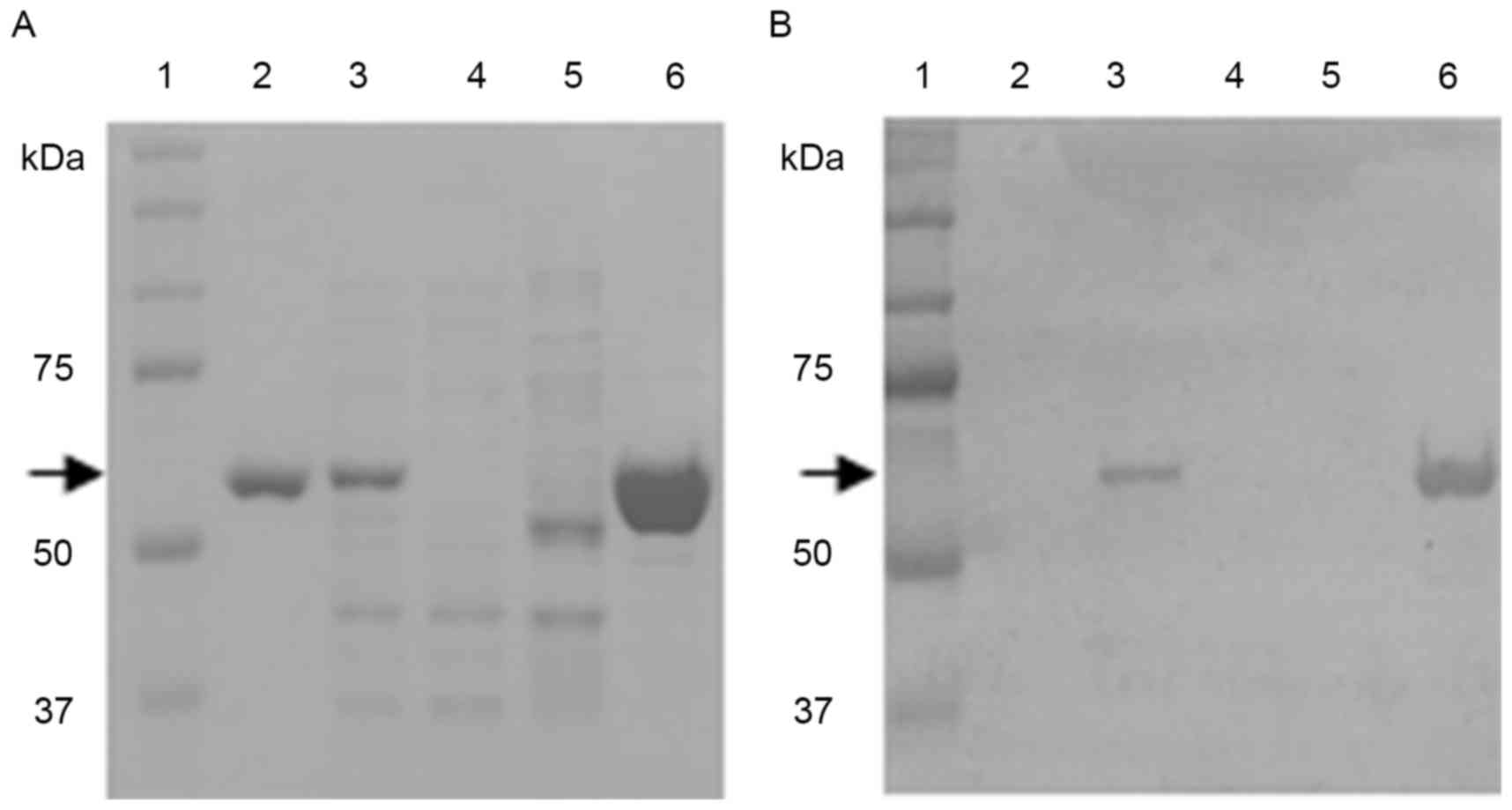
The identity of the final product purified by the column
chromatography method was validated by SDS-PAGE and western blot
analyses (Fig. 1A and B). The lanes
of the gel loaded with the commercial rhAMF, the soluble fraction
from the pET22b-AMF-transformed E. coli lysate, and the 250
mM imidazole elution fraction revealed clear bands stained with
Coomassie brilliant blue R-250 at ~55 kDa, which is the expected
molecular weight of rhAMF. The bands were identified to be rhAMF by
western blot analysis using a monoclonal antibody against the
His-tag. The commercial rhAMF was not detected by the western blot
analysis since it does not contain a His-tag (Fig. 1B). Since the enzyme activity of E.
coli-derived rhAMF was not lower than that of rhAMFs from other
sources, and the preparation of E. coli-derived rhAMF is
easier than that from HEK293F cells, the following experiment was
conducted using E. coli-derived rhAMF.
 | Table I.Enzyme activity of rhAMF. |
Table I.
Enzyme activity of rhAMF.
| Commercial
rhAMF | E.
coli-derived rhAMF | HEK293F-derived
rhAMF |
|---|
| 3.65 | 4.64 | 3.50 |
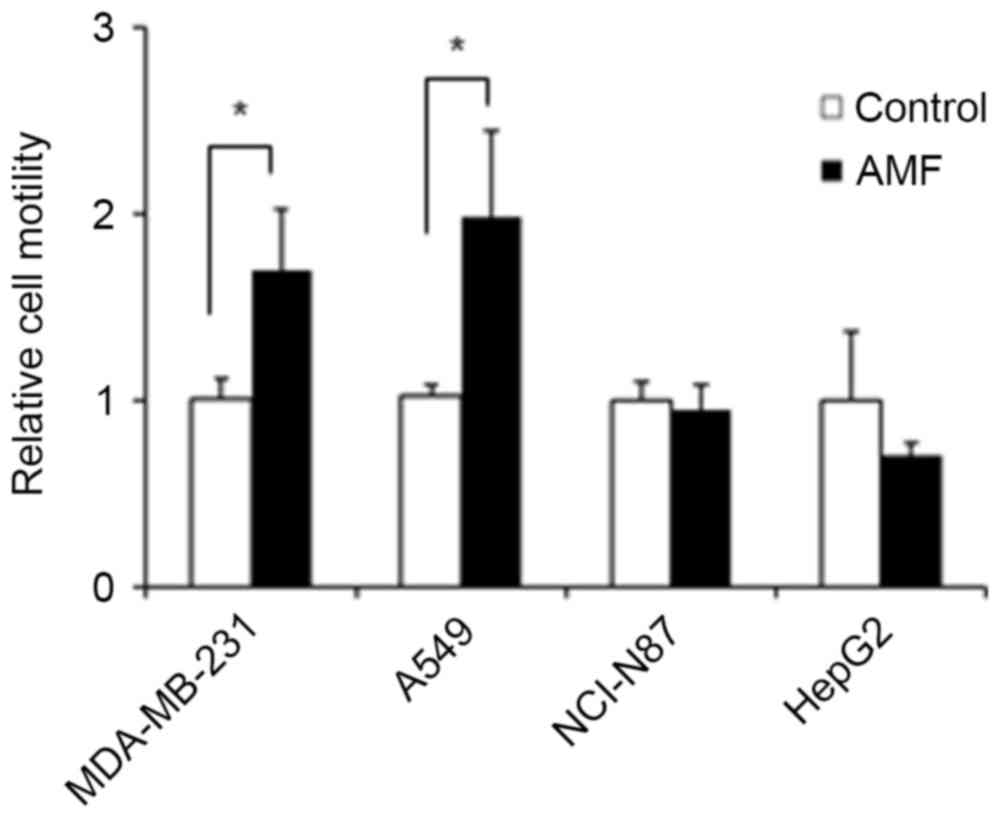
Effects of E. coli-derived rhAMF on
the migration of cancer cells
E. coli-derived rhAMF was examined for its
chemoattractive activity upon cancer cells using a Boyden chamber.
As shown in Fig. 2, the migration of
MDA-MB-231 and A549 cells was significantly increased by treatment
with rhAMF. The cell motility of MDA-MB-231 and A549 cells as a
result of treatment with rhAMF increased by ~1.6- and 1.9-fold,
respectively, compared with that of the control cells. However, the
migration of NCI-N87 and HepG2 cells was not significantly changed
by treatment with rhAMF.
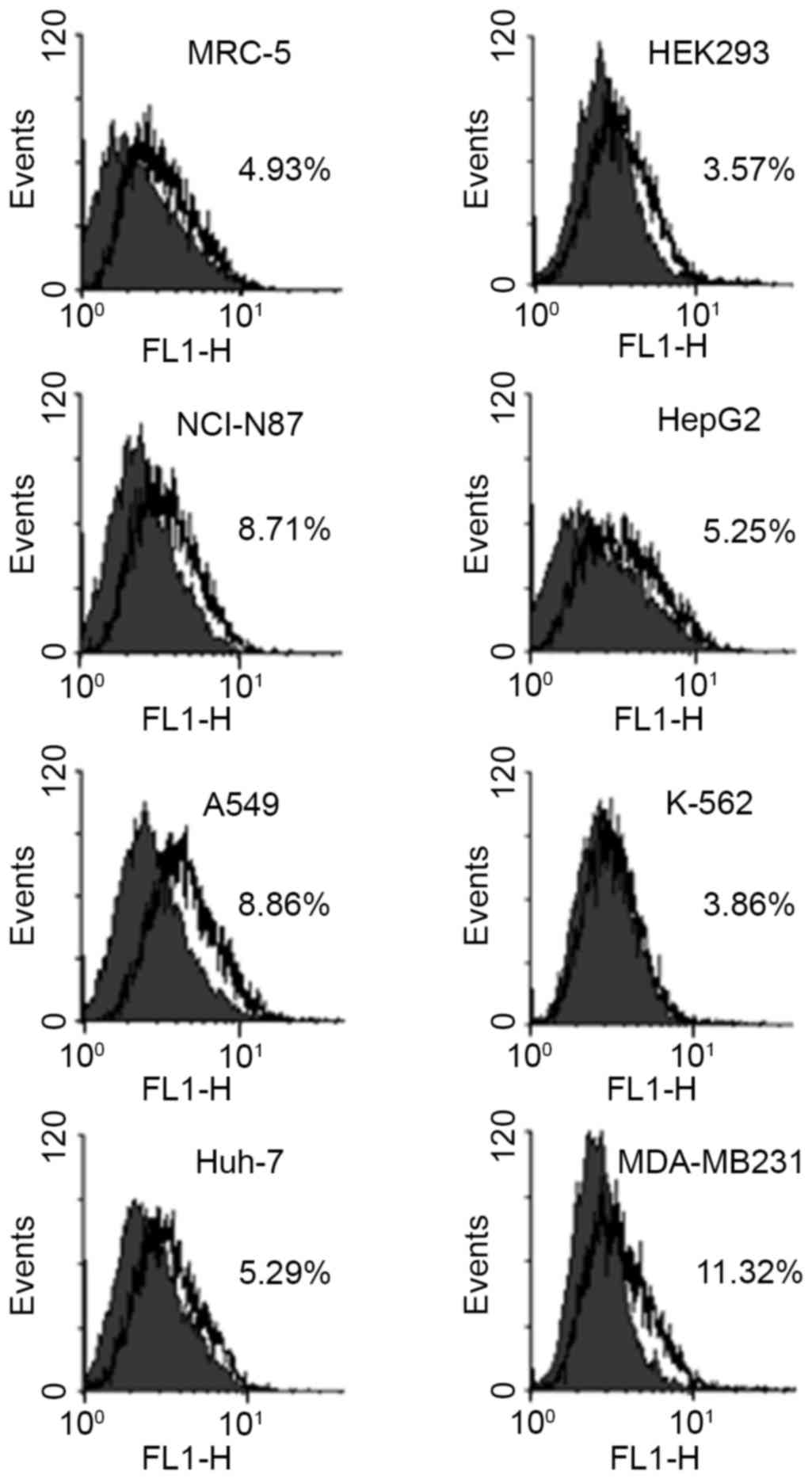
Effect of the expression of the AMF
receptor on the secretion of AMF by cancer cells
In order for a signal to be transmitted into cells
by extracellular AMF, the presence of AMFR is required. The
relative amount of AMFR was measured in the normal human fibroblast
MRC-5 and embryonic kidney HEK293 cells, and in the human gastric
carcinoma NCI-N87, hepatocellular carcinoma HepG2, lung carcinoma
A549, chronic myelogenous leukemia K-562, hepatocellular carcinoma
Huh-7, and breast cancer MDA-MB-231 cells using flow cytometry
(Fig. 3). The percentage values of
cells that express AMFR in HEK293 and MRC-5 cells were 3.57 and
4.93%, respectively. In the case of cancer cells, while the
percentage values of cells that express AMFR in K-562, Huh-7, and
HepG2 cells were similar to those in the normal cell lines, those
in NCI-N87, A549, and MDA-MB-231 cells were 8.71, 8.86, and 11.32%,
respectively, which were 1.8 to 3.2 times higher compared with
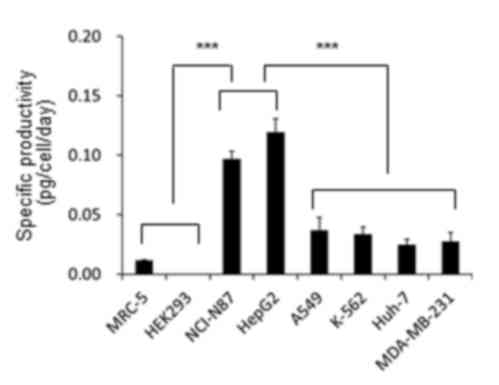
those in the normal cells. In contrast to the expression of AMFR,
there were huge differences in the amount of AMF secreted by these
cells (Fig. 4). The AMF-specific
productivities of NCI-N87 and HepG2 cells were statistically
greater (P<0.001) compared with the other groups of cells. While
the AMF-specific productivities of NCI-N87 and HepG2 cells were
0.097±0.006 and 0.119±0.011 pg/cell/day, respectively, those of
A549, K-562, Huh-7 and MDA-MB-232 cells were no higher than
0.037±0.011 pg/cell/day. The AMF-specific productivities of the
normal cell lines, MRC-5 and HEK293, were 0.012±0.001 and
0.000±0.000 pg/cell/day, respectively. These results were in
contrast to those demonstrated in Fig.
2, in that NCI-N87 and HepG2 cells secreted endogenous AMF much
more actively than the other cell lines, including MDA-MB-231 and
A549 cells, whose motilities were more greatly affected by
treatment with rhAMF.
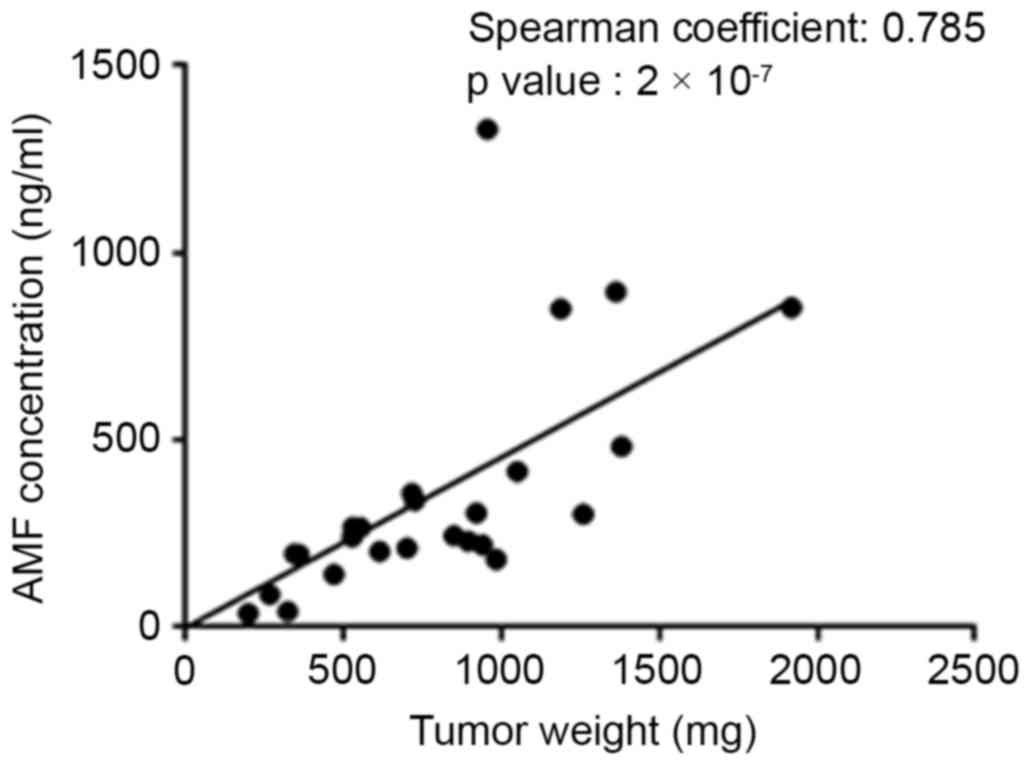
Correlation between plasma AMF
concentration and tumor growth
To investigate whether there was a correlation
between tumor growth and plasma concentration of AMF,
A549-xenografted Balb/c nude mice were treated with sunitinib
(21), a small-molecule
multi-targeted receptor tyrosine kinase inhibitor, and cetuximab
(22,23), a chimeric anti-EGFR monoclonal
antibody (Fig. 5). Blood and tumors
were collected at the end of the experiments, the plasma
concentration of AMF and the tumor weights were measured, and the
correlation between plasma AMF concentration and tumor weight was
analyzed by the Spearman's rank correlation method (Fig. 5). The results reveal that the plasma
AMF concentration became lower as the tumor weight decreased upon
treatment with sunitinib and cetuximab. As demonstrated with a
Spearman's rank coefficient of 0.785 obtained from the analysis of
the correlation between the plasma AMF concentration and tumor
weight, there was a relatively good correlation between the two
factors.
Selection of human monoclonal anti-AMF
antibodies
In total, 4 human monoclonal antibodies (9A-4H, 4H,
7F4 and 8F) specific for AMF were selected from a naive human scFv
phage display library using biopanning. The binding kinetics of the
anti-AMF human monoclonal antibodies with E. coli-derived
and HEK293F-derived rhAMF were measured using surface plasmon
resonance (Table II). The
equilibrium dissociation constant (KD) values of these 4
monoclonal antibodies with E. coli-derived rhAMF were
similar to those with HEK293F-derived rhAMF, however, the
KD value of 8F with E. coli-derived rhAMF may not
be obtained due to a lack of detectable dissociation from the
immobilized ligand. Among these 4 antibodies, 9A-4H and 7F4 had
somewhat lower affinity for the two rhAMFs than the 8F and 4H
antibodies. Since the binding kinetics of these antibodies with
rhAMFs derived from two different sources were similar, the
following experiments, to investigate the effect of AMF on the
migration of cancer cells, were performed with E.
coli-derived rhAMF, which may be easily prepared compared with
the method using the human cell line.
 | Table II.Surface plasmon resonance binding
kinetics of anti-AMF human monoclonal antibodies with rhAMF. |
Table II.
Surface plasmon resonance binding
kinetics of anti-AMF human monoclonal antibodies with rhAMF.
| Immobilized
ligands | Analytes | Ka,
M−1s−1a | Kd,
s−1b | KD,
nMc |
|---|
|
E.coli-derived rhAMF | 9A-4H |
7.92±3×103 |
1.07±2×10−3 | 135±5 |
|
| 7F4 |
4.50±5×104 |
3.71±3×10−3 | 82.5±9 |
|
| 8F |
1.23±2×105 | ND | ND |
|
| 4H |
5.67±5×105 |
4.89±2×10−3 | 8.64±7 |
| HEK293F-derived
rhAMF | 9A-4H |
1.22±3×104 |
9.35±3×10−4 | 77.0±2 |
|
| 7F4 |
1.06±2×105 |
6.23±5×10−3 | 58.9±8 |
|
| 8F |
1.65±4×105 |
1.69±5×10−3 | 10.3±2 |
|
| 4H |
4.84±3×105 |
8.93±4×10−3 | 18.5±1 |
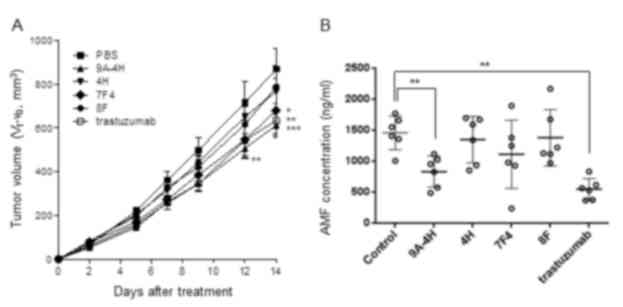
Effects of the monoclonal antibodies
on tumor growth and plasma AMF concentration
Among the 4 monoclonal antibodies (9A-4H, 4H, 7F4,
and 8F), only 9A-4H and 7F4 significantly inhibited the growth of
tumors in NCI-N87-xenografted mice (Fig.
6A). Monoclonal antibody 9A-4H caused the greatest inhibition
among the four monoclonal antibodies, with an inhibition rate
slightly higher than that caused by trastuzumab, a HER2 inhibitor,
which was included as a positive control. The concentration of AMF
in plasma collected from the mice at the end of the experiments
demonstrated a similar pattern to the inhibition of tumor growth
(Fig. 6B). The mean plasma AMF
concentration of the Balb/c nude mice treated with 9A-4H and
trastuzumab were significantly decreased compared with that of the
control Balb/c nude mice (P<0.01). Although it was not
statistically significant, the mean concentration of AMF in the
plasma of the mice treated with 7F4 was decreased compared with the
control mice.
Discussion
The most important characteristics of cancer cells
are uncontrolled proliferation and metastatic ability, which allow
a tumor to grow indefinitely and transfer to distant organs,
respectively. Although there has been certain progress in the
improvement of 5-year survival rates of cancer patients due to
tremendous effort to elucidate avenues to treat cancers, the
majority of cancers, with a few exceptions including thyroid and
non-melanoma skin cancer, are still a great threat to humans.
Although molecularly-targeted therapeutics, including imatinib
(24) and bevacizumab (25), have provided specific evidence that
current approaches to cancer treatment are being somewhat
successful, newer and improved therapeutics are always requested in
the clinic since resistant cancers are being continuously exposed
to traditional and the most recent anticancer agents (26). In view of these matters, AMF, which is
highly secreted by various cancers, is a valuable anticancer drug
target as it is involved in cell proliferation, survival,
metastasis and angiogenesis (8–11). The
signal stimulated by AMF is transmitted into the cell through AMFR.
In previous studies using immunocytochemistry (ICC) staining in
tumors obtained from patients, positivity of AMFR expression in the
tumor cells was defined when >10% of the cells exhibited AMFR
staining (27,28). However, in the present study using
flow cytometry analysis, only MDA-MB-231 cells, among which 11.32%
were positive, satisfied this criterion. NCI-N87 and A549 cells
contained 8.71 and 8.86% positive cells, which were slightly lower
than that of MDA-MB-231 cells, however, the values were increased
compared with those of the normal MRC-5 and HEK293 cell lines. The
10% guideline used to define positivity in ICC staining may not be
appropriate for the present study, which used flow cytometry
analysis that may be able to analyze the proportion of cells more
precisely. It appears that NCI-N87 and A549 cells, as well as
MDA-MB-231 cells, are defined as positive in expressing AMFR since
the expression level of AMFR in these cell lines was increased
compared with that in normal cell lines.
Although it is known that AMF is secreted by various
types of cancer, the amount of AMF secreted into the media by
cancer cells was greatly variable. Cancer cells originating from
digestive organs, including NCI-N87 and HepG2 cells, secreted much
more AMF than the other types of cancer cells examined in the
present study. However, it is too early to decide if there is an
association between the amount of AMF secretion and the organ from
which the cancer cells originated. In contrast to the amount of AMF
secreted by the cancers, the motility of the cells affected by AMF
revealed a different pattern. While the motility of NCI-N87 and
HepG2 cells was not increased by treatment with exogenous AMF, the
motility of MDA-MB-231 and A549 cells was significantly increased.
Since there are no studies to date regarding the analysis of the
association between the amount of AMF secretion and the amount of
AMFR expression in the same cells, it is difficult to say how this
happened. One possibility is that AMFR in the cells highly
expressing their own AMF, including NCI-N87 and HepG2 cells, may be
desensitized. Another possibility is that AMF secreted by cancer
cells may produce effects in normal cells near the tumor (29), and subsequently, the normal cells may
provide molecules required by the tumor. Thus, in addition to the
other previously reported roles of AMF, AMF produced by NCI-N87 and
HepG2 cells may serve a role as a paracrine factor, effecting
normal cells such as the endothelial cells of blood vessels.
There are studies that AMF has been detected in the
urine and plasma of cancer patients (6,7), however,
what happens to the level of AMF in the plasma of humans and animal
treated with anticancer agents has not been reported. The present
study reveals, for the first time, that the plasma concentration of
AMF decreased with a reduction in tumor weights in A549-xenografted
mice treated with sunitinib or cetuximab. In order to be able to
answer the question whether this decrease in plasma AMF
concentration contributed to the reduction in tumor weight, further
study using AMF inhibitors is necessary.
An antibody specific to AMFR, the 3F3A anti-AMFR IgM
monoclonal antibody, has been widely used to identify the presence
of this receptor (30), however, it
does not have an inhibitory effect on the action of AMF. Antitumor
effects of an antibodies specific to AMF have not yet been
reported, nevertheless the present study demonstrates that at least
two monoclonal antibodies specific to AMF, which were selected from
a phage display library, revealed antitumor activity in the
NCI-N87-xenografted mouse model, to the same extent as trastuzumab.
In addition to tumor growth inhibition, the monoclonal antibody
9A-4H, specific to rhAMF, reduced the plasma concentration of AMF.
This result demonstrates that 9A-4H may not only inhibit the action
of AMF but may also decrease the secretion of AMF by tumors.
Although it is difficult to say how this inhibition of AMF by 9A-4H
is connected to the decrease in the plasma concentration of AMF,
there is a possibility that the binding of 9A-4H interferes with
the paracrine or autocrine activity of AMF secreted by the tumor,
which is required for further tumor growth. As a result, the growth
of tumors decreases, with a subsequent reduction in the amount of
AMF secretion.
Trastuzumab is an anticancer agent that was approved
for use in patients with HER2-overexpressing breast cancer and
HER2-overexpressing metastatic gastric or gastroesophageal junction
adenocarcinoma (31). The present
study also revealed that trastuzumab decreased the plasma AMF
concentration as well as the weight of tumors produced from a
gastric cancer cell line. A previous study demonstrated that AMF
secretion was inhibited in HER2 knocked down breast cancer cells,
and positively correlated with the overexpression of HER2 (32). The reduction in the plasma
concentration of AMF as a result of trastuzumab may have occurred,
at least in part, due to its inhibitory effect on HER2 expressed in
HER2-positive NCI-N87 cells. Taken together, the present study
demonstrates that AMF secretion correlated with tumor weight, and
the inhibition of AMF by specific antibodies induced suppression of
tumor growth. Although further study is required to obtain a clear
understanding regarding the role of AMF in vivo, these
results suggest that monoclonal antibody 9A-4H may be a valuable
drug candidate for the treatment of gastric cancer.
Acknowledgements
This research was supported by the Area-Wide
Economic Regional Coalition and Cooperation Program through the
Korea Institute for the Advancement of Technology of the Ministry
of Trade, Industry and Energy, by the Basic Science Research
Program through the National Research Foundation of Korea and by
the Ministry of Education (grant no. NRF-2014R1A1A2059237).
References
|
1
|
Liotta LA, Mandler R, Murano G, Katz DA,
Gordon RK, Chiang PK and Schiffmann E: Tumor cell autocrine
motility factor. Proc Natl Acad Sci USA. 83:pp. 3302–3306. 1986;
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gurney ME, Apatoff BR, Spear GT, Baumel
MJ, Antel JP, Bania MB and Reder AT: Neuroleukin: A lymphokine
product of lectin-stimulated T cells. Science. 234:574–581. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu W, Seiter K, Feldman E, Ahmed T and
Chiao JW: The differentiation and maturation mediator for human
myeloid leukemia cells shares homology with neuroleukin or
phosphoglucose isomerase. Blood. 87:4502–4506. 1996.PubMed/NCBI
|
|
4
|
Cao MJ, Osatomi K, Matsuda R, Ohkubo M,
Hara K and Ishihara T: Purification of a novel serine proteinase
inhibitor from the skeletal muscle of white croaker (Argyrosomus
argentatus). Biochem Biophys Res Commun. 272:485–489. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yakirevich E and Naot Y: Cloning of a
glucose phosphate isomerase/neuroleukin-like sperm antigen involved
in sperm agglutination. Biol Reprod. 62:1016–1023. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomm SA, Keevil BG, Thatcher N, Hasleton
PS and Swindell RS: The value of tumour markers in lung cancer. Br
J Cancer. 58:797–804. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baumann M, Kappl A, Lang T, Brand K,
Siegfried W and Paterok E: The diagnostic validity of the serum
tumor marker phosphohexose isomerase (PHI) in patients with
gastrointestinal, kidney, and breast cancer. Cancer Invest.
8:351–356. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsutsumi S, Yanagawa T, Shimura T,
Fukumori T, Hogan V, Kuwano H and Raz A: Regulation of cell
proliferation by autocrine motility factor/phosphoglucose isomerase
signaling. J Biol Chem. 278:32165–32172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsutsumi S, Hogan V, Nabi IR and Raz A:
Overexpression of the autocrine motility factor/phosphoglucose
isomerase induces transformation and survival of NIH-3T3
fibroblasts. Cancer Res. 63:242–249. 2003.PubMed/NCBI
|
|
10
|
Haga A, Funasaka T, Deyashiki Y and Raz A:
Autocrine motility factor stimulates the invasiveness of malignant
cells as well as up-regulation of matrix metalloproteinase-3
expression via a MAPK pathway. FEBS Lett. 582:1877–1882. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsutsumi S, Yanagawa T, Shimura T, Kuwano
H and Raz A: Autocrine motility factor signaling enhances
pancreatic cancer metastasis. Clin Cancer Res. 10:7775–7784. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funasaka T, Haga A, Raz A and Nagase H:
Tumor autocrine motility factor is an angiogenic factor that
stimulates endothelial cell motility. Biochem Biophys Res Commun.
285:118–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silletti S, Watanabe H, Hogan V, Nabi IR
and Raz A: Purification of B16-F1 melanoma autocrine motility
factor and its receptor. Cancer Res. 51:3507–3511. 1991.PubMed/NCBI
|
|
14
|
Kanbe K, Chigira M and Watanabe H: Effects
of protein kinase inhibitors on the cell motility stimulated by
autocrine motility factor. Biochim Biophys Acta. 1222:395–399.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsutsumi S, Gupta SK, Hogan V, Collard JG
and Raz A: Activation of small GTPase Rho is required for autocrine
motility factor signaling. Cancer Res. 62:4484–4490.
2002.PubMed/NCBI
|
|
16
|
Chiu CG, St-Pierre P, Nabi IR and Wiseman
SM: Autocrine motility factor receptor: A clinical review. Expert
Rev Anticancer Ther. 8:207–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kho DH, Zhang T, Balan V, Wang Y, Ha SW,
Xie Y and Raz A: Autocrine motility factor modulates EGF-mediated
invasion signaling. Cancer Res. 74:2229–2237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Funasaka T, Hu H, Yanagawa T, Hogan V and
Raz A: Down-regulation of phosphoglucose isomerase/autocrine
motility factor results in mesenchymal-to-epithelial transition of
human lung fibrosarcoma cells. Cancer Res. 67:4236–4243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niinaka Y, Harada K, Fujimuro M, Oda M,
Haga A, Hosoki M, Uzawa N, Arai N, Yamaguchi S, Yamashiro M and Raz
A: Silencing of autocrine motility factor induces
mesenchymal-to-epithelial transition and suppression of
osteosarcoma pulmonary metastasis. Cancer Res. 70:9483–9493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Che Q, Bian Y, Zhou Q, Jiang F, Tong
H, Ke J, Wang K and Wan XP: Autocrine motility factor promotes
epithelial-mesenchymal transition in endometrial cancer via MAPK
signaling pathway. Int J Oncol. 47:1017–1024. 2015.PubMed/NCBI
|
|
21
|
Papaetis GS and Syrigos KN: Sunitinib: A
multitargeted receptor tyrosine kinase inhibitor in the era of
molecular cancer therapies. BioDrugs. 23:377–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ciardiello F, de Vita F, Orditura M,
Comunale D and Galizia G: Cetuximab in the treatment of colorectal
cancer. Future Oncol. 1:173–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hitt R, Martin P and Hidalgo M: Cetuximab
in squamous cell carcinoma of the head and neck. Future Oncol.
2:449–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iqbal N and Iqbal N: Imatinib: A
breakthrough of targeted therapy in cancer. Chemother Res Pract.
2014:3570272014.PubMed/NCBI
|
|
25
|
Braghiroli MI, Sabbaga J and Hoff PM:
Bevacizumab: Overview of the literature. Expert Rev Anticancer
Ther. 12:567–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holohan C, van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takanami I, Takeuchi K, Watanabe H,
Yanagawa T, Takagishi K and Raz A: Significance of autocrine
motility factor receptor gene expression as a prognostic factor in
non-small-cell lung cancer. Int J Cancer. 95:384–387. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohta Y, Tanaka Y, Hara T, Oda M, Watanabe
S, Shimizu J and Watanabe Y: Clinicopathological and biological
assessment of lung cancers with pleural dissemination. Ann Thorac
Surg. 69:1025–1029. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Funasaka T and Raz A: The role of
autocrine motility factor in tumor and tumor microenvironment.
Cancer Metastasis Rev. 26:725–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nabi IR, Watanabe H and Raz A:
Identification of B16-F1 melanoma autocrine motility-like factor
receptor. Cancer research. 50:409–414. 1990.PubMed/NCBI
|
|
31
|
Bang YJ, van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kho DH, Nangia-Makker P, Balan V, Hogan V,
Tait L, Wang Y and Raz A: Autocrine motility factor promotes HER2
cleavage and signaling in breast cancer cells. Cancer Res.
73:1411–1419. 2013. View Article : Google Scholar : PubMed/NCBI
|