Introduction
Retinoblastoma, the most common primary malignancy
in the retina, usually occurs in childhood and accounts for 2–4% of
all childhood malignancies. The morbidity rate is
~1:15,000–1:20,000 in children <5 years of age (1,2). In 2015,
it was estimated that there were 2,580 new cases in the United
States (3). Various factors are
involved in the carcinogenesis and development of retinoblastoma,
including genetic and epigenetic alterations to oncogenes and tumor
suppressor genes, and a large number of these have previously been
demonstrated to regulate cell growth, the cell cycle and apoptosis
(4,5).
In developed countries, >95% of patients with retinoblastoma
have a good prognosis. However, in patients with advanced disease,
the prognosis remains unsatisfactory due to metastasis to distant
organs (6,7). Currently, the main therapeutic
strategies for patients with retinoblastoma are surgery (removal of
the eyes), thermotherapy, cryotherapy, chemotherapy (systemic and
local delivery) and radiotherapy (8).
Despite advances in the development of therapeutic strategies for
patients with retinoblastoma, a proportion of retinoblastoma cases
respond only transiently to these treatments (9). Given this, it is necessary to further
explore the molecular mechanisms underlying the initiation and
progression of retinoblastoma and to develop effective treatments,
including molecular targeted therapy, a novel potential option for
individualized therapy.
Accumulating evidence suggests that abnormal
expression levels of a variety of microRNAs (miRNAs) are often
present in various types of human cancer, including retinoblastoma,
indicating the potential functions of miRNAs in carcinogenesis and
cancer progression (10–12). miRNAs, which are a class of
single-stranded, short (~23 bases in length) and non-protein-coding
RNAs, act as negative regulators of their target mRNAs by binding
to the 3′ untranslated region (UTR) of the mRNA, resulting in
target degradation or translational repression (5,13). An
increasing number of studies have revealed that miRNAs are involved
in a wide variety of physiological and pathological processes,
including cell proliferation, the cell cycle, apoptosis,
differentiation, angiogenesis, migration, invasion and metastasis
(14,15). miRNAs may act as oncogenes or tumor
suppressors in cancer, depending on their target mRNA (16). miRNAs that are downregulated in cancer
normally act as tumor suppressors, whereas upregulated miRNAs
usually act as oncogenes (17).
Therefore, the identification of target mRNAs of miRNAs is
important in order to further understand the roles of miRNAs in
cancer initiation and progression. Due to the regulatory functions
of miRNAs in cancer initiation and progression, targeting of miRNAs
should be investigated as a novel therapeutic strategy.
miR-497, a member of the miR-15/16/195/424/497
family, has been demonstrated to be aberrantly expressed in
numerous types of cancer (18).
However, its expression, function and associated molecular
mechanisms in retinoblastoma have not been previously studied. The
results of the present study indicate that miR-497 is downregulated
in human retinoblastoma tissues and cell lines, and that ectopic
expression of miR-497 can suppress proliferation, migration and
invasion in retinoblastoma cells via blocking vascular endothelial
growth factor A (VEGFA). These findings indicate that miR-497 may
be a potential targeted therapy for retinoblastoma.
Materials and methods
Human retinoblastoma tissue
samples
The present study was approved by the Human Subjects
Committee of Xi'an XD Group Hospital (Xi'an, China). Written
informed consent was also obtained from all patients prior to
enrollment in the study. In total, 23 human retinoblastoma tissue
samples and 5 normal retinal tissues were used in the present
study, and were obtained from patients with retinoblastoma who had
undergone surgery in Xi'an XD Group Hospital. These patients had
not received thermotherapy, cryotherapy, chemotherapy or
radiotherapy prior to surgery. Tissues were immediately snap-frozen
in liquid nitrogen and transferred to a −80°C freezer until
use.
Cell culture
The Y79 and WERI-Rb-1 human retinoblastoma cell
lines were purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were maintained in Gibco RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% Gibco fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) in humidified atmosphere of
5% CO2 at 37°C.
Cell transfection
Mature miR-497 mimics and the negative control miRNA
(NC) were synthesized and purchased from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). To assess the functions of miR-497 in
retinoblastoma, miR-497 mimics and NC were transfected into Y79 and
WERI-Rb-1 cells. Prior to transfection for 12 h, cells were seeded
on 6-well plates at a density of 8×105 and cultured in
RPMI-1640 without FBS and antibiotics at 37°C. Cell transfection
and co-transfection (with luciferase reporter constructs, as
described subsequently) were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
After transfection for 4–6 h, cell culture medium was replaced with
RPMI-1640 supplemented with 10% FBS.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA samples from retinoblastoma tissue
samples, normal retinal tissues and retinoblastoma cell lines were
isolated using TRIzol® reagent (Invitrogen, Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Total RNA was reverse transcribed to cDNA using a PrimeScript RT
Reagent kit (Takara Biotechnology, Co., Ltd., Dalian, China).
RT-qPCR analyses were conducted to detect the expression levels of
VEGFA mRNA using Power SYBR-Green PCR Master Mix (Thermo Fisher
Scientific, Inc.). The thermocycling conditions of qPCR were as
follows: 95°C for 10 min; 40 cycles of 95°C for 15 sec; and 60°C
for 1 min. This reaction included 2 µl cDNA (200 ng), 2 µl forward
primer and 2 µl reverse primer.
miR-497 expression was determined using a SYBR
Premix Ex Taq mix (Takara Biotechnology, Co., Ltd.). This stage was
performed with cycling conditions as follows: 5 min at 95°C;
followed by 40 cycles of 95°C for 30 sec; and 65°C for 45 sec. An
ABI 7500 sequence detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for thermal cycling. This
reaction included 2 µl cDNA (200 ng), 2 µl forward primer and 2 µl
reverse primer. The primer sequences used for qPCR were: miR-497
forward, 5-CCAGTCTCAGGGTCCGAGGTATTC-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; U6 forward, 5′-CGCTTCGGCAGCACATATACTA-3′
and reverse, 5′-GCGAGCACAGAATTAATACGAC-3′; VEGFA forward,
5′-ACTTTCTGCTGTCTTGGGTG-3′ and reverse, 5′-CTGCATGGTGATGTTGGACT-3′;
and GAPDH forward, 5′-TGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-CCAGTAGAGGCAGGGATGAT-3′. Relative expression levels were
determined normalized to the expression level of GAPDH or U6.
Relative expression levels were evaluated using the
2−ΔΔCq method (19). All
samples were analyzed in triplicate.
Cell Counting Kit-8 assay
Following a 24-h transfection with miR-497 or NC,
transfected cells were collected and resuspended in culture medium,
and 3,000 transfected cells in 100 µl medium were seeded into each
well in 96-well plates. Following various incubation times (24–96
h), cells were treated with CCK-8 reagent (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan), according to manufacturer's
protocol. In brief, 10 µl CCK-8 assay solution was added to each
well and incubated in at 37°C for 2 h. Absorbance at 450 nm was
detected using the Thermo Scientific Microplate Reader (Thermo
Fisher Scientific, Inc.). Each sample was analyzed in
triplicate.
Cell migration and invasion
assays
The cell migration and invasion assays were
performed using Transwell chambers with 8-µm pores (Corning
Incorporated, Corning, NY, USA)., The Transwell chambers were
coated with Matrigel matrix (BD Biosciences, Franklin Lakes, NJ,
USA) for the invasion assay.
Following a 48-h transfection, transfected cells
(miR-497 and NC) were collected and resuspended in culture medium
without FBS. Transfected cells (1×105) in 300 µl
FBS-free medium were added to the upper chambers, and 500 µl
culture medium containing 20% FBS was added to the lower chambers.
Following incubation at 37°C for 24 h, the Transwell chambers were
stained with 0.5% crystal violet (Beyotime Institute of
Biotechnology, Haimen, China) for 15 min. Subsequently,
non-migrated and non-invaded cells were removed using cotton swabs.
Migrated and invaded cells were imaged and counted using an
inverted microscope (Olympus Corporation, Tokyo, Japan). Each
experiment was repeated at least three times.
Bioinformatic predication
The potential targets of miR-497 were analyzed using
miRanda (http://www.microrna.org) and TargetScan
(http://www.targetscan.org/).
Western blot analysis
Following a 72-h transfection, protein was extracted
from transfected cells (miR-497 and NC) using RIPA lysis buffer (50
mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% Na-deoxycholate; 150 mM NaCl;
1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; 1 µg/ml each of
aprotinin, leupeptin, pepstatin; 1 mM Na3VO4;
1 mM NaF). Protein concentration was detected using a bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology). Subsequently,
equal amounts of protein were separated by 10% SDS-PAGE (Bioworld
Technology, Inc., St. Louis Park, MN, USA) and electro-transferred
to polyvinylidene difluoride membranes (Beyotime Institute of
Biotechnology). The membranes were blocked with 5% non-fat milk for
1 h at room temperature, and then incubated at 4°C overnight with a
mouse anti-human VEGFA monoclonal primary antibody (dilution,
1:1,000; cat no. ab155944; Abcam, Tokyo, Japan) and mouse
anti-human β-actin monoclonal primary antibody (dilution, 1:1000;
cat no. sc-130,065; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Subsequently, the membranes were incubated with a
corresponding horseradish peroxidase-conjugated secondary antibody
(dilution, 1:5,000; cat no. sc-2005; Santa Cruz Biotechnology,
Inc.) in TBS with Tween-20 (Beyotime Institute of Biotechnology).
The bands were detected using an enhanced chemiluminescence
solution (Pierce; Thermo Fisher Scientific, Inc.) and imaged using
the FluorChem imaging system (Alpha Innotech, San Leandro, CA,
USA). Protein expression was quantified using Quantity One software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin was used
as the internal control. This assay was repeated three times.
Dual-Luciferase reporter assay
In order to explore whether VEGFA was a direct
target of miR-497, a Dual-Luciferase Reporter Assay (Promega
Corporation, Madison, WI, USA) was performed. Cells were
transfected with miR-497 mimics or NC and co-transfected with pGL3
Luciferase Reporter Vectors (Promega Corporation) containing
wild-type (Wt) or mutated (Mut) 3′-UTR sequences of VEGFA
(pGL3-VEGFA-3′-UTR-Wt and pGL3-VEGFA-3′-UTR-Mut, respectively).
After 48 h, Dual-Luciferase Reporter Assays were performed to
detect firefly and Renilla luciferase activities. Renilla
luciferase activities were evaluated as an internal control. Each
sample was assayed in triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments, and were statistically
analyzed using two-tailed Student's t test or one-way analysis of
variance with SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA). Student-Newman-Keuls method was used to compare between
two groups in multiple groups. Two-tailed P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-497 expression is downregulated in
retinoblastoma tissues and cell lines
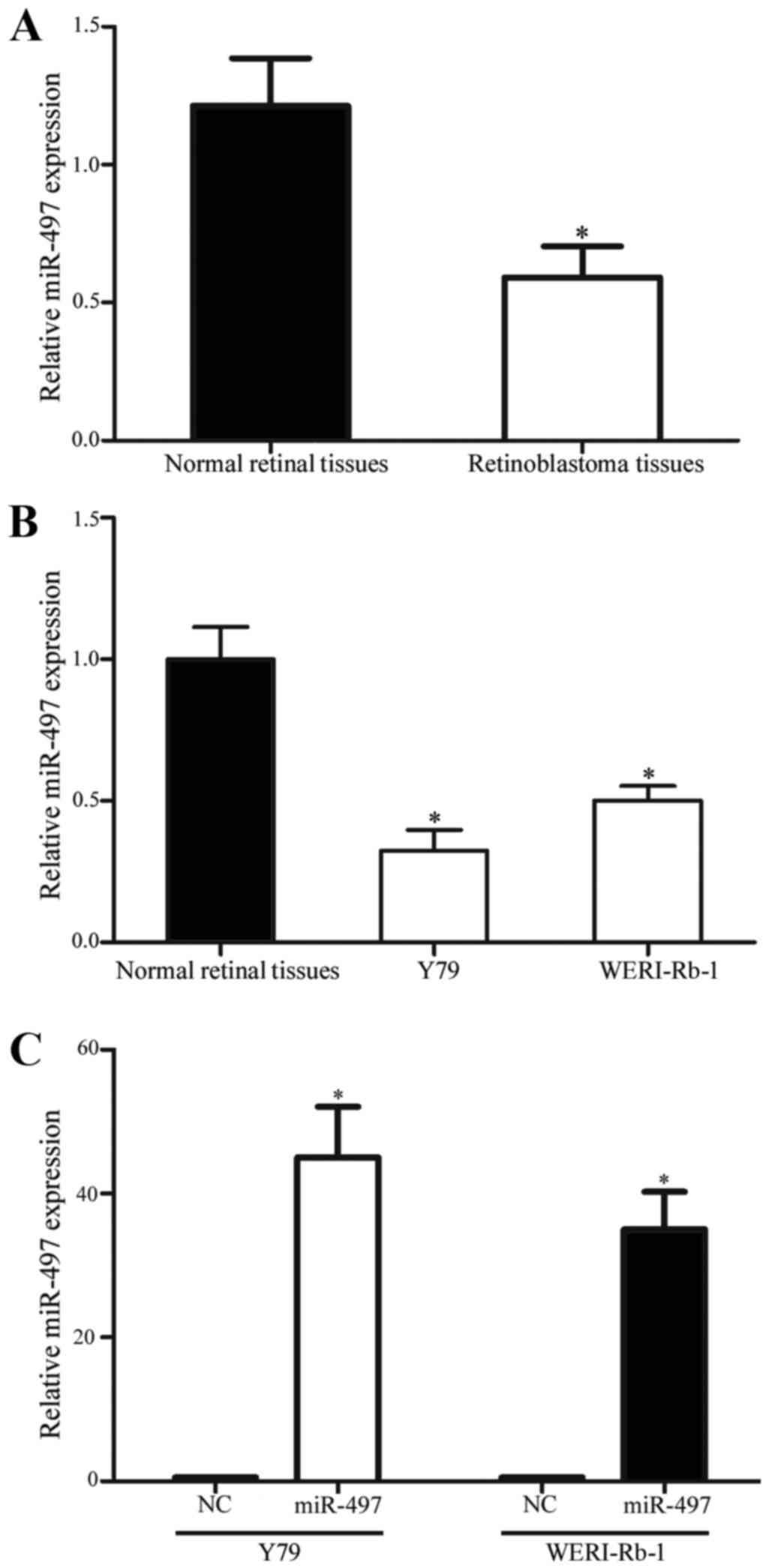
RT-qPCR was performed to evaluate miR-497 expression
in 23 retinoblastoma tissues and 5 normal retinal tissues. As shown
in Fig. 1A, miR-497 was significantly
downregulated in retinoblastoma tissues compared with in normal
retinal tissues (P<0.05). In addition, the present study
examined the expression levels of miR-497 in Y79 and WERI-Rb-1
retinoblastoma cell lines. The results revealed that miR-497 was
also downregulated in Y79 and WERI-Rb-1 cell lines in comparison
with 5 normal retinal tissues (P<0.05; Fig. 1B). Furthermore, the expression levels
of miR-497 in Y79 and WERI-Rb-1 cells following transfection with
miR-497 mimics or NC were evaluated. As shown in Fig. 1C, miR-497 was upregulated in the
miR-497 mimic transfectants compared with the NC transfectants
(P<0.05).
miR-497 suppresses cell proliferation
in Y79 and WERI-Rb-1 cells
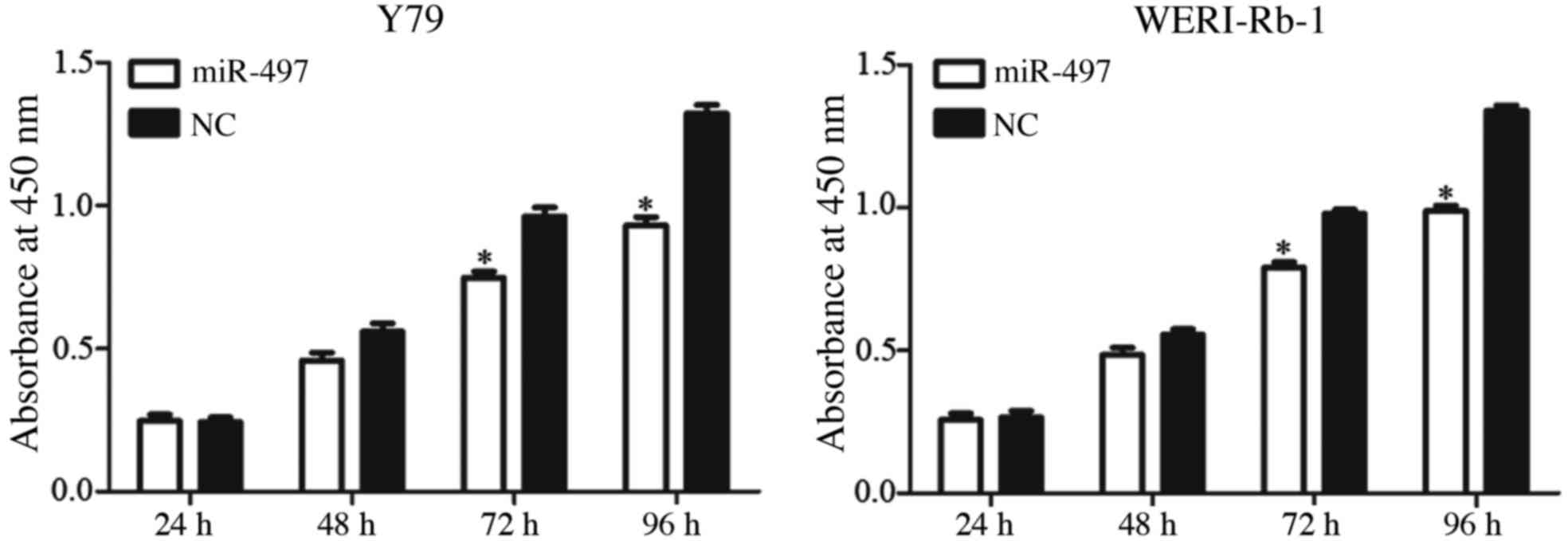
A CCK-8 assay was performed to assess the role of
miR-497 in retinoblastoma cell proliferation. As presented in
Fig. 2, restoration of miR-497 via
transfection with mimics significantly suppressed Y79 and WERI-Rb-1
cell proliferation after 72 and 96 h (P<0.05). After 96 h, cell
proliferation was decreased by 30.48±5.7% in miR-497-transfected
Y79 cells and 26.21±4.5% in miR-497-transfected WERI-Rb-1 cells
compared with the NC-transfected cells (P<0.05).
miR-497 suppresses cell migration and
invasion in Y79 and WERI-Rb-1 cells
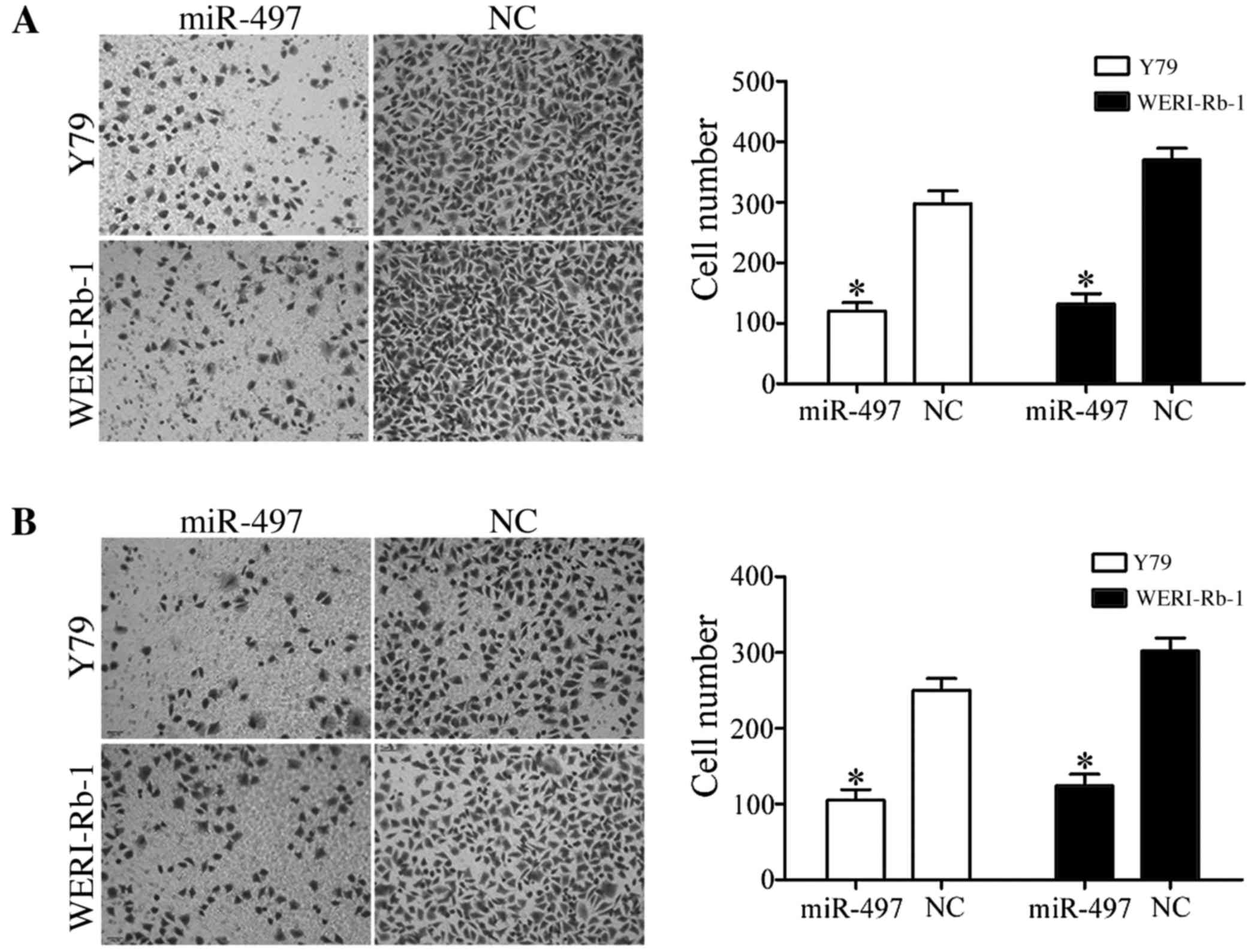
To investigate the effect of miR-497 on cell
migration, Y79 and WERI-Rb-1 cells transfected with miR-497 mimics
or NC were seeded into Transwell chambers. Using a Transwell
chamber coated with Matrigel matrix, the effect of miR-497 on cell
invasion was also investigated. As presented in Fig. 3, in the two cell lines, the
percentages of migrated and invaded cells were decreased in cells
transfected with miR-497 compared with cells transfected with NC
(all P<0.05).
VEGFA is a potential direct target
gene of miR-497 in vitro
Accumulating studies have suggested that miRNAs
exert their functions by directly regulating target mRNA expression
levels (20). Thus, in order to
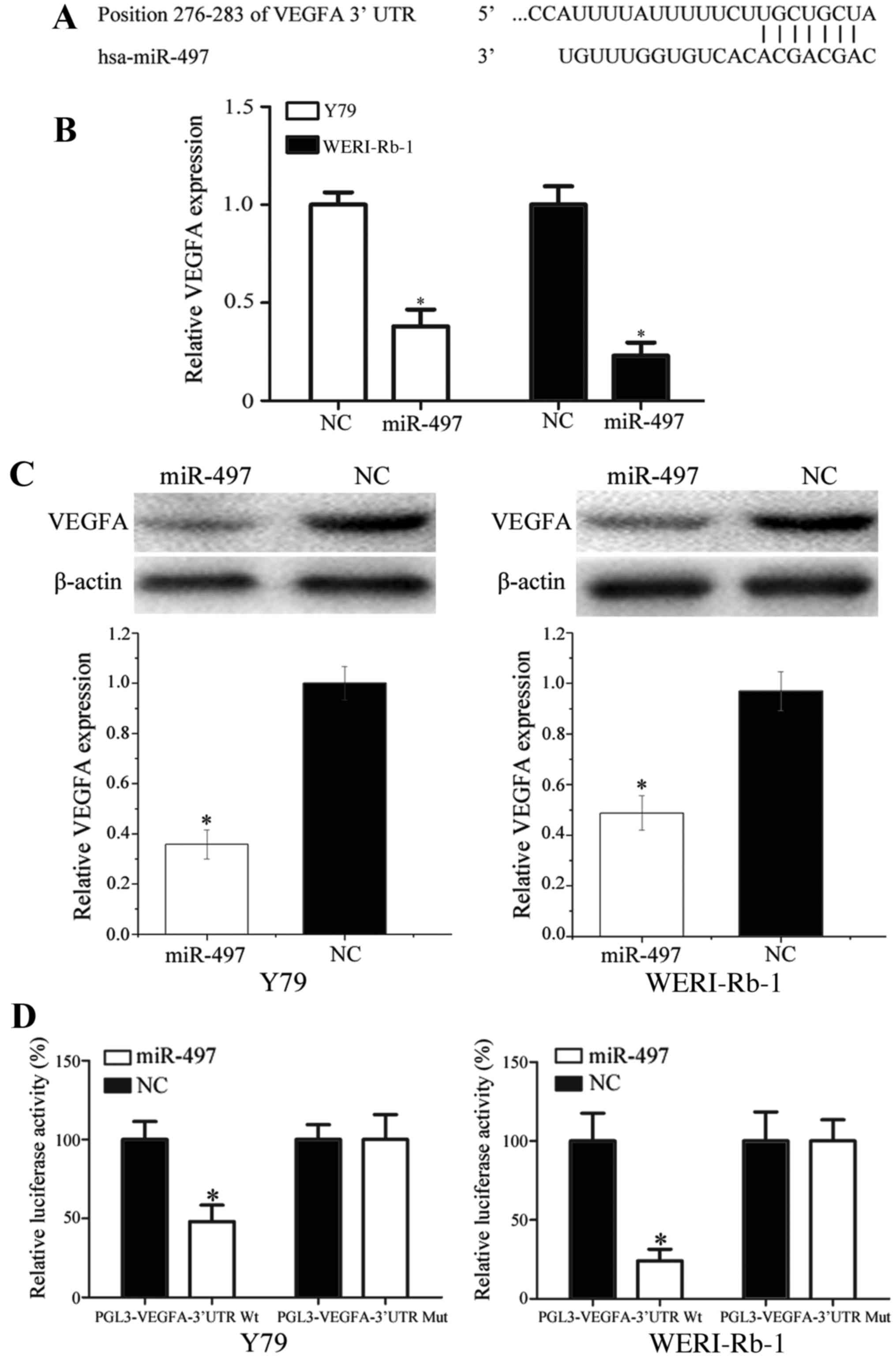
identify the target gene of miR-497, miRanda (http://www.microrna.org) and TargetScan (http://www.targetscan.org/) were used. As presented in
Fig. 4A, VEGFA was identified as a
potential direct target of miR-497; the analysis revealed that
position 276–283 of the VEGFA 3′-UTR comprised a region matching
the miR-497 seed sequence.
To investigate the effect of miR-497 on VEGFA
expression, miR-497 mimics were transfected into Y79 and WERI-Rb-1
cells. The results demonstrated that forced expression of miR-497
significantly decreased VEGFA expression at the mRNA and protein
levels (Fig. 4B and C;
P<0.05).
To confirm that VEGFA was a direct target of
miR-497, Y79 and WERI-Rb-1 cells were co-transfected with miR-497
mimics or NC, and PGL3-VEGFA-3′-UTR-Wt or PGL3-VEGFA-3′-UTR-Mut. It
was revealed that miR-497 mimics suppressed the luciferase activity
of PGL3-VEGFA-3′-UTR-Wt but not PGL3-VEGFA-3′-UTR-Mut in Y79 and
WERI-Rb-1 cells (Fig. 4D; P<0.05).
Taken together, these results suggest that VEGFA is directly
targeted by miR-497 in vitro.
Discussion
miR-497, a member of miR-15/16/195/424/497 family,
is located on chromosome 17p13.1 (21). The loss or deletion of chromosome
17p13.1 has been demonstrated in various types of cancer,
indicating that miR-497 may be downregulated in cancer due to
genomic DNA loss or deletion (22–24). An
increasing number of previous studies have demonstrated that the
expression level of miR-497 is downregulated in numerous cancer
types, including renal cancer (25),
pancreatic cancer (26),
hepatocellular carcinoma (27),
endometrial cancer (28),
nasopharyngeal carcinoma (29), and
breast (30), colorectal (21), gastric (31) and prostate cancer (32). In renal cancer, miR-497 was shown to
be significantly downregulated, and the low miR-497 expression
level was associated with tumor stage, histological grade and lymph
node metastases (25). In pancreatic
cancer, the expression level of miR-497 was significantly decreased
in comparison with adjacent non-tumorous tissues. Furthermore,
downregulation of miR-497 was an independent adverse prognostic
factor for pancreatic cancer and was recommended for investigation
as a prognostic marker (26). Guo
et al (31) revealed that
miR-497 was also downregulated in the plasma of bladder cancer
patients compared with healthy patients, which indicated that
miR-497 expression in the plasma could be explored as a promising
novel circulating biomarker. However, to the best of our knowledge,
the expression level of miR-497 has not been previously studied in
retinoblastoma. The present study demonstrated that miR-497 was
downregulated in retinoblastoma tissues and cell lines, and
provides further evidence regarding the expression level of miR-497
in cancer.
miR-497 has been verified as a tumor suppressor in
cancer. In prostate cancer, miR-497 has been demonstrated to
regulate the nuclear factor-κB (NF-κB) signaling pathway by
targeting inhibitor of NF-κB kinase subunit β, decreasing cell
growth, migration and invasion (33).
In lung cancer, miR-497 inhibited cell growth by downregulation of
cyclin E1 (CCNE1) (24). In ovarian
cancer, upregulation of miR-497 significantly decreased
angiogenesis via blockade of VEGFA (34). In human cervical carcinoma, exogenous
expression of miR-497 suppressed cell proliferation, migration and
invasion through downregulation of insulin-like growth factor 1
receptor (IGF-1R) and CCNE1 (35,36).
Furthermore, in colorectal cancer, ectopic expression of miR-497
targeted IGF-1R to suppress cell survival, growth and invasion, and
to enhance cell sensitivity to apoptosis (21). The present study revealed that miR-497
inhibited cell proliferation, migration and invasion via blockade
of VEGFA. Taken together, these finding indicate that miR-497 could
be developed as a novel molecular marker and targeted therapy in
the future. Exogenous expression of miR-497 or provision of
analogous pharmaceutical compounds exogenously may be effective
therapies for cancers resulting from the overexpression of
miR-497's target oncogenic transcripts.
Identification of miR-497 target genes is essential
for understanding its functions in retinoblastoma carcinogenesis
and progression, and for exploring novel targeted therapies for
retinoblastoma. In the present study, an important molecular
mechanism involving miR-497 and VEGFA was demonstrated in
vitro. Firstly, miRanda and TargetScan predicted that VEGFA was
a potential target of miR-497. Secondly, RT-qPCR and western blot
analysis revealed that miR-497 decreased VEGFA expression at the
mRNA and protein levels. Finally, the Dual-Luciferase reporter
assay revealed that miR-497 directly targeted the VEGFA 3′-UTR.
These findings indicate that miR-497 contributed to retinoblastoma
carcinogenesis and progression via directly targeting VEGFA.
VEGFA is a 35–45 kDa heparin-binding glycoprotein
and a central mediator of inflammation and angiogenesis (37). Increasing evidence has suggested that
VEGFA is upregulated in numerous cancer subtypes, including
retinoblastoma (38). VEGFA has been
revealed to be involved in vasculogenesis, angiogenesis, cell
proliferation, migration, invasion and tumor angiogenesis (39–41). A
number of anti-VEGFA monoclonal antibodies that specifically bind
to the VEGFA receptor to inhibit VEGFA signaling are in development
at present, and certain of these antibodies are currently in
clinical trials for cancer treatment (37). Therefore, regarding its
cancer-associated functions, VEGFA is worthy of attention as a
potential target for inhibition in retinoblastoma. The present
study revealed that miR-497 inhibited proliferation, migration and
invasion of retinoblastoma cells by negative regulation of VEGFA.
This suggested that miR-497 could be investigated as a targeted
therapy for retinoblastoma.
VEGFA has been demonstrated to be regulated by
multiple miRNAs in cancer. For example, in lung cancer, miR-126 was
demonstrated to suppress lung cancer cell proliferation via
blockade of VEGFA (42). In
hepatocellular carcinoma, miR-146a was shown to be downregulated
and significantly associated with liver cancer metastasis, whereas
its upregulation inhibited hepatocellular carcinoma cell invasion
and metastasis by repression of VEGFA expression (43). In breast cancer, ectopic expression of
miR-185 decreased cell growth and invasion and contributed to
breast cancer formation by directly targeting VEGFA (44). In osteosarcoma, miR-145 and miR-410
targeted VEGFA to suppress cell growth and metastasis (37,45). The
present study verified that miR-497 negatively regulated the
expression level of VEGFA to inhibit cell proliferation, migration
and invasion in retinoblastoma. Taken together, these findings
indicate that an miR-497/VEGFA-based targeted therapy may be a
novel therapy for retinoblastoma.
In summary, the present study revealed that miR-497
was downregulated in retinoblastoma, and that forced expression of
miR-497 significantly decreased cell proliferation, migration and
invasion in retinoblastoma cells by directly targeting VEGFA. These
findings indicated that miR-497 served an important role in
inhibiting retinoblastoma carcinogenesis and progression by
targeting VEGFA and should be investigated as a potential novel
targeted therapy for retinoblastoma.
References
|
1
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
MacCarthy A, Draper GJ, Steliarova-Foucher
E and Kingston JE: Retinoblastoma incidence and survival in
European children (1978–1997). Report from the automated childhood
cancer information system project. Eur J Cancer. 42:2092–2102.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beta M, Venkatesan N, Vasudevan M,
Vetrivel U, Khetan V and Krishnakumar S: Identification and
insilico analysis of retinoblastoma serum microRNA profile and gene
targets towards prediction of novel serum biomarkers. Bioinform
Biol Insights. 7:21–34. 2013.PubMed/NCBI
|
|
5
|
Wang J, Wang X, Wu G, Hou D and Hu Q:
MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle
progression and apoptosis of human retinoblastoma cells by
targeting PAX6. FEBS Lett. 587:1779–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shields CL and Shields JA: Diagnosis and
management of retinoblastoma. Cancer Control. 11:317–327.
2004.PubMed/NCBI
|
|
7
|
Xu X, Jia R, Zhou Y, Song X, Wang J, Qian
G, Ge S and Fan X: Microarray-based analysis: Identification of
hypoxia-regulated microRNAs in retinoblastoma cells. Int J Oncol.
38:1385–1393. 2011.PubMed/NCBI
|
|
8
|
Friedman DL, Himelstein B, Shields CL,
Shields JA, Needle M, Miller D, Bunin GR and Meadows AT:
Chemoreduction and local ophthalmic therapy for intraocular
retinoblastoma. J Clin Oncol. 18:12–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta M, Sethi S, Pushker N, Kashyap S,
Sen S, Bajaj MS and Ghose S: Retinoblastoma. Singapore Med J.
53:128–135. 2012.PubMed/NCBI
|
|
10
|
Shen F, Mo MH, Chen L, An S, Tan X, Fu Y,
Rezaei K, Wang Z, Zhang L and Fu SW: MicroRNA-21 down-regulates Rb1
expression by targeting PDCD4 in retinoblastoma. J Cancer.
5:804–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Wang X, Li Z, Liu H and Teng Y:
MicroRNA-183 suppresses retinoblastoma cell growth, invasion and
migration by targeting LRP6. FEBS J. 281:1355–1365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalgard CL, Gonzalez M, deNiro JE and
O'Brien JM: Differential microRNA-34a expression and tumor
suppressor function in retinoblastoma cells. Invest Ophthalmol Vis
Sci. 50:4542–4551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96
Suppl:R40–R44. 2007.PubMed/NCBI
|
|
15
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun YC, Wang J, Guo CC, Sai K, Wang J,
Chen FR, Yang QY, Chen YS, Wang J, To TS, et al: MiR-181b
sensitizes glioma cells to teniposide by targeting MDM2. BMC
Cancer. 14:6112014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Finnerty JR, Wang WX, Hébert SS, Wilfred
BR, Mao G and Nelson PT: The miR-15/107 group of microRNA genes:
Evolutionary biology, cellular functions, and roles in human
diseases. J Mol Biol. 402:491–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harries LW: Long non-coding RNAs and human
disease. Biochem Soc Trans. 40:902–906. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bailey-Wilson JE, Amos CI, Pinney SM,
Petersen GM, de Andrade M, Wiest JS, Fain P, Schwartz AG, You M,
Franklin W, et al: A major lung cancer susceptibility locus maps to
chromosome 6q23-25. Am J Hum Genet. 75:460–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng RC, Chang JW, Hsien FJ, Chang YH,
Hsiao CF, Chen JT, Chen CY, Jou YS and Wang YC: Genomewide loss of
heterozygosity and its clinical associations in non small cell lung
cancer. Int J Cancer. 117:241–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang
Y, Yang C and Jiang Y: miR-497 and miR-34a retard lung cancer
growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget.
6:13149–13163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X, Zhao Z, Xu W, Hou J and Du X:
Down-regulation of miR-497 is associated with poor prognosis in
renal cancer. Int J Clin Exp Pathol. 8:758–764. 2015.PubMed/NCBI
|
|
26
|
Xu J, Wang T, Cao Z, Huang H, Li J, Liu W,
Liu S, You L, Zhou L, Zhang T and Zhao Y: MiR-497 downregulation
contributes to the malignancy of pancreatic cancer and associates
with a poor prognosis. Oncotarget. 5:6983–6993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF
and Wang HY: Checkpoint kinase 1 is negatively regulated by miR-497
in hepatocellular carcinoma. Med Oncol. 31:8442014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiroki E, Akahira J, Suzuki F, Nagase S,
Ito K, Suzuki T, Sasano H and Yaegashi N: Changes in microRNA
expression levels correlate with clinicopathological features and
prognoses in endometrial serous adenocarcinomas. Cancer Sci.
101:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen HC, Chen GH, Chen YH, Liao WL, Liu
CY, Chang KP, Chang YS and Chen SJ: MicroRNA deregulation and
pathway alterations in nasopharyngeal carcinoma. Br J Cancer.
100:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang
Y, Zou C, Zhang X, Liu S, Wang X, et al: Analysis of MiR-195 and
MiR-497 expression, regulation and role in breast cancer. Clin
Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Li B, Li L and Wang T:
MicroRNA-497 suppresses proliferation and induces apoptosis in
prostate cancer cells. Asian Pac J Cancer Prev. 14:3499–3502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong XJ, Duan LJ, Qian XQ, Xu D, Liu HL,
Zhu YJ and Qi J: Tumor-suppressive microRNA-497 targets IKKb to
regulate NF-κB signaling pathway in human prostate cancer cells. Am
J Cancer Res. 5:1795–1804. 2015.PubMed/NCBI
|
|
34
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
35
|
Han J, Huo M, Mu M, Liu J and Zhang J:
miR-497 suppresses proliferation of human cervical carcinoma HeLa
cells by targeting cyclin E1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
30:597–600. 2014.(In Chinese). PubMed/NCBI
|
|
36
|
Luo M, Shen D, Zhou X, Chen X and Wang W:
MicroRNA-497 is a potential prognostic marker in human cervical
cancer and functions as a tumor suppressor by targeting the
insulin-like growth factor 1 receptor. Surgery. 153:836–847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan L, Wu Q, Xing X, Wei Y and Shao Z:
MicroRNA-145 targets vascular endothelial growth factor and
inhibits invasion and metastasis of osteosarcoma cells. Acta
Biochim Biophys Sin (Shanghai). 44:407–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Youssef NS and Said AM:
Immunohistochemical expression of CD117 and vascular endothelial
growth factor in retinoblastoma: Possible targets of new therapies.
Int J Clin Exp Pathol. 7:5725–5737. 2014.PubMed/NCBI
|
|
39
|
Zhuang Y and Wei M: Impact of vascular
endothelial growth factor expression on overall survival in
patients with osteosarcoma: A meta-analysis. Tumour Biol.
35:1745–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Zheng Q, Wu H, Guo X, Li J and Hao
S: Rapamycin increases pCREB, Bcl-2, and VEGF-A through ERK under
normoxia. Acta Biochim Biophys Sin (Shanghai). 45:259–267. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wiszniak S, Mackenzie FE, Anderson P,
Kabbara S, Ruhrberg C and Schwarz Q: Neural crest cell-derived VEGF
promotes embryonic jaw extension. Proc Natl Acad Sci USA. 112:pp.
6086–6091. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang X, Chen BB, Zhang MH and Wang XR:
MicroRNA-126 inhibits the proliferation of lung cancer cell line
A549. Asian Pac J Trop Med. 8:239–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Zhang Y, Sun XX, Ma X and Chen
ZN: microRNA-146a inhibits cancer metastasis by downregulating VEGF
through dual pathways in hepatocellular carcinoma. Mol Cancer.
14:52015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang R, Tian S, Wang HB, Chu DP, Cao JL,
Xia HF and Ma X: MiR-185 is involved in human breast carcinogenesis
by targeting Vegfa. FEBS Lett. 588:4438–4447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao D, Jia P, Wang W and Zhang G:
VEGF-mediated suppression of cell proliferation and invasion by
miR-410 in osteosarcoma. Mol Cell Biochem. 400:87–95. 2015.
View Article : Google Scholar : PubMed/NCBI
|