Introduction
Neuroblastoma is the third most prevalent malignancy
in children and accounts for >10% of cancer-associated mortality
in children in the USA (1). Despite
aggressive therapy, the long-term survival rates for these children
remains low at 40%. The long-term survival rate for children with
high-risk neuroblastomas also remains poor (2). The role of irradiation in the treatment
of high-risk neuroblastoma has been established (3,4). As with
all pediatric malignancies, the aim of radiation therapy in
neuroblastoma is the eradication of the tumor, whilst minimizing
collateral damage to normal tissue. Secreted protein acidic and
rich in cysteine (SPARC) is part of a group of non-structural
components of the extracellular matrix (ECM) that modulates
interactions between cells and their environment (5–7). The role
of SPARC in tumorigenesis is suggested to be cell type specific due
to its diverse function in the microenvironment. In certain types
of cancer, including breast, pancreatic and glioblastoma, high
levels of SPARC expression have been identified to associate with
disease progression and poor prognosis (8,9). In other
types of cancer, including ovarian (10), colorectal (11) and neuroblastoma (12–14), SPARC
functions as a tumor suppressor. It has been reported that there is
an inverse correlation between SPARC expression and neuroblastoma
progression (14), indicating that
SPARC overexpression may be an effective option for the treatment
of neuroblastoma. A literature review has established that
tumorigenic cell lines exhibit low or undetectable levels of SPARC,
whereas non-tumorigenic cells express high levels of SPARC
(9). A previous study identified heat
shock protein 27 (HSP27) as a mediator of SPARC activity (15). This suggests that HSP27 may be a
downstream effector of SPARC-regulated cell morphology and
migration. In the present study SPARC overexpression is
demonstrated to suppress radiation-induced HSP27, resulting in
radio-sensitization of neuroblastoma cells.
Materials and methods
Cell lines and culture conditions
The SK-N-BE(2)
neuroblastoma cell line was obtained from the American Type Culture
Collection (Manassas, VA, USA) and NB1691, also a neuroblastoma
cell line, was donated by Dr Peter Houghton (St. Jude Children's
Research Hospital, Memphis, TN, USA). These cells were cultured in
Opti-Mem medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 5% fetal bovine serum (Atlanta Biologicals, Inc.,
Flowery Branch, GA, USA) and 1% penicillin/streptomycin in a
humidified atmosphere containing 5% CO2 at 37°C.
Antibodies
Antibodies were obtained from the following sources:
SPARC, (cat. no., OAAN00424; Aviva Systems Biology Corporation, San
Diego, CA, USA); was used at a dilution of 1:1,000 for western blot
analysis; β-actin (cat. no., sc-130300; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was used at a dilution of 1:1,000 and HSP27
(cat. no., ab2790; Abcam, Cambridge, MA) was used at a dilution of
1:200 for immunohistochemistry. Secondary antibodies used were
HRP-conjugated anti-rabbit at a dilution of 1:200 for
immunohistochemistry and 1:2,000 for western blot analysis (cat.
no., sc-2030; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
HRP-conjugated anti-mouse antibody at a dilution of 1:200 for
immunohistochemistry (cat. no., 7076; Cell Signaling Technologies,
Danvers, MA).
Radiation dosage
The RS2000 radiator (Rad Source Technologies, Inc.,
Suwanee, GA, USA) was used, which was operated at a maximum of 150
kV/50 mA, for all radiation treatments. All cells were treated with
a single (5 Gy) dose of radiation that was administered following
cell transfection. To radiate subcutaneous tumors in mice, 2 doses
at 2 and 3 Gy with a maximum cumulative dose of 5 Gy was
administered. The mice were shielded using lead sheets, exposing
only the subcutaneous tumors.
Irradiation and SPARC overexpression
in vitro
SK-N-BE(2) and NB1691
cells were transfected with SPARC overexpression plasmid RC209964
(OriGene Technologies, Inc., Rockville, MD, USA) using
Lipofectamine® (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol for in vitro
experiments. Cells were incubated in Opti-Mem medium supplemented
with 5% FBS and 1% penicillin/streptomycin in a humidified
atmosphere containing 5% CO2 at 37°C for 24 h.
Subsequent to this incubation cells were not treated or treated
with 5 Gy of ionizing radiation and incubated for another 24 h in
Opti-Mem medium supplemented with 5% FBS and 1%
penicillin/streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C.
Western blot analysis
Following SPARC overexpression and radiation
treatments, cells were obtained and total protein was isolated
using Mammalian Protein Extraction reagent (Thermo Fisher
Scientific, Inc.). Equal quantities of protein (10 µg/lane)
measured using Pierce 660 nm Protein Assay (cat. no., 1861426
Thermo Fisher Scientific, Inc.) were separated in reducing
conditions on 10% polyacrylamide gels. Following SDS-PAGE, the
proteins were transferred on to a polyvinylidene difluoride
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes
were blocked with standard TBS Tween-20 containing 5% non-fat
skimmed milk for 1 h at room temperature followed by immunoprobing
with primary antibody for 2 h at room temperature in TBS Tween-20
containing 5% non-fat skimmed milk. This was followed by washing
(x4 with TBS Tween-20) followed by blocking again with TBS Tween-20
containing 5% non-fat skimmed milk for 1 h at room temperature.
This was followed by the addition of appropriate horseradish
peroxidase-labeled secondary antibody in TBS Tween-20 containing 5%
non-fat skimmed milk and incubated for 1 h at room temperature
followed by washing (x4 with TBS Tween-20). All incubation and
washings were done on a rocking platform set at 2-strokes per min.
Specific protein bands were visualized using enhanced
chemiluminescence detection reagents (cat. no., 32106; Thermo
Fisher Scientific, Inc.).
Expression analysis of protein 21
(p21) and HSP27 using a spotted antibody array
An antibody array for the detection of HSP27 and p21
was obtained from RayBiotech Human Apoptosis array C1 (catalogue
no., AAH-APO-1; RayBiotech, Inc., Norcross, GA, USA) and processed
according to the manufacturer's protocol. Briefly, 80% confluent
petri plates of SK-N-BE(2) and NB1691
cells were transfected with SPARC overexpression plasmid, followed
by radiation treatment as previously described. Total protein was
isolated using Mammalian Protein Extraction reagent (Thermo Fisher
Scientific, Inc.) and equal quantities of protein (500 µg) measured
using Pierce 660 nm Protein Assay (cat. no., 1861426 Thermo Fisher
Scientific, Inc.) were added to the provided antibody array and
processed according to the manufacturer's protocol, expression of
HSP27 and p21 was determined by measuring signal intensities
compared to untreated controls.
Cell cycle analysis
Cell cycle distribution of SPARC overexpressing and
irradiated SK-N-BE(2) cells, and
NB1691 neuroblastoma cells was analyzed using flow cytometry (FACS
Calibur System; BD Bioscience, San Jose, CA, USA) with excitation
at a wavelength of 488 nm and an emission of 639 nm, following
propidium iodide staining according to standard protocols (9). A total of 10,000 cells were sorted to
determine cell cycle phase using the Cell Quest Pro software
version 5.2.1 (BD Bioscience, San Jose, CA, USA).
Cell proliferation assay
An MTT cell proliferation assay (cat. no.,
50-213-524, Thermo Fisher Scientific, Inc.) was performed using
SPARC overexpressing and irradiated SK-N-BE(2) and NB1691 neuroblastoma cells plated in
96 well plates (density, 2,000 cells/well). After 72 h of
incubation in Opti-Mem medium supplemented with 5% FBS and 1%
penicillin/streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C, MTT was added at a concentration of 0.5
mg/ml to each well. Plates were incubated for 3 h at 37°C.
Following incubation, 100 µl of dimethyl sulfoxide was added and
the absorbance was measured at a wavelength of 550 nm and presented
as the survival percentage.
In vitro scratch assay
The in vitro scratch assay was performed as
previously described (16,17). Briefly, SK-N-BE(2) and NB1691 neuroblastoma cells were plated
in 24-well plates (density, 10,000 cells/well). The cells were
transfected with SPARC overexpression plasmid followed by treatment
with 5 Gy ionizing radiation after 24 h at 37°C. The cell monolayer
was scratched with a 100 µl pipette tip and the migration into the
gap was measured over time using the EVOS-FL cell imaging system,
magnification, ×4 and graphically presented.
Determination of mitochondrial
membrane potential (Δψ)
The mitochondrial Δψ was measured using the
MitoProbe™ JC-1 Assay kit (cat. no., M34152, Thermo Fisher
Scientific, Inc.). Briefly, SK-N-BE(2) and NB1691 neuroblastoma cells were plated
in 24 well plates (density, 20,000 cells/well). The cells were
transfected with SPARC overexpression plasmid followed by treatment
with 5 Gy of ionizing radiation. A total of 48 h after irradiation,
cells were stained with JC-1 dye at a final concentration of 2 µM
and the cells were incubated at 37°C, 5% CO2, for 30 min
and sorted using flow cytometry (10,000 cells) and fluorescence
emission at a wavelength of ~590 nm was quantified and graphically
presented. Staurosporine (cat. no., 11055682001 Roche Diagnostics
Mannheim, Germany) was used as a positive control (5 µM) to
determine experimental validity (18,19).
Caspase assay
Caspase activation was detected using the CellEvent™
Caspase-3/7 Green ReadyProbes from Molecular Probes™ (cat. no.,
R3711; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. SK-N-BE (2)
and NB1691 cells were plated in chamber slides and transfected with
Lipofectamine®, according to standard protocols using
(20) the SPARC overexpression
plasmid. A total of 24 h after transfection, cells were exposed to
5 Gy of ionizing radiation and incubated for 24 h in Opti-Mem
medium supplemented with 5% FBS and 1% penicillin/streptomycin in a
humidified atmosphere containing 5% CO2 at 37°C.
Following this 24 h incubation, media was removed followed by the
addition of 60 µl of CellEvent Caspase-3/7 Green ReadyProbe. Cells
were observed using a confocal microscope (aperture setting 2).
Nuclei were visualized using propidium iodide. The percent of cells
exhibiting activated caspase 3/7 was determined by counting the
number of cells with green florescence in 10 random fields.
Radiation and SPARC overexpression in
vivo
All animal studies were according to instructional
approved protocols by the IRB of the University Of Illinois College
Of Medicine at Peoria (Peoria, USA). Female athymic Nude mice
(Foxn1nu) obtained from Jackson Laboratories (Bar
Harbor, ME) were subcutaneously implanted on the right rear flank
with 1×106 SK-N-BE(2) or
NB1691 cells harvested at the log phase (n=5) using a 20 gauge
needle attached to a syringe at a maximum volume of 200 µl in serum
free OptiMem media. The tumors were allowed to grow until 5 mm in
diameter and followed by intratumoral injections of 100 µg of SPARC
overexpression plasmid solution in phosphate buffer saline (NaCl
137 mmol/l, KCl 2.7 mmol/l, Na2HPO4 10 mmol/l, KH2PO4 1.8 mmol/l)
on day 5, 15 and 20. Controls were untreated or treated with 100 µg
of empty plasmid vector (pGEM) in phosphate buffer saline. Tumors
were irradiated on day 7 at 2 Gy and on day 17 at 3 Gy each with a
cumulative dose of 5 Gy. Mice were euthanized by carbon dioxide
asphyxiation when they lost >20% of body weight or had altered
behavior when ambulating, feeding or grooming. Tumors were obtained
on day 30 and fixed in 10% phosphate-buffered formaldehyde for a
minimum of 24 h at room temperature and processed for paraffin
embedding using an automated tissue processor (Tissue-Tek VIP
Vacuum Infiltration Processor; Sakura Finetek USA, Inc., Torrance,
CA) and sectioning using a manual microtome set at 6 µm (Leitz
1512, Ramsey, MN), prior to sectioning the paraffin blocks were
cooled to −4°C. Serial section were taken and allowed to float over
warm deionized water (42°C) and collected on subbed slides
(21).
Immunohistochemistry
Tissue sections (thickness, 6-µm) obtained from the
paraffin blocks were. The paraffin sections were deparaffinized in
100% xylene (20 min at room temperature) followed by 100% ethanol
(2x, 15 min each). The sections were then rehydrated in serial
ethanol concentrations (100, 90, 70, 50, 30 and 20%) for 10 min
each. Subsequent to rehydration the sections were incubated in
standard PBS for 10 min at room temperature followed by incubation
for 30 sec in PBS at 80°C for antigen retrieval. The sections were
then treated with 1% H2O2 at room temperature
for 5 min to inactivate peroxidases if any. The sections were then
incubated in 1% BSA solution in PBS at room temperature for 1 h
followed by incubation with monoclonal HSP27 antibody (1:200
dilution for 2 h at room temperature; Santa Cruz Biotechnology,
Inc.). The sections were then washed in PBS at room temperature
(3x) followed by incubation with appropriate horseradish
peroxidase-conjugated secondary antibody at a dilution of 1:200 in
1% BSA in PBS for 1 h. The slides were the washed in PBS three
times (5 min each at room temperature) followed by the addition of
DAB Peroxidase (HRP) Substrate (cat. no., SK-4100, Vector Labs
Burlingame, CA). The DAB reaction proceeded until the desired color
developed, and was stopped by washing with PBS at room temperature.
Nucleus was counterstained with hematoxylin. Negative control
slides were obtained by treating with non-specific Immunoglobulin
G. Sections were mounted and analyzed using the EVOS-FL cell
imaging system. The intensity of HSP27 expression per unit area was
measured in arbitrary pixel units using Image J (version 1.4) and
represented as raw units.
Tumor density measurement
Hematoxylin stained tumor sections were analyzed and
an average number of tumor cells in random 10 different fields in
an area of 100 µm2 was measured and graphically
presented (300 dpi image at magnification ×10).
RNA isolation
SPARC-overexpressing NB1691 and SK-N-BE(2) cells were treated with 5 Gy of ionizing
radiation as described above. Total RNA was isolated using the
TRIzol method as per standard protocols (cat. no., 15596026; Thermo
Fisher Scientific, Inc.) (22) and
RNA quantity and quality were measured by NanoDrop ND-1000.
RNA labeling and array hybridization
(Arraystar, Inc.)
Sample labeling and array hybridization of total RNA
isolated from SPARC-overexpressing NB1691 and SK-N-BE (2) treated with or without 5 Gy of ionizing
radiation were performed according to the Agilent One-Color
Microarray-Based Gene Expression Analysis protocol (Agilent
Technologies, Inc., Santa Clara, CA, USA). The array consisted of
27,958 Entrez Gene RNAs, content sourced from RefSeq Build 36.3,
Ensemble Release 52, Unigene Build 216 and GenBank (April 2009) on
a 4×44K slide formats (http://www.agilent.com/cs/library/usermanuals/Public/G4140-90040_GeneExpression_OneColor_6.9.pdf).
Total RNA from each sample was linearly amplified and
simultaneously labeled with Cy3-UTP (cat. no., NEL580; PerkinElmer,
Inc., Waltham, MA, USA). The labeled cRNAs were purified by RNeasy
Mini kit as per manufacturer's protocol (Qiagen, Inc., Valencia,
CA, USA). A total of 1 µg of each labeled cRNA was fragmented by
adding 11 µl 10X Blocking Agent and 2.2 µl of 25X Fragmentation
Buffer, then heated at 60°C for 30 min, and finally 55 µl 2X GE
Hybridization buffer was added to dilute the labeled cRNA. A total
of 100 µl hybridization solution was dispensed into the gasket
slide and assembled to the gene expression microarray slide. The
slides were incubated for 17 h at 65°C in an Agilent Hybridization
Oven (Agilent Technologies, Inc.). The hybridized arrays were
washed using Gene Expression wash buffer with 0.005% Triton-X102
twice in wash buffer 1 at room temperature for 1 min and once in
wash buffer 2 at 37°C for 1 min followed by scanning using the
Agilent DNA Microarray Scanner (part no. G2505C; Agilent
Technologies, Inc.). RNA labeling, hybridization and data analysis
was performed by Arraystar Inc (Arraystar, Inc., Rockville, MD,
USA) using Agilent Feature Extraction software (version 11.0.1.1;
Agilent Technologies, Inc.).
Gene ontology enrichment data
analysis
This analysis was performed using Agilent Feature
Extraction software (version 11.0.1.1; Agilent Technologies, Inc.)
was used to analyze the acquired array images. Quantile
normalization and subsequent data processing were performed with
using the GeneSpring GX software (version 12.0; Agilent
Technologies, Inc.). Following quantile normalization of the raw
data, genes exhibiting enriched expression were selected for
further data analysis. Differentially expressed genes with
statistical significance were identified through Fold Change
filtering set to 1. Gene Ontology analysis was performed using the
standard enrichment computation method to functionally profile gene
sets and was graphically presented using Gene Ontology Consortium
tools (23) (http://geneontology.org/).
Statistical methods
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using Student's
t-test. P≤0.05 was considered to indicate a statistically
significant difference. Regression analysis was used to determine
the statistical validity of migration. All experiments were
performed in triplicate, or as indicated. For the pathway analysis
the enrichment P-value of the PathwayID used the EASE method, EASE
Score is a modified Fisher's exact probability by penalizing
(subtract) the count of positive agreement by 1.
Results
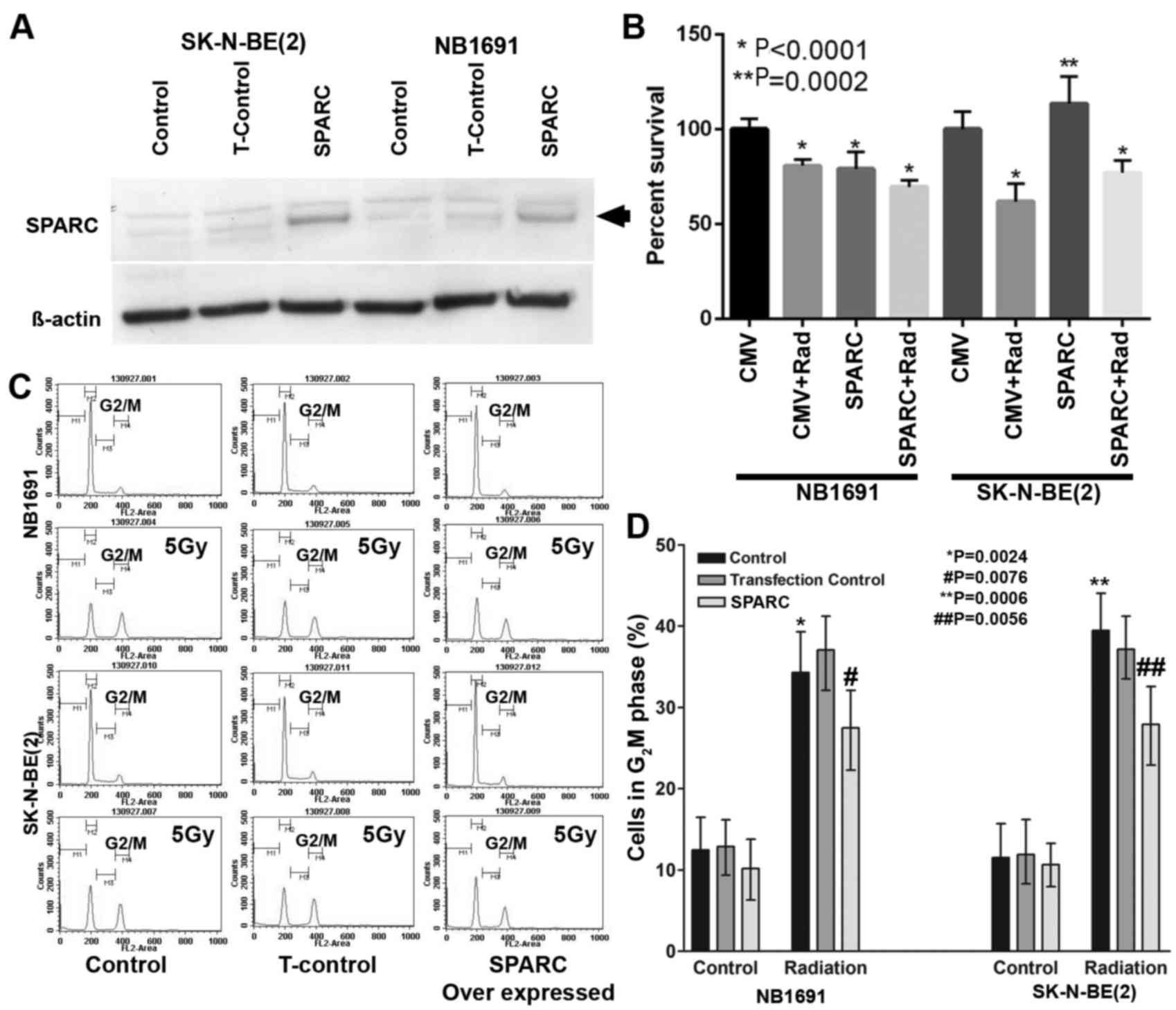
Overexpression of SPARC suppresses
proliferation and radiation-induced G2M arrest
To determine whether overexpression of SPARC
suppresses proliferation and radiation-induced G2M arrest, SPARC
was overexpressed in NB1691 and SK-N-BE(2) neuroblastoma cells (Fig. 1A). The MTT assay demonstrated that in
SPARC overexpressing and irradiated NB1691 cells, the proliferation
rate decreased significantly (P<0.0001), whereas in SK-N-BE2
cells an increase in proliferation was observed in SPARC
overexpressing cells compared with the controls (Fig. 1B), notably SPARC overexpressed and
radiation treated SK-N-BE2 cells showed decreased survival compared
with the untreated controls (Fig.
1B), this observation may be due to the varied p53 status of
NB1691 (wt) and SK-N-BE(2) (mt).
Furthermore, it was observed that radiation-induced accumulation of
cells in the G2 phase and the addition of SPARC overexpression
suppressed G2 phase accumulation in NB1691 and SK-N-BE(2) (Fig. 1C).
Quantitative analysis of G2M phase identified that radiation alone
in NB1691 cells resulted in 34±6% cells in the G2M phase
(P=0.0024), whereas the addition of SPARC overexpression and
radiation exhibited a 28±4% (P=0.0076) increase in G2M phase cells
compared with controls of which 12±3% cells were in the G2M phase.
Similar results were observed when SK-N-BE(2) cells were treated with radiation alone,
which resulted in a G2M phase accumulation of 39±4% (P=0.0006) and
the overexpression of SPARC and radiation exhibited a G2M phase
accumulation of 28±5% (P=0.0056; Fig.
1D).
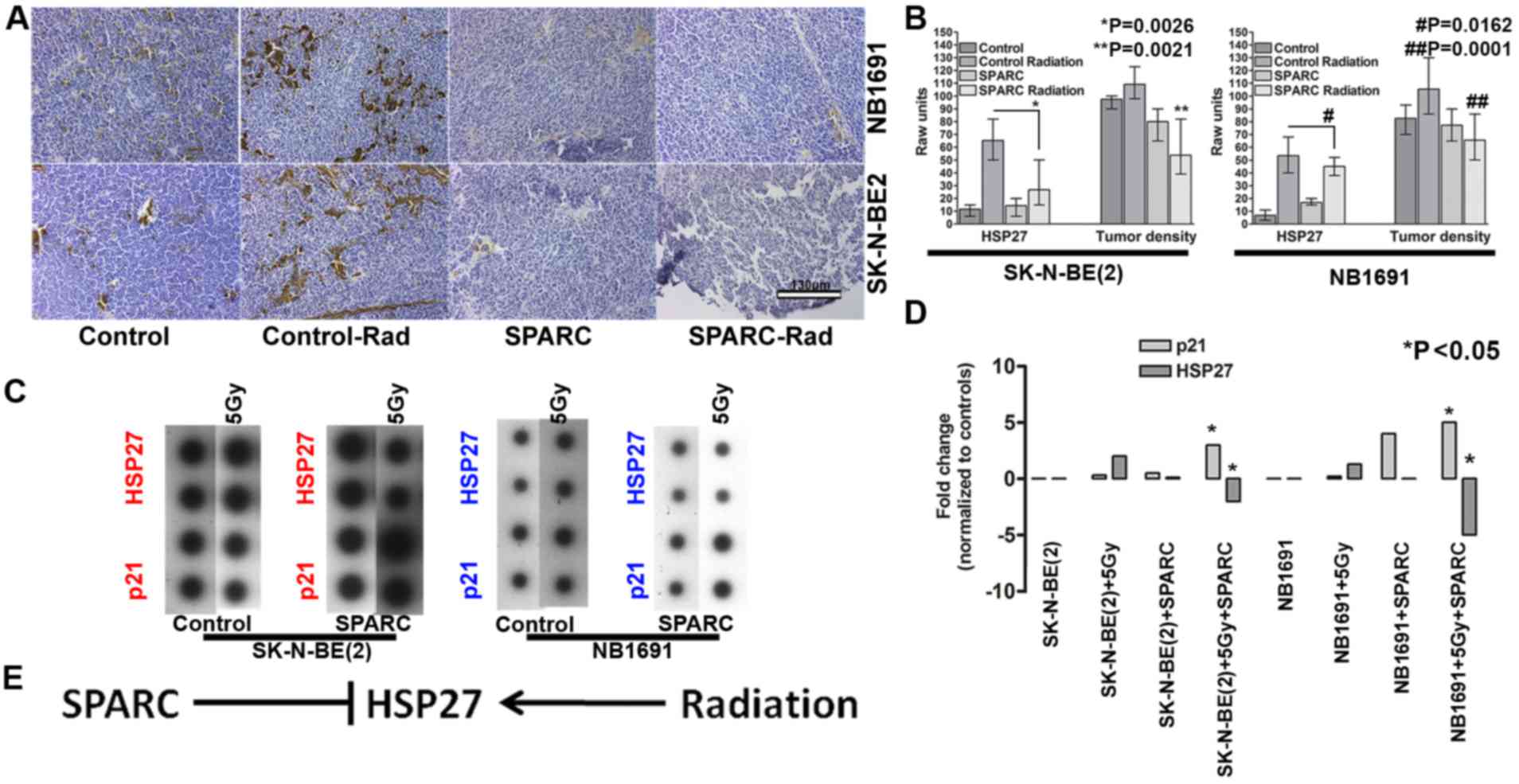
SPARC overexpression suppresses
radiation-induced HSP27
To determine whether the overexpression of SPARC
influences the expression of HSP27 in vivo, the nude mice
subcutaneous tumor model of neuroblastoma was used in the current
study (11). The results demonstrated
that radiation exposure increased HSP27 expression in NB1691 and
SK-N-BE(2) subcutaneous tumors. SPARC
overexpression reduced the radiation-induced HSP27 expression
indicating that SPARC may negatively regulate HSP27 (Fig. 2A). Quantitative analysis of HSP27
expression in the tumors identified radiation-induced HSP27
expression in 55±6% of NB1691 cells, whereas the addition of SPARC
reduced the radiation-induced HSP27 expression to 12±2% of cells
compared with untreated controls of which the expression rate of
HSP27 was determined to be 8±2% (P=0.0162; Fig. 2B). In SK-N-BE(2) cell tumors that were exposed to
radiation, HSP27 expression was increased to 59±2% and the addition
of SPARC reduced the radiation-induced HSP27 expression to 16±4%
(P=0.0026). To additionally determine whether SPARC causes a
reduction in tumor burden, relative tumor density was evaluated by
measuring the number of cells per unit area in a hematoxylin
stained section. Untreated NB1691 tumors exhibited the highest
tumor density at 138±10 cells per 100 µm2. The addition
of radiation treatment decreased the tumor density to 112±8 cells
per 100 µm2. Further addition of SPARC overexpression
reduced the tumor density further to 68±5 cells per 100
µm2 (P=0.0001 Fig. 2B)
compared with the radiated controls. Tumor density of
NB1691-derived tumors following treatment with SPARC alone was
similar to the densities of irradiated tumors (110±6 cells per 100
µm2). SK-N-BE(2)-derived
tumors exhibited similar tumor density patterns with the control
group at 135±7 cells per 100 µm2, SPARC overexpressing
tumors at 100±12 cells per 100 µm2, radiation treated
tumors at 94±5 cells per 100 µm2 and SPARC and radiation
treated tumors at 65±11 cells per 100 µm2 (P=0.0021;
Fig. 2B). Similarly, in vitro
cells treated with radiation induced the expression of the
radiation response gene HSP27, in NB1691 and SK-N-BE(2) cells. Overexpression of SPARC accompanied
with irradiation suppressed HSP27 expression in NB1691 (5-fold) and
SK-N-BE(2) (2-fold) cells.
Furthermore, it was also observed that the expression of p21
increased in SPARC overexpressing irradiated (5 Gy) cells: 4-fold
in NB1691 cells; 2.5-fold SK-N-BE(2)
cells (Fig. 2C and D). There was an
inverse association between the expression of HSP27 and p21, in
SPARC overexpressing irradiated environment (P<0.05), this is in
accordance with a previous study that established that HSP27
protects cells from the p21 apoptotic pathway (Fig. 2E) (12).
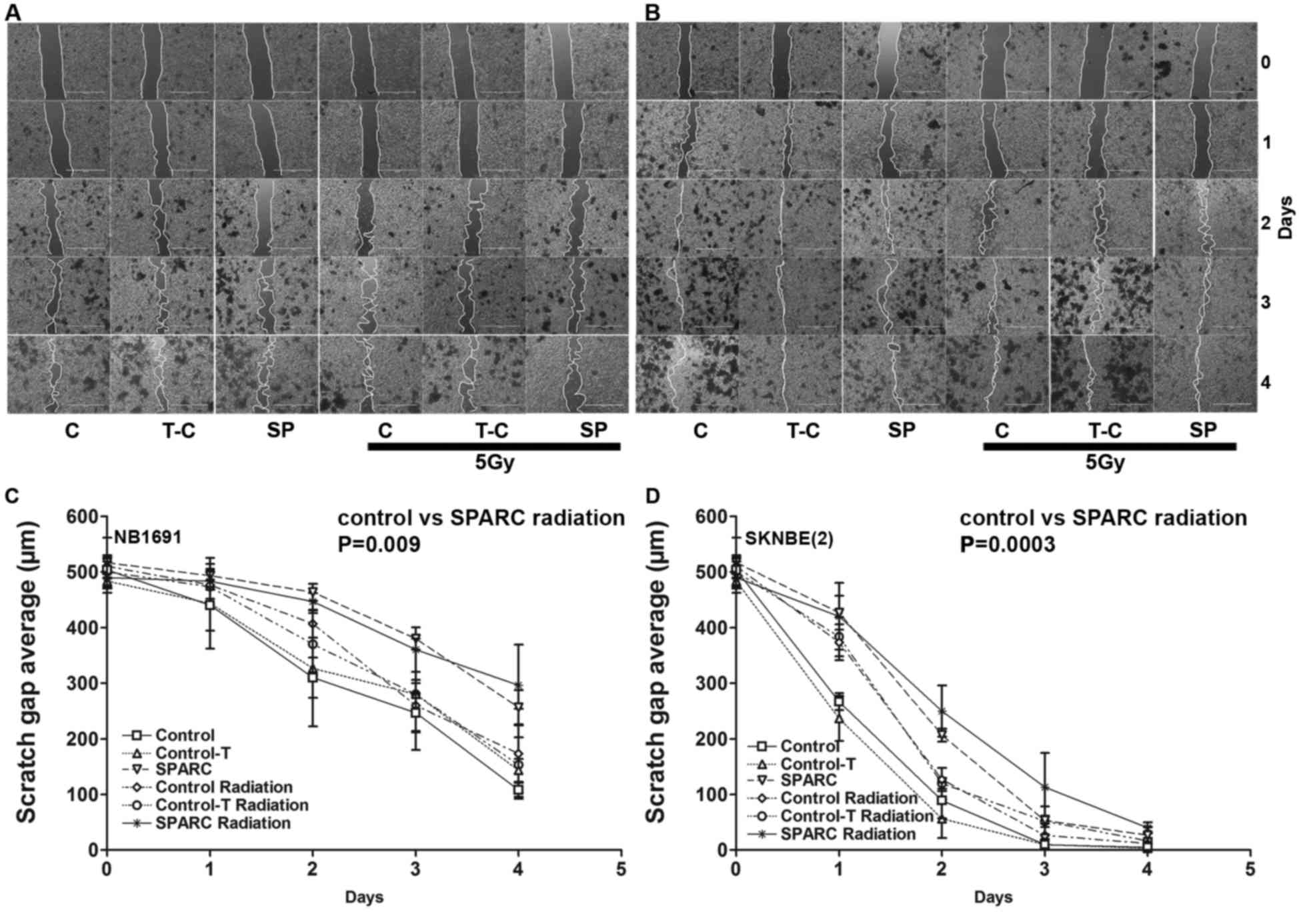
SPARC overexpression and irradiation
suppresses tumor cell migration
To determine whether SPARC overexpression suppresses
tumor cell migration, the in vitro wound healing assay
(13) was performed. The
overexpression of SPARC and irradiation reduced the migration
potential of NB1691 and SK-N-BE(2)
cells (Fig. 3A and B). Quantitative
analysis demonstrated that SPARC overexpressing cells had a reduced
migration potential in NB1691 (300±30 µm; R2=0.77) and
SK-N-BE(2) (40±8 µm;
R2=0.90) after 4 days of migration compared with
controls (NB1691, 100±10 µm; SK-N-BE(2), 3±1 µm; Fig. 3C
and D).
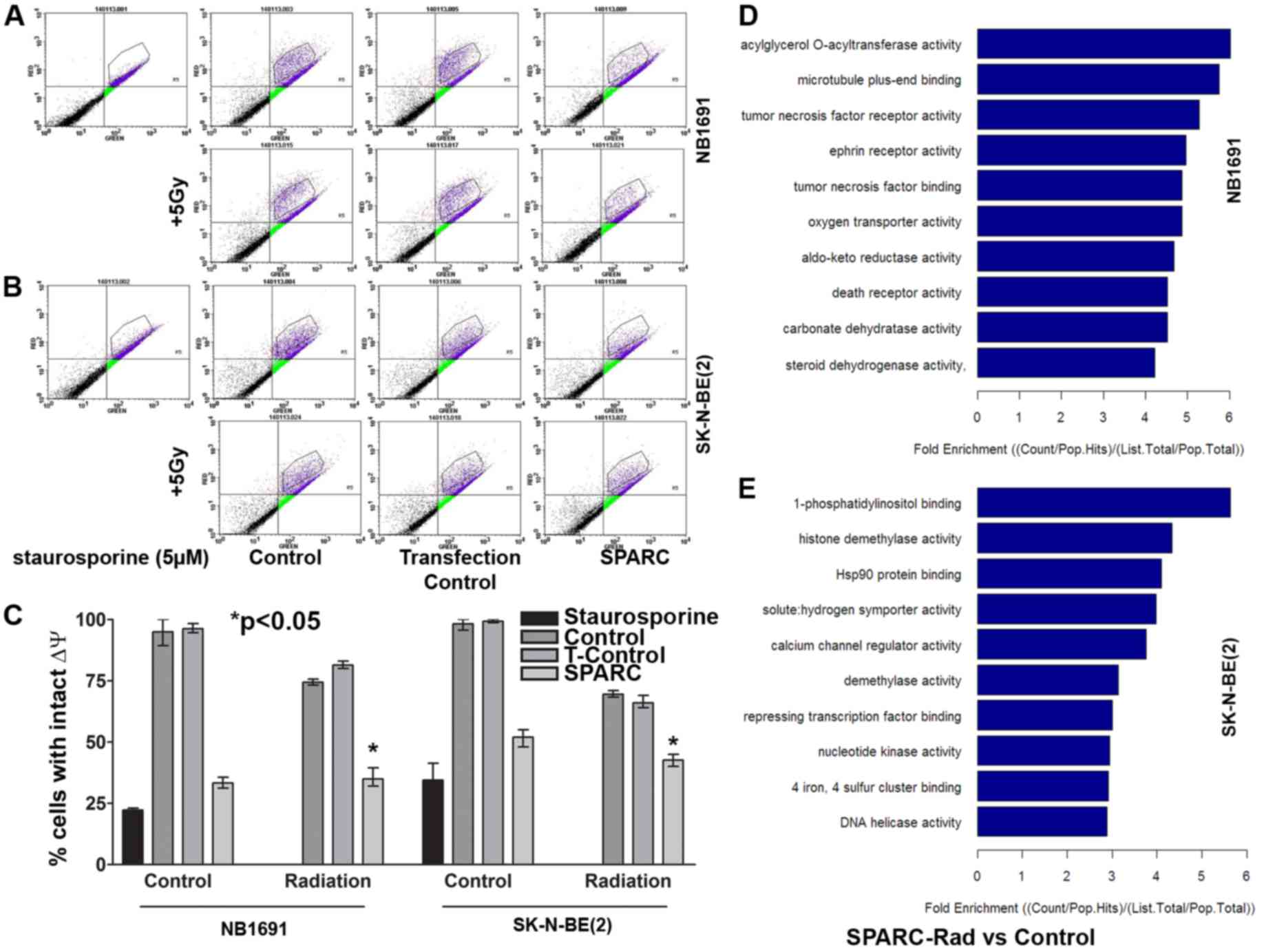
SPARC overexpression and irradiation
induces mitochondrial Δψ collapse and induces caspase mediated cell
death
A previous study established that targeting HSP27
promotes radio sensitization (14).
Previous studies have also demonstrated that HSP27 protects against
mitochondrial-induced apoptosis (15,16).
Therefore the present study aimed to determine whether SPARC
facilitates the suppression of HSP27-mediated protection of
mitochondrial Δψ. NB1691 and SK-N-BE(2) cells with overexpressed SPARC were
irradiated and the mitochondrial Δψ was determined from MitoProbe™
JC-1 stained cells evaluated using flow cytometry analysis. SPARC
overexpression reduced the mitochondrial Δψ to 70±3% and the
addition of irradiation reduced the mitochondrial Δψ to 64±6% in
NB1691 cells when compared to untreated control (P<0.05). In
SK-N-BE(2) cells, SPARC
overexpression alone reduced the mitochondrial Δψ to 46±3% and the
addition of irradiation reduced the mitochondrial Δψ to 53±2% when
compared with the untreated control (P<0.05) (Fig. 4A-C).
Overexpression of SPARC accompanied
with irradiation induces differential molecular function response
genes in NB1691 and SK-N-BE(2)
neuroblastoma cells
To determine the molecular function response genes
in NB1691 and SK-N-BE(2) cells
overexpressing SPARC and treated with irradiation, genome
expression analysis was performed using the Allegiant whole genome
array (Arrarstar Service). SPARC overexpression accompanied with
irradiation induced the enrichment of genes associated with the
death receptor pathway (<4 fold) and TNF receptor-associated
molecules (<5 fold) in NB1691 cells. In SK-N-BE(2) cells, an enrichment of genes associated
with HSP90 binding (<4 fold) and calcium channel regulator
activity (<4 fold) were observed (Fig.
4D and E).
Overexpression of SPARC accompanied
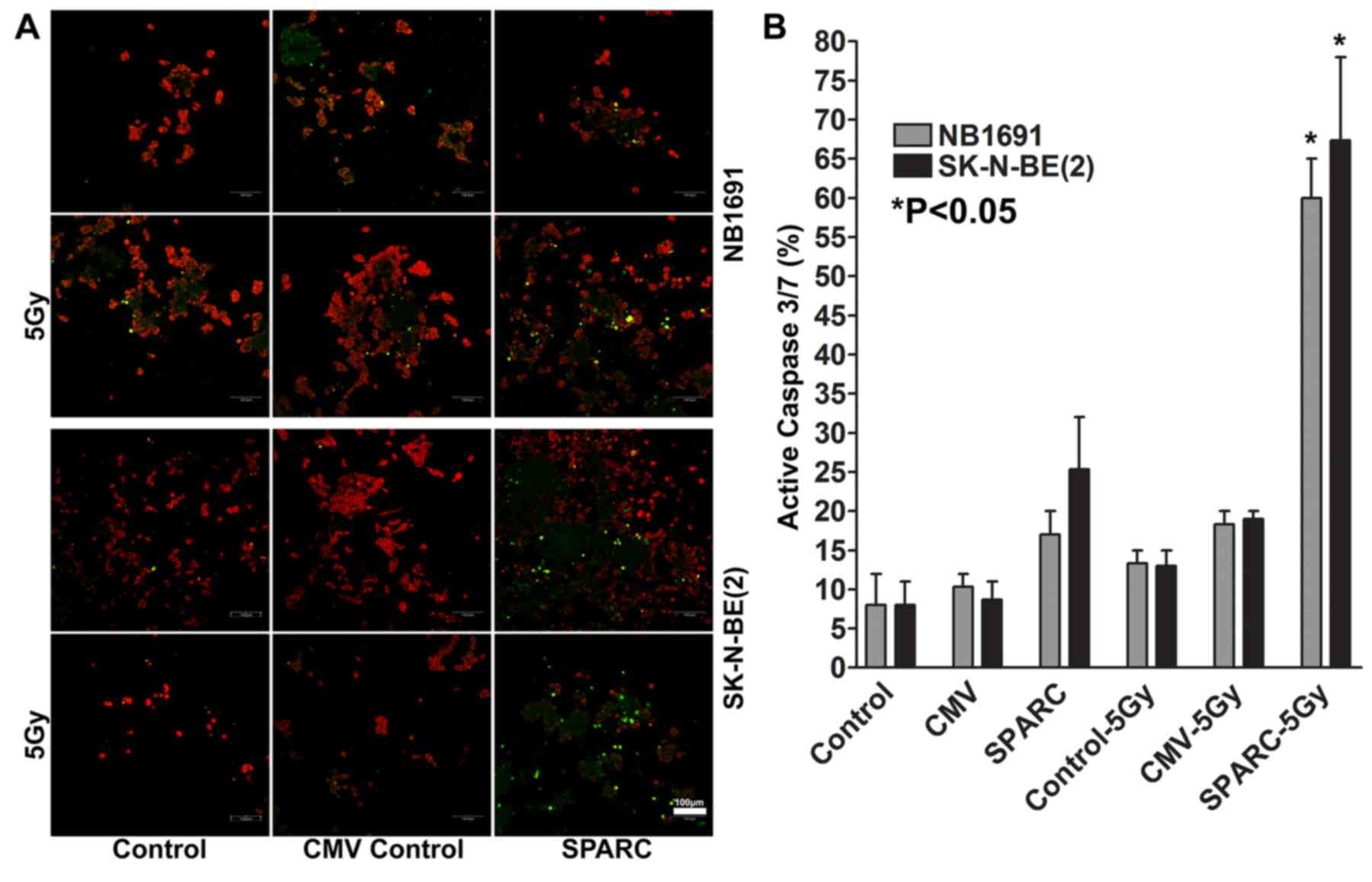
with irradiation induces activation of caspase 3/7
Caspase activation studies demonstrated that SPARC
overexpression increased apoptotic events by 16±4% in NB1691 cells
and by 24±8% in SK-N-BE(2)
neuroblastoma cells, and the addition of irradiation increased the
number of apoptotic events by 60±5% in NB1691 when compared to
untreated controls (P<0.05) and by 67±12% in SK-N-BE(2) cells compared with untreated controls
(P<0.05; Fig. 5A and B).
Discussion
SPARC, which is also known as osteonectin, belongs
to the matricellular family of secreted proteins (24,25). A
number of biological functions in human cancer have been reported
to involve SPARC. In neuroblastoma, SPARC expression has been found
to be associated with impaired tumor growth and angiogenesis
(26,27). A previous study demonstrated that the
overexpression of SPARC induced autophagy-mediated apoptosis in
primitive neuroectodermal tumor cells (13). The present study investigates the
underlying mechanisms prior to the induction of apoptosis. SK-N-BE2
and NB1691 neuroblastoma cell lines were subjected to
overexpression of SPARC with and without ionizing radiation
treatment. To determine the potential for resistance to
irradiation, the cells were screened for established radiation
response molecules and it was observed that HSP27 was overexpressed
in NB1691 and SK-N-BE(2) cells
following radiation treatment. HSP27, also known as heat shock
protein beta-1, is an inhibitor of apoptosis (28,29).
Previous studies have reported that HSP27 protects against
caspase-3 activation thereby inhibiting caspase-3 mediated
apoptosis (30–32). Previous studies have also demonstrated
that the overexpression of HSP27 reduces the levels of apoptosis
(33) and inhibition of HSP27
radio-sensitizes tumor cells (34).
Notably, a previous study suggested that HSP27-mediates gemcitabine
sensitivity in pancreatic cancer cells indicating a pro-apoptotic
role of HSP27 as well as the extrinsic apoptosis pathway function
(35). Another study has identified
that the downregulation of HSP27 increases the sensitivity of
pancreatic cancer cells to gemcitabine (36). These contradictory results demonstrate
that tumors cells differ and targeting tumors based on one single
observation may not be clinically relevant. In the present study,
two cell lines with varied p53 backgrounds were used. NB1691 is
wild type for p53, and SK-N-BE(2) has
a p53 mutation, and using these cell lines, it was observed that
the effects of SPARC overexpression were not contradictory with
previous studies where the status of p53 or other mutations were
not indicated (35,36). Studies that were published within the
previous decade, reported that HSP27 may serve a role in the
regulation of cellular senescence via the modulation of the p53
pathway, in which HSP27 downregulation was associated with the
stabilization of p53 suggesting that HSP27 expression may
suppresses p53 function (37).
Although the current study observed that radiation-induced
expression of HSP27 did not promote the inhibition of apoptosis as
demonstrated previously (38). This
indicates that radiation accompanied by SPARC overexpression may be
a viable therapeutic option for neuroblastoma. Notably, the results
of the current study also identified that SPARC induces the
reduction of the mitochondrial Δψ and activates caspases, and
radiation exposure alone did not significantly influence
mitochondrial Δψ. This suggests that SPARC may activate the
mitochondrial-associated cell death pathway and promote the
radiation-induced interjection of HSP27 that reverses apoptotic
induction. However, as SPARC overexpression suppresses
radiation-induced HSP27 and also activates caspases, this may
suppress any rescue of SPARC-induced mitochondrial Δψ
collapse-mediated cell death (39,40).
In conclusion, the results of the present study
demonstrate that SPARC overexpression suppresses radiation-induced
G2M arrest and HSP27, independently of p53 status in human
neuroblastoma cells.
Acknowledgements
The present study was supported by the National
Cancer Institute (Rockville, MD, USA; grant no. R01CA147792) and
the Department of Internal Medicine University of Illinois College
of Medicine, Peoria, IL 61605, USA. Services provided by Arraystar
(Arraystar, Inc., Rockville, MD, USA) for micro array analysis and
gene ontology analysis is acknowledged.
Glossary
Abbreviations
Abbreviations:
|
SPARC
|
secreted protein acidic and rich in
cysteine
|
|
HSP27
|
heat shock protein 27
|
|
ECM
|
extracellular matrix
|
References
|
1
|
Louis CU and Shohet JM: Neuroblastoma:
Molecular pathogenesis and therapy. Annu Rev Med. 66:49–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinto NR, Applebaum MA, Volchenboum SL,
Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F,
Schleiermacher G, Park JR, et al: Advances in risk classification
and treatment strategies for neuroblastoma. J Clin Oncol.
33:3008–3017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
PDQ Pediatric Treatment Editorial Board:
Neuroblastoma treatment (PDQ(R)): Health professional version. PDQ
cancer information summaries, Bethesda (MD). 2002.
|
|
4
|
Luksch R, Castellani MR, Collini P, de
Bernardi B, Conte M, Gambini C, Gandola L, Garaventa A, Biasoni D,
Podda M, et al: Neuroblastoma (peripheral neuroblastic tumours).
Crit Rev Oncol Hematol. 107:163–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huynh MH, Sage EH and Ringuette M: A
calcium-binding motif in SPARC/osteonectin inhibits chordomesoderm
cell migration during xenopus laevis gastrulation: Evidence of
counter-adhesive activity in vivo. Dev Growth Differ. 41:407–418.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark CJ and Sage EH: A prototypic
matricellular protein in the tumor microenvironment-where there's
SPARC, there's fire. J Cell Biochem. 104:721–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagaraju GP and Sharma D: Anti-cancer role
of SPARC, an inhibitor of adipogenesis. Cancer Treat Rev.
37:559–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han W, Cao F, Chen MB, Lu RZ, Wang HB, Yu
M, Shi CT and Ding HZ: Prognostic value of SPARC in patients with
pancreatic cancer: A systematic review and meta-analysis. PLoS One.
11:e01458032016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng J and Tang L: SPARC in tumor
pathophysiology and as a potential therapeutic target. Curr Pharm
Des. 20:6182–6190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Said N and Motamed K: Absence of
host-secreted protein acidic and rich in cysteine (SPARC) augments
peritoneal ovarian carcinomatosis. Am J Pathol. 167:1739–1752.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang MJ and Tai IT: A novel interaction
between procaspase 8 and SPARC enhances apoptosis and potentiates
chemotherapy sensitivity in colorectal cancers. J Biol Chem.
282:34457–34467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chlenski A, Guerrero LJ, Peddinti R, Spitz
JA, Leonhardt PT, Yang Q, Tian Y, Salwen HR and Cohn SL:
Anti-angiogenic SPARC peptides inhibit progression of neuroblastoma
tumors. Mol Cancer. 9:1382010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhoopathi P, Chetty C, Gujrati M, Dinh DH,
Rao JS and Lakka S: Cathepsin B facilitates autophagy mediated
apoptosis in SPARC overexpressed primitive neuroectodermal tumor
cells. Cell Death Differ. 17:1529–1539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhoopathi P, Gorantla B, Sailaja GS, Gondi
CS, Gujrati M, Klopfenstein JD and Rao JS: SPARC overexpression
inhibits cell proliferation in neuroblastoma and is partly mediated
by tumor suppressor protein PTEN and AKT. PLoS One. 7:e360932012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golembieski WA, Thomas SL, Schultz CR,
Yunker CK, McClung HM, Lemke N, Cazacu S, Barker T, Sage EH, Brodie
C and Rempel SA: HSP27 mediates SPARC-induced changes in glioma
morphology, migration, and invasion. Glia. 56:1061–1075. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blattes GB, Mestieri LB, Böttcher DE,
Fossati AC, Montagner F and Grecca FS: Cell migration, viability
and tissue reaction of calcium hypochlorite based-solutions
irrigants: An in vitro and in vivo study. Arch Oral Biol. 73:34–39.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grada A, Otero-Vinas M, Prieto-Castrillo
F, Obagi Z and Falanga V: Research techniques made simple: Analysis
of collective cell migration using the wound healing assay. J
Invest Dermatol. 137:e11–e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salvioli S, Dobrucki J, Moretti L, Troiano
L, Fernandez MG, Pinti M, Pedrazzi J, Franceschi C and Cossarizza
A: Mitochondrial heterogeneity during staurosporine-induced
apoptosis in HL60 cells: Analysis at the single cell and single
organelle level. Cytometry. 40:189–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Perchellet EM, Ward MM, Lou K, Hua
DH and Perchellet JP: Rapid collapse of mitochondrial transmembrane
potential in HL-60 cells and isolated mitochondria treated with
anti-tumor 1,4-anthracenediones. Anticancer Drugs. 16:953–967.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hawley-Nelson P and Ciccarone V:
Transfection of cultured eukaryotic cells using cationic lipid
reagents. Curr Protoc Neurosci Appendix. 1:Appendix 1F. 2001.
View Article : Google Scholar
|
|
21
|
Paul CA, Beltz B and Berger-Sweeney J:
Subbing slides. CSH Protoc. 2008:pdb.prot4804. 2008.
|
|
22
|
Chadderton T, Wilson C, Bewick M and Glück
S: Evaluation of three rapid RNA extraction reagents: Relevance for
use in RT-PCR's and measurement of low level gene expression in
clinical samples. Cell Mol Biol (Noisy-le-Grand). 43:1227–1234.
1997.PubMed/NCBI
|
|
23
|
Mi H, Muruganujan A, Casagrande JT and
Thomas PD: Large-scale gene function analysis with the PANTHER
classification system. Nat Protoc. 8:1551–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lane TF and Sage EH: The biology of SPARC,
a protein that modulates cell-matrix interactions. FASEB J.
8:163–173. 1994.PubMed/NCBI
|
|
25
|
Porter PL, Sage EH, Lane TF, Funk SE and
Gown AM: Distribution of SPARC in normal and neoplastic human
tissue. J Histochem Cytochem. 43:791–800. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorantla B, Bhoopathi P, Chetty C,
Gogineni VR, Sailaja GS, Gondi CS and Rao JS: Notch signaling
regulates tumor-induced angiogenesis in SPARC-overexpressed
neuroblastoma. Angiogenesis. 16:85–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ribatti D: Anti-angiogenesis in
neuroblastoma. Crit Rev Oncol Hematol. 86:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arya R, Mallik M and Lakhotia SC: Heat
shock genes-integrating cell survival and death. J Biosci.
32:595–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bao XQ and Liu GT: Bicyclol protects HepG2
cells against D-galactosamine-induced apoptosis through inducing
heat shock protein 27 and mitochondria associated pathway. Acta
Pharmacol Sin. 31:219–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pandey P, Farber R, Nakazawa A, Kumar S,
Bharti A, Nalin C, Weichselbaum R, Kufe D and Kharbanda S: Hsp27
functions as a negative regulator of cytochrome c-dependent
activation of procaspase-3. Oncogene. 19:1975–1981. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li R, Li J, Sang D and Lan Q:
Phosphorylation of AKT induced by phosphorylated Hsp27 confers the
apoptosis-resistance in t-AUCB-treated glioblastoma cells in vitro.
J Neurooncol. 121:83–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu HS, Lin JH, Huang WC, Hsu TW, Su K,
Chiou SH, Tsai YT and Hung SC: Chemoresistance of lung cancer
stemlike cells depends on activation of Hsp27. Cancer.
117:1516–1528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Zhou HS, Cheng Q, Lei L and Hu B:
Overexpression of HSP27 in cultured human aortic smooth muscular
cells reduces apoptosis induced by low-frequency and low-energy
ultrasound by inhibition of an intrinsic pathway. Genet Mol Res.
12:6588–6601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guttmann DM, Hart L, Du K, Seletsky A and
Koumenis C: Inhibition of Hsp27 radiosensitizes head-and-neck
cancer by modulating deoxyribonucleic acid repair. Int J Radiat
Oncol Biol Phys. 87:168–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo Y, Ziesch A, Hocke S, Kampmann E, Ochs
S, de Toni EN, Göke B and Gallmeier E: Overexpression of heat shock
protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic
cancer cells through S-phase arrest and apoptosis. J Cell Mol Med.
19:340–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Zhang XQ, Huang SL, Chen M, Shen
SS, Ding XW, Lv Y and Zou XP: The effects of HSP27 on
gemcitabine-resistant pancreatic cancer cell line through snail.
Pancreas. 44:1121–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Callaghan-Sunol C, Gabai VL and Sherman
MY: Hsp27 modulates p53 signaling and suppresses cellular
senescence. Cancer Res. 67:11779–11788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sailaja GS, Bhoopathi P, Gorantla B,
Chetty C, Gogineni VR, Velpula KK, Gondi CS and Rao JS: The
secreted protein acidic and rich in cysteine (SPARC) induces
endoplasmic reticulum stress leading to autophagy-mediated
apoptosis in neuroblastoma. Int J Oncol. 42:188–196.
2013.PubMed/NCBI
|
|
39
|
Fulda S: Targeting apoptosis for
anticancer therapy. Semin Cancer Biol. 31:84–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim B, Srivastava SK and Kim SH: Caspase-9
as a therapeutic target for treating cancer. Expert Opin Ther
Targets. 19:113–127. 2015. View Article : Google Scholar : PubMed/NCBI
|